Abstract
Polyphenols are organic chemical compounds naturally present in plants, renowned for their anti-inflammatory, antioxidant, immunomodulatory, anticancer, and cardiovascular protective properties. Their bioactivity and bioavailability can vary widely depending on the methods of food processing and interactions with the gut microbiome. These factors can induce changes in polyphenols, affecting their ability to achieve their intended health benefits. Thus, it is essential to develop and apply food processing methods that optimize polyphenol content while maintaining their bioactivity and bioavailability. This review aims to explore how various food processing techniques affect the quantity, bioactivity, and bioavailability of polyphenols, as well as their interactions with the gut microbiome, which may ultimately determine their health effects.
1. Introduction
Polyphenols are naturally occurring organic compounds found in plants that are essential for human health. They are recognized for their anti-inflammatory, antioxidant, immunomodulatory, anticancer, and cardiovascular protective properties [1]. Their impact on health is primarily determined by their bioavailability, which is shaped by various food processing techniques and interactions with gut microbiota [2,3].
The gut microbiota, composed of millions of microorganisms within the digestive tract, is crucial in the metabolism of polyphenols, affecting their transformation and subsequent bioavailability [4,5]. The interactions between polyphenols and the microbiota can result in the formation of bioactive metabolites, which confer significant health benefits, including anti-inflammatory, antioxidant properties, and the protection of the gut barrier [6]. Thus, the relationship between polyphenols and gut microbiota is fundamental to human health.
In recent years, new insights have emerged regarding the interaction of polyphenols with the gut microbiota and the effects of various food processing techniques on the polyphenol content and bioavailability [3,7]. The available evidence suggests that processing techniques, including thermal treatment, drying, fermentation, and cold processing, can markedly alter the polyphenol content, consequently affecting their bioavailability and bioactivity [8,9]. Nevertheless, further extensive research is essential to enhance our comprehension of these processes and their implications for human health.
The objective of this review is to provide a comprehensive examination of the impact of diverse food processing techniques on the bioactivity and bioavailability of polyphenols, as well as their interactions with the gut microbiota. The objective of this review is to present updated information and perspectives on recent studies, which may offer new avenues for research on polyphenols and their health effects. This literature review is primarily concerned with studies conducted within the last five years. Nevertheless, some older studies of fundamental importance are also discussed.
2. Review
Polyphenols are organic chemical compounds that naturally occur in plants, constituting one of the most abundant and diverse groups of natural compounds in the plant kingdom. These compounds are classified as secondary metabolites, produced through the shikimate pathway derived from phenylpropanoids and/or the polyketide pathway [10]. With over 80,000 identified polyphenols, their molecular weights can reach up to 30,000 Da [10]. The polyphenol content in food products varies considerably, with certain items like dried herbs and cocoa powder containing over 1 g of polyphenols per 100 g of product [11,12]. In contrast, some plant-based foods, such as tomatoes, cucumbers, potatoes, and lemons, have significantly lower polyphenol content [12].
2.1. Classification of Polyphenols
Although polyphenols share common phenolic structural features, they exhibit significant structural diversity, resulting in their classification into several subgroups. Polyphenols can be categorized based on the number of phenol rings they contain and the structural elements that link these rings. The four primary structural groups of polyphenols are flavonoids, phenolic acids, lignans, and stilbenes (see Figure 1) [13,14,15]. Among these, flavonoids are the largest group, comprising approximately 60% of all polyphenols, followed by phenolic acids, which account for about 30% [14].
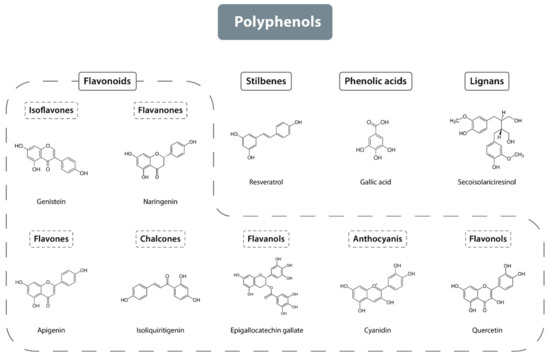
Figure 1.
Classification of polyphenols.
2.1.1. Flavonoids
Flavonoids, a prominent subgroup within the polyphenol family, comprise over 6000 distinct chemical structures. The fundamental structure of flavonoids consists of two aromatic rings linked by three carbon atoms, forming an oxidized heterocyclic ring. Flavonoids can be categorized into two primary types based on the saturation of the central heterocyclic ring [16]. Saturated flavonoids include compounds such as flavanones, dihydroflavonols, and flavan-3-ols, while unsaturated flavonoids, characterized by a double bond between the 2 and 3 carbon atoms, include anthocyanidins, flavones, flavonols, and isoflavones. Chalcones represent a distinct subclass of flavonoids due to their unique chemical structure, characterized by an open-chain configuration instead of the closed heterocyclic ring typical of other flavonoids [17]. Although this structural classification is the most common, flavonoids can also be categorized based on molecular size and the degree of substitution on the A and B rings (flavonoids are composed of 15 carbon atoms arranged in three rings, labeled A, B, and C) [17].
Flavonoids in plants can exist either in free form or bound to sugars. Methylation of flavonoids can enhance their cellular uptake and stability, while glycosylation improves their solubility, metabolism, and distribution, as well as facilitating their transport across cell membranes [18].
Flavonoids are abundant in plants, nuts, and fruits, where they contribute to the fragrance, taste, and appearance of these foods. They serve various beneficial functions, including attracting pollinating insects, neutralizing reactive oxygen species, and acting as UV filters [19]. A diet rich in flavonoids is based on plant-based foods, including beverages such as beer, wine, and tea, as well as foods like fruits, vegetables, nuts, and edible flowers [20]. The phenolic compound content in plants is influenced by several factors, including the plant species, growth and storage conditions, temperature, soil type, environment, and harvesting methods [20].
Flavonoids are categorized into seven main classes based on their structural transformations: flavones, flavanones, isoflavones, flavonols, chalcones, flavanols, and anthocyanins (see Figure 1) [21,22]. Among these, flavones are one of the largest classes and are primarily found in parsley, red pepper, chamomile, celery, mint, and ginkgo [23]. Notable flavonols include quercetin, galangin, kaempferol, and myricetin, which are present in onions, asparagus, broccoli, lettuce, tomatoes, and apples [24]. Flavanones are predominantly found in citrus fruits [25], isoflavones are mainly in legume seeds [26], flavanols are present in fruits such as apples, pears, bananas, and blueberries [27]. Anthocyanins are found in various vegetables and fruits including blackcurrants, grapes, and berries, though they are not present in all fruits and vegetables, as their occurrence is linked to purple, red, and black pigmentation [28]. Chalcones occur in plants from the Fabaceae, Zingiberaceae, Moraceae, and Cannabaceae families [29].
2.1.2. Phenolic Acids
Phenolic acids are generated as by-products from the degradation of cell wall polymers or through microbial activity, and are predominantly found in fruits, vegetables, grains, and seeds. They are primarily classified into two groups: derivatives of hydroxybenzoic acid and derivatives of cinnamic acid. Hydroxybenzoic acid derivatives include vanillic acid and gallic acid, whereas cinnamic acid derivatives encompass ferulic acid and caffeic acid [30].
2.1.3. Lignans
Lignans are a diverse group of naturally occurring compounds synthesized via the shikimic acid pathway [31]. Their basic structure comprises two or more phenylpropanoid units. The monomers that contribute to lignan formation include cinnamic acid, cinnamyl alcohol, allylbenzene, and propenylbenzene [32]. Based on the molecular arrangement of these monomers, lignans are classified into two categories: classical lignans and neolignans. Neolignans are distinguished by their more varied structures compared to classical lignans [33]. To date, more than 200 classical lignans and 100 neolignans have been identified [34].
Lignans are present in a variety of plant-based products, including fruits, seeds, flowers, and leaves. While most lignans are found in their free form, some are bound to sugars, forming glycosides and other derivatives [33].
2.1.4. Stilbenes
Stilbenes consist of two phenyl groups linked by a two-carbon methylene bridge. Most stilbenes are produced as a defensive response to injury or infection. They are predominantly synthesized in plants such as peanuts, grapes, rhubarb, and berries. A prominent compound in this group is resveratrol [35,36].
2.2. Bioavailability of Polyphenols
The impact of polyphenols on health is largely determined by their bioavailability. Bioavailability refers to the proportion of polyphenols that, after being released from the food matrix, is metabolized and absorbed, and subsequently reaches target cells or tissues to exert its bioactivity and produce beneficial health effects [2]. It is crucial to differentiate between bioavailability and bioactivity. Bioactivity pertains to the fraction of polyphenols that, after being released from the food matrix, reaches the gastrointestinal system, undergoes digestion, is absorbed by intestinal epithelial cells, and is subjected to metabolic changes in the intestines and liver [3,7]. This concept does not encompass the utilization of polyphenols in target cells or tissues, which is addressed by bioavailability [37]. Among polyphenols, isoflavones exhibit the highest bioavailability, followed by phenolic acids, flavanols, flavanones, flavonols, and anthocyanins. Conversely, the bioavailability of flavonoids tends to be relatively low due to their reduced bioactivity [38,39].
The bioavailability of polyphenols is influenced by several factors. Principal among these are the initial polyphenol content in the food, the processing methods employed, and the gut microbiota. The polyphenol content is also affected by the storage conditions of the raw material, including temperature, duration, and environmental factors. Furthermore, industrial and domestic processing methods, such as drying or fermentation, can alter the bioavailability of polyphenols in the final product (see Figure 2) [2,40].
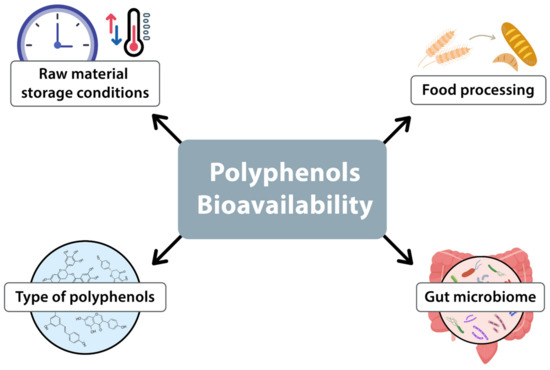
Figure 2.
Factors influencing the bioavailability of polyphenols.
The chemical structure of polyphenols plays a significant role in determining their bioavailability. In food, polyphenols are frequently present as polymers or glycosides. Glycosides are composed of an aglycone (the non-sugar component) and a glycone (the sugar component). Most polyphenols are resistant to adverse conditions during digestion and undergo hydrolysis primarily when exposed to intestinal enzymes or gut microbiota [41].
The bioavailability of polyphenols is regulated by both systemic and intestinal factors. Simple forms of polyphenols can be absorbed in the small intestine, while more complex forms reach the large intestine, where they are transformed into bioactive metabolites by the gut microbiota. Anthocyanins are unique in that they are absorbed directly from the stomach with the assistance of bilitranslocase, a specific membrane transporter [42,43,44].
In the intestine, polyphenols may interact with other dietary components, such as binding with macromolecules like dietary fiber. This interaction can lead to the formation of colloidal structures and chemical complexes, which may either enhance or reduce their bioavailability [45]. Polyphenol molecules that can be absorbed from the small intestine are typically aglycones. However, since most polyphenols in food are found as esters, glycosides, or polymers, they often reach the large intestine. There, they are transformed by the gut microbiota to facilitate their subsequent absorption [45].
2.3. Food Processing and Polyphenol Bioavailability
Food processing aims to extend the shelf life of products, enhance their taste and texture, and improve the bioavailability of active ingredients present in raw materials. However, some processing techniques can lead to a reduction in polyphenol content. Consequently, the final polyphenol content in a product is significantly influenced by the type of food processing methods used, the duration of processing, and the nature of the raw materials employed [8].
Among the various food processing methods, thermal treatment is one of the most commonly used. This includes processes such as boiling, steaming, frying, braising, baking, drying, sterilization, pasteurization, canning, roasting, and toasting. The impact of these methods on polyphenol content largely depends on the specific technique applied. High temperatures can either increase bioavailability by damaging plant cell walls or reduce polyphenol content through oxidation [9].
2.3.1. Thermal Processing
Thermal processing is commonly employed in both domestic and industrial food preparation. Heat treatment leads to the breakdown of cell walls, which can facilitate the migration of polyphenolic compounds to different parts of the plant, potentially enhancing their bioavailability. However, this process also increases the susceptibility of polyphenols to oxidation. Furthermore, the thermostability of polyphenols varies, with some being more or less stable under heat [3,46].
Cooking, as a form of thermal processing, is often associated with significant losses of polyphenols, primarily because these compounds are water-soluble and can leach into the cooking liquid [46]. In contrast, cooking in oil tends to result in lower polyphenol losses [47]. The volume of the cooking solution also impacts polyphenol retention, with smaller amounts of water reducing the extent of polyphenol leaching [48]. Methods such as frying, steaming, and baking generally lead to lower losses of polyphenols compared to boiling [46].
Thermal processing methods such as cooking, grilling, and baking have been shown to reduce polyphenol content and diminish antioxidant activity in faba beans [49]. However, alternative processes such as cold and hot soaking, as well as sprouting for 3 to 5 days, may increase the phenolic content in faba beans. Despite this, thermal processing is more effective in reducing antinutritional factors compared to soaking and fermenting [49]. The impact of various thermal processing methods on polyphenol content is presented in Table 1.

Table 1.
Recent case examples of variations in polyphenol levels due to thermal processing.
The polyphenol content can vary depending on the thermal processing method and the type of raw material. Significant losses are generally observed during boiling, as polyphenols tend to leach into the cooking water. Furthermore, food processing techniques such as baking and frying may lead to higher levels of polyphenols, likely due to the breakdown of the food matrix. Careful selection of temperature and time can further improve polyphenol retention, emphasizing the importance of optimization.
2.3.2. Drying
Drying is a process that reduces the water content in a product to a level that inhibits microbial growth and reproduction, thus preventing spoilage and extending shelf life. Various drying methods include freeze-drying, vacuum drying, sun drying, and air-drying [3].
Freeze-drying has been shown to be the most effective method for preserving polyphenol content in products, whereas high-temperature drying is generally associated with greater losses of these compounds [66]. However, it is not only the temperature, but also the duration of the drying process that influences polyphenol retention. The final polyphenol content in a product is significantly affected by the specific types of polyphenols present [3].
Moreover, the timing of harvest can influence both polyphenol levels and antioxidant activity. Extracts from olive leaves harvested in autumn and summer have been shown to contain higher levels of polyphenols, a trend that remains evident even after drying at high temperatures. This increase in polyphenol content is likely associated with higher solar radiation exposure during these seasons [67]. The impact of various drying methods on polyphenol content is presented in Table 2.

Table 2.
Recent examples of polyphenol alterations during drying processes.
Drying can help preserve polyphenols in food, but it is essential to choose the appropriate temperature, duration, and processing method. Employing lower temperatures or freeze-drying appears to be effective, as these methods are associated with reduced polyphenol losses or even an increase in their content in the final product.
2.3.3. Fermentation
Fermentation is a biological process in which microorganisms such as bacteria, yeast, and molds convert nutrients into metabolically active biomolecules. This important technology is widely used in food and beverage production, as well as in biotechnology, enhancing preservation, taste, texture, and nutritional value of products [85,86]. During fermentation, polyphenols can become more bioactive and bioavailable due to the action of enzymes involved in the process. Additionally, these polyphenols may support the growth of beneficial microbes while inhibiting harmful microorganisms [87,88].
During fermentation, microorganisms may degrade the glycosidic bonds of high-molecular-weight polyphenols, potentially influencing their direct utilization by intestinal microbes [87]. Additionally, enzymes associated with polyphenols, such as tannases, esterases, phenolic acid decarboxylases, and glycosidases, may be released during fermentation. These enzymes can liberate polyphenols that were previously bound within the food matrix, thereby enhancing their bioavailability and bioactivity [87,89]. Furthermore, fermentation can transform high-molecular-weight polyphenols into low-molecular-weight forms. This transformation often results from the enzymatic activity of microorganisms involved in the fermentation process. For instance, in the fermentation of pomegranate, ellagic tannins may be reduced to intermediate products like punicalin and gallagic acid, eventually yielding ellagic acid [90].
The fermentation process leads to changes in the composition and levels of polyphenols, which can impact their activity since different polyphenols exhibit varying levels of effectiveness. Fermentation typically converts high-molecular-weight polyphenols into smaller molecular forms, which are generally associated with higher antioxidant activity [87]. Fermented products often show increased bioactivity and bioavailability of polyphenols [91,92,93]. For instance, a study with human subjects revealed that consuming fermented soy altered isoflavone composition, which enhanced their absorption and increased the bioavailability of specific plasma metabolites [94].
The presence of polyphenols in a product can also positively impact the fermentation process by fostering the growth of beneficial microorganisms and suppressing pathogenic ones [95].
Fermenting foods can increase the polyphenol content in the final product and enhance their bioavailability and bioactivity. This process is associated with higher polyphenol levels due to the enzymatic breakdown of high-molecular-weight polyphenols. Additionally, the presence of polyphenols during fermentation can inhibit the growth of pathogenic microorganisms. The impact of various fermentation methods on polyphenol content is presented in Table 3.

Table 3.
Recent examples of variations in polyphenol content during fermentation.
Fermentation can significantly impact the polyphenol content in products, with critical factors including fermentation time, type of raw material, and the bacterial strains used. Selecting the appropriate fermentation duration is crucial for maximizing the benefits of polyphenols while minimizing their degradation. The type of raw material is also vital, as some materials contain higher levels of polyphenols that can be released or enhanced during fermentation. Additionally, the choice of bacterial strains, such as lactic acid bacteria, can enhance the bioavailability of polyphenols. Moreover, whether the fermentation process is controlled is an important consideration, as controlled conditions provide consistency that can help preserve or even increase polyphenol content.
2.3.4. Cold Processing
Freezing is a method of cold food processing aimed at slowing down biochemical and physicochemical reactions, thereby extending the shelf life of products [113]. This technique generally has minimal impact on polyphenol content. For instance, freezing fresh raspberries at −30 °C did not alter their polyphenol levels. Additionally, freezing has been recognized as a highly promising initial processing method for improving the functional quality of black garlic [114]. Similarly, freezing organic butternut squash did not negatively affect its polyphenol content; rather, it was associated with an increase in bioaccessible polyphenols [115].
The speed of the freezing process can influence the polyphenol content of a product. Slow freezing tends to produce larger ice crystals, which can cause more damage to cell structures, while rapid freezing results in smaller ice crystals and potentially less cellular damage [116]. However, research on this topic is limited, and existing studies sometimes indicate that freezing may negatively affect polyphenol content and bioactivity. For example, apples frozen at −50 °C showed reduced levels of polyphenols and decreased antioxidant activity [117]. Thus, additional research is necessary to identify the optimal freezing temperatures and processing methods to maximize polyphenol content, bioavailability, and bioactivity in the final product. The impact of various cold processing methods on polyphenol content is presented in Table 4.

Table 4.
Examples of alterations in polyphenol content attributed to cold processing.
Cold processing methods can have a substantial impact on the polyphenol content in products. Generally, lower temperatures aid in preserving polyphenols by reducing enzymatic degradation and oxidation. However, the effects can vary based on the type of product and the specific polyphenols involved. In some instances, cold processing may enhance the bioavailability or concentration of polyphenols, while in others, it could result in minimal losses. Therefore, careful control of temperature and processing time is essential for optimizing polyphenol content in cold-treated products.
2.4. The Impact of Digestion on Polyphenol Content
For maintaining health and adequate nutrition, effective digestion and the breakdown of food into simpler constituents are essential [122]. During digestion, proteins are converted into amino acids, fats into fatty acids, and carbohydrates and glycerol into glucose. Additionally, other molecules, including plant metabolites, are transformed within the digestive tract according to their original structures and chemical properties [122,123]. Polyphenols in plant-based foods may be present in both free and bound forms. A crucial factor affecting their absorption is their release from the food matrix and dissolution during digestion [124]. The quantity of bioavailable polyphenols can differ markedly from their concentration in the food [125]. This disparity is largely influenced by the food matrix, interactions between polyphenols and other compounds, and the digestion process itself [126].
Chewing plays a crucial role in breaking down plant cell walls, which otherwise significantly impedes digestion. Saliva, which mixes with food during this process, can facilitate the dissolution of polyphenols present in fruits and fruit juices, potentially enhancing their bioavailability [127]. Additionally, proteins in human saliva may bind with certain polyphenols, thereby improving their absorption [128]. Hydrophobic polyphenols, in particular, have a greater tendency to interact with these proteins [126]. During the breakdown of plant cell walls, polyphenols may also bind to dietary fiber, creating a barrier that can affect their availability in the acidic environment of the stomach [129]. Despite this, such interactions can influence the fermentation of polyphenols by gut microbiota in the large intestine, leading to the production of metabolites with additional health benefits [129,130].
Most polyphenols remain stable during digestion in the mouth and stomach [131]. Their stability is attributed to their resistance to the conditions in these digestive stages, with hydrolysis primarily occurring through the action of intestinal enzymes and the gut microbiome [8]. Low-molecular-weight polyphenols can be directly absorbed in the small intestine; however, their absorption is limited to only 5–10%. In contrast, high-molecular-weight polyphenols are transported to the large intestine, where they are metabolized by the microbiota, allowing for their transformation into compounds with greater bioavailability [132].
In the intestine, polyphenols can interact with other substances, such as dietary fiber, which may influence their bioavailability [45]. Once absorbed, polyphenols and their metabolites are transported via the portal vein to the liver, where they undergo phase II metabolism, including methylation, sulfation, and glucuronidation [133]. This digestive process results in significant transformations of polyphenols and the formation of metabolites, which then reach target cells and exert their bioactivity, ultimately impacting human health [39].
2.5. Polyphenols and Their Interaction with the Gut Microbiome
The gut microbiome comprises millions of microorganisms residing in the digestive tract, playing a crucial role in sustaining human health by affecting digestion, metabolism, and the functionality of various bodily systems [4,5]. These microorganisms aid in breaking down undigested food, producing essential compounds such as vitamins and short-chain fatty acids, and preventing the colonization of harmful pathogens [5,134]. The composition of the gut microbiota is shaped by factors including genetics, medications, diet, age, and external conditions such as stress exposure [29,135,136].
Dietary polyphenols can significantly influence gut microorganisms by fostering beneficial alterations in their composition, thereby contributing to a healthier gut microbiota profile. These compounds can directly stimulate or inhibit the growth of specific bacterial strains [137]. Once metabolized by the gut microbiota, polyphenols are converted into metabolites with powerful antioxidant and anti-inflammatory properties, which support the production of short-chain fatty acids (SCFAs) and help maintain the integrity of the gut barrier. Furthermore, the microbiota play a role in suppressing intestinal inflammation and stimulating the production of neurotransmitters that influence the central nervous system [6]. One of the primary mechanisms by which polyphenols modulate the gut microbiome is through their prebiotic effect [138,139]. They can act as prebiotics by promoting the growth of beneficial bacteria, such as Lactobacillus and Bifidobacterium species, which in turn enhances the production of SCFAs like butyrate [140]. For example, catechins found in green tea are known to stimulate the growth of Bifidobacterium, leading to increased SCFA production, which is essential for maintaining intestinal health (see Figure 3) [141,142].
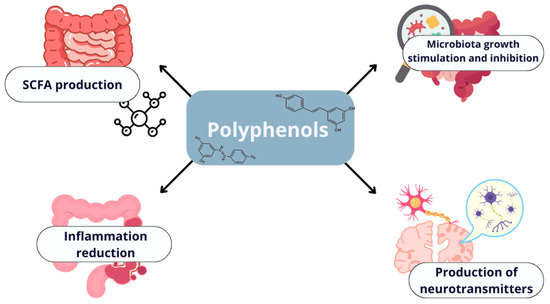
Figure 3.
The impact of polyphenols on the gut microbiome.
Gut microbiota dysbiosis can be induced by several factors, including an unhealthy diet, smoking, excessive alcohol consumption, and stress. This imbalance can result in reduced nutrient absorption, diminished production of SCFAs and vitamins, and may also lead to inflammation, thereby heightening the risk of gastrointestinal disorders and diseases [143]. However, the consumption of polyphenols may help regulate gut microbiome bacteria, potentially supporting overall gut health [144].
The combination of fresh grape pomace with a probiotic containing the Lactiplantibacillus plantarum strain may exhibit antimicrobial effects against E. coli, Listeria monocytogenes, and Bacillus megaterium [145]. Additionally, quercetin may enhance gut microbiome activity by modulating immune responses [146]. Metabolites derived from gallic acid are absorbed into the bloodstream, where they subsequently influence gut activity and potentially help reduce gut dysbiosis [147,148]. Polyphenols found in orange juice, such as hesperidin and naringin, promote the growth of beneficial bacteria like Bifidobacterium spp. and Lactiplantibacillus spp., which in turn increase SCFA production [149]. Similarly, polyphenols in cocoa exhibit positive effects on the gut microbiome; the metabolites produced from cocoa polyphenols stimulate the growth of beneficial bacteria such as Lactobacillus and Bifidobacterium, while inhibiting the growth of Clostridium perfringens [138].
The influence of polyphenols on the gut microbiome is particularly important in the context of metabolic diseases such as obesity [150,151,152] and type 2 diabetes [153,154,155,156]. Research has demonstrated that polyphenols can alter the composition of the gut microbiota by encouraging the growth of metabolically beneficial bacteria like Akkermansia muciniphila, which can enhance insulin sensitivity and reduce inflammation [157,158,159].
The metabolites derived from polyphenols can help reduce intestinal inflammation, promote the growth of beneficial microorganisms, inhibit the proliferation of pathogenic bacteria, and boost SCFA production. Therefore, maintaining a diet rich in plant-based foods high in polyphenols is essential for supporting gut health.
2.6. Strengths and Limitations
This article explores the relationship between dietary polyphenol content, the methods of their processing, and their impact on bioavailability and bioactivity. Additionally, it addresses the reciprocal interactions between polyphenols and the gut microbiota. The primary objective was to conduct an analysis of the current literature, synthesizing the information and methodologies that have informed previous research and contributed to the current understanding in this field.
One of the limitations of this review is its reliance on the existing literature, which unfortunately includes a limited number of in vivo studies specifically assessing the bioavailability and bioactivity of polyphenols. Additionally, there is a scarcity of data comparing polyphenol content in specific foods and dishes based on different processing methods. To draw more comprehensive conclusions, further research is needed to closely examine polyphenol content in processed products and to identify the most effective food processing methods.
Additionally, while this article reviews the current understanding of how food processing affects the bioactivity and bioavailability of polyphenols, as well as their interaction with the gut microbiome, more detailed studies are needed to further explore these topics. Such research would offer a more comprehensive and nuanced understanding of these complex interactions.
2.7. Summary
Polyphenols are organic compounds renowned for their wide-ranging health benefits, including anti-inflammatory, antioxidant, immunomodulatory, anticancer, and cardioprotective effects [1]. The efficacy of these compounds is substantially influenced by their bioavailability and bioactivity, which can be impacted by various factors such as the timing of harvest, soil type, sunlight exposure, and the processing methods applied to the raw material [3,7,37].
Thermal processing, including methods such as boiling, steaming, and baking, is among the most commonly used food processing techniques. Its impact on polyphenol content can vary; it may either preserve or degrade these compounds [3,46]. Drying, especially through freeze-drying, has been found to be effective in preserving polyphenols [3,66,67,68]. Fermentation, on the other hand, modifies food in ways that release polyphenols trapped within the matrix, enhancing their bioavailability and bioactivity [87,89]. Freezing typically does not significantly affect polyphenol content, though the results can vary depending on the specific temperatures used [116,160].
Proper digestion and the breakdown of food into simple components like amino acids, fatty acids, and glucose are essential for maintaining health and ensuring adequate nutrition [122]. Polyphenols, found in plant-based foods, can exist in either free or bound forms, with their bioavailability depending on their release from the food matrix during digestion [72]. Chewing and the presence of saliva can enhance the bioavailability of polyphenols, particularly those with hydrophobic characteristics, by facilitating their dissolution and binding to protein [126,127,128]. Most polyphenols remain stable during digestion in the mouth and stomach, with the majority of absorption occurring in the small intestine, although this process is somewhat limited [131,132]. In the intestines, polyphenols undergo further modifications by the gut microbiome, potentially leading to the formation of health-promoting metabolites [129,130].
During digestion, polyphenols are metabolized in the intestines, where the gut microbiome plays a crucial role in their transformation. This process generates metabolites such as phenolic acids, which may offer various health benefits, including anti-inflammatory effects and antioxidant properties [129,130].
Research into the relationships between gut microbiota, diet, health, and polyphenols is still in its early stages and continues to evolve [161]. A notable gap exists in studies examining how various food processing methods influence gut microbiota, highlighting a promising area for future investigation. Addressing this gap could deepen our understanding of how food processing techniques affect overall health.
2.8. Conclusions
Polyphenols in food offer a diverse range of health benefits, including anti-inflammatory, antioxidant, immunomodulatory, anticancer, and cardioprotective effects. However, their effectiveness is influenced by their bioavailability and bioactivity, which can be altered by various food processing methods. Additionally, the impact of polyphenols is closely linked to digestion and the gut microbiota. The relationship between polyphenols and the microbiota is reciprocal, with each component influencing and modulating the other.
Polyphenols can contribute to reducing inflammation in the gut, enhancing the production of short-chain fatty acids, and fostering the growth of beneficial microbes. Further research is necessary to better understand how different food processing techniques influence polyphenol content and quality, which will inform the selection of optimal processing methods. Equally important is a deeper investigation into the effects of polyphenols on the gut microbiome, which could lead to a more comprehensive understanding of their role in health maintenance.
The quantity of polyphenols present is largely dependent on the nature of the product being processed and the specific method employed. Appropriate processing techniques may result in minimal polyphenol reduction or even an increase in content, emphasizing the critical importance of method selection. Lyophilization and fermentation are notably effective approaches.
Author Contributions
Conceptualization, M.S., A.M.W. and I.M.-C.; investigation, M.S.; writing—original draft preparation, M.S.; writing—review and editing, A.M.W., I.M.-C. and S.K.; visualization, M.S.; supervision, A.M.W., I.M.-C. and S.K.; funding acquisition, A.M.W. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Cheng, Y.-C.; Sheen, J.-M.; Hu, W.L.; Hung, Y.-C. Polyphenols and Oxidative Stress in Atherosclerosis-Related Ischemic Heart Disease and Stroke. Oxid. Med. Cell. Longev. 2017, 2017, 8526438. [Google Scholar] [CrossRef] [PubMed]
- Ifie, I.; Marshall, L.J. Food Processing and Its Impact on Phenolic Constituents in Food. Cogent Food Agric. 2018, 4, 1507782. [Google Scholar] [CrossRef]
- Arfaoui, L. Dietary Plant Polyphenols: Effects of Food Processing on Their Content and Bioavailability. Molecules 2021, 26, 2959. [Google Scholar] [CrossRef]
- Sejbuk, M.; Siebieszuk, A.; Witkowska, A.M. The Role of Gut Microbiome in Sleep Quality and Health: Dietary Strategies for Microbiota Support. Nutrients 2024, 16, 2259. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Zhou, J.; Wang, L. Role and Mechanism of Gut Microbiota in Human Disease. Front. Cell. Infect. Microbiol. 2021, 11, 625913. [Google Scholar] [CrossRef]
- Luo, C.; Wei, X.; Song, J.; Xu, X.; Huang, H.; Fan, S.; Zhang, D.; Han, L.; Lin, J. Interactions between Gut Microbiota and Polyphenols: New Insights into the Treatment of Fatigue. Molecules 2022, 27, 7377. [Google Scholar] [CrossRef]
- Heaney, R.P. Factors Influencing the Measurement of Bioavailability, Taking Calcium as a Model. J. Nutr. 2001, 131, 1344S–1348S. [Google Scholar] [CrossRef]
- Marín, L.; Miguélez, E.M.; Villar, C.J.; Lombó, F. Bioavailability of Dietary Polyphenols and Gut Microbiota Metabolism: Antimicrobial Properties. BioMed Res. Int. 2015, 2015, 905215. [Google Scholar] [CrossRef]
- Minatel, I.O.; Borges, C.V.; Ferreira, M.I.; Gomez, H.A.G.; Chen, C.-Y.O.; Lima, G.P.P. Phenolic Compounds: Functional Properties, Impact of Processing and Bioavailability. In Phenolic Compounds—Biological Activity; Soto-Hernndez, M., Palma-Tenango, M., Garcia-Mateos, M.D.R., Eds.; InTech: Vienna, Austria, 2017; ISBN 978-953-51-2959-2. [Google Scholar]
- Lacroix, S.; Klicic Badoux, J.; Scott-Boyer, M.-P.; Parolo, S.; Matone, A.; Priami, C.; Morine, M.J.; Kaput, J.; Moco, S. A Computationally Driven Analysis of the Polyphenol-Protein Interactome. Sci. Rep. 2018, 8, 2232. [Google Scholar] [CrossRef]
- Pérez-Jiménez, J.; Neveu, V.; Vos, F.; Scalbert, A. Identification of the 100 Richest Dietary Sources of Polyphenols: An Application of the Phenol-Explorer Database. Eur. J. Clin. Nutr. 2010, 64, S112–S120. [Google Scholar] [CrossRef]
- Neveu, V.; Perez-Jimenez, J.; Vos, F.; Crespy, V.; Du Chaffaut, L.; Mennen, L.; Knox, C.; Eisner, R.; Cruz, J.; Wishart, D.; et al. Phenol-Explorer: An Online Comprehensive Database on Polyphenol Contents in Foods. Database 2010, 2010, bap024. [Google Scholar] [CrossRef] [PubMed]
- Li, A.-N.; Li, S.; Zhang, Y.-J.; Xu, X.-R.; Chen, Y.-M.; Li, H.-B. Resources and Biological Activities of Natural Polyphenols. Nutrients 2014, 6, 6020–6047. [Google Scholar] [CrossRef]
- Wang, Q.; Yang, B.; Wang, N.; Gu, J. Tumor Immunomodulatory Effects of Polyphenols. Front. Immunol. 2022, 13, 1041138. [Google Scholar] [CrossRef] [PubMed]
- De Araújo, F.F.; De Paulo Farias, D.; Neri-Numa, I.A.; Pastore, G.M. Polyphenols and Their Applications: An Approach in Food Chemistry and Innovation Potential. Food Chem. 2021, 338, 127535. [Google Scholar] [CrossRef]
- Saini, N.; Gahlawat, S.K.; Lather, V. Flavonoids: A Nutraceutical and Its Role as Anti-Inflammatory and Anticancer Agent. In Plant Biotechnology: Recent Advancements and Developments; Gahlawat, S.K., Salar, R.K., Siwach, P., Duhan, J.S., Kumar, S., Kaur, P., Eds.; Springer: Singapore, 2017; pp. 255–270. ISBN 978-981-10-4731-2. [Google Scholar]
- Dias, M.C.; Pinto, D.C.G.A.; Silva, A.M.S. Plant Flavonoids: Chemical Characteristics and Biological Activity. Molecules 2021, 26, 5377. [Google Scholar] [CrossRef] [PubMed]
- Šamec, D.; Karalija, E.; Šola, I.; Vujčić Bok, V.; Salopek-Sondi, B. The Role of Polyphenols in Abiotic Stress Response: The Influence of Molecular Structure. Plants 2021, 10, 118. [Google Scholar] [CrossRef]
- Panche, A.N.; Diwan, A.D.; Chandra, S.R. Flavonoids: An Overview. J. Nutr. Sci. 2016, 5, e47. [Google Scholar] [CrossRef]
- Rodríguez-García, C.; Sánchez-Quesada, C.; Gaforio, J.J. Dietary Flavonoids as Cancer Chemopreventive Agents: An Updated Review of Human Studies. Antioxidants 2019, 8, 137. [Google Scholar] [CrossRef]
- Rehan, M. Biosynthesis of Diverse Class Flavonoids via Shikimate and Phenylpropanoid Pathway. In Biosynthesis [Working Title]; IntechOpen: Vienna, Austria, 2021. [Google Scholar]
- Zakaryan, H.; Arabyan, E.; Oo, A.; Zandi, K. Flavonoids: Promising Natural Compounds against Viral Infections. Arch. Virol. 2017, 162, 2539–2551. [Google Scholar] [CrossRef]
- Luca, S.V.; Macovei, I.; Bujor, A.; Miron, A.; Skalicka-Woźniak, K.; Aprotosoaie, A.C.; Trifan, A. Bioactivity of Dietary Polyphenols: The Role of Metabolites. Crit. Rev. Food Sci. Nutr. 2020, 60, 626–659. [Google Scholar] [CrossRef]
- Ali, A.; Parisi, A.; Normanno, G. Polyphenols as Emerging Antimicrobial Agents. In Emerging Modalities in Mitigation of Antimicrobial Resistance; Akhtar, N., Singh, K.S., Prerna Goyal, D., Eds.; Springer International Publishing: Cham, Switzerland, 2022; pp. 219–259. ISBN 978-3-030-84125-6. [Google Scholar]
- Najmanová, I.; Vopršalová, M.; Saso, L.; Mladěnka, P. The Pharmacokinetics of Flavanones. Crit. Rev. Food Sci. Nutr. 2020, 60, 3155–3171. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Wang, X.; Cheng, Y.; Gao, H.; Chen, X. A Review of Classification, Biosynthesis, Biological Activities and Potential Applications of Flavonoids. Molecules 2023, 28, 4982. [Google Scholar] [CrossRef] [PubMed]
- Ding, T.; Cao, K.; Fang, W.; Zhu, G.; Chen, C.; Wang, X.; Wang, L. Evaluation of Phenolic Components (Anthocyanins, Flavanols, Phenolic Acids, and Flavonols) and Their Antioxidant Properties of Peach Fruits. Sci. Hortic. 2020, 268, 109365. [Google Scholar] [CrossRef]
- Fang, J. Classification of Fruits Based on Anthocyanin Types and Relevance to Their Health Effects. Nutrition 2015, 31, 1301–1306. [Google Scholar] [CrossRef] [PubMed]
- Vicente, O.; Boscaiu, M. Flavonoids: Antioxidant Compounds for Plant Defence… and for a Healthy Human Diet. Not. Bot. Horti Agrobot. Cluj-Napoca 2018, 46, 14–21. [Google Scholar] [CrossRef]
- Di Lorenzo, C.; Colombo, F.; Biella, S.; Stockley, C.; Restani, P. Polyphenols and Human Health: The Role of Bioavailability. Nutrients 2021, 13, 273. [Google Scholar] [CrossRef]
- Teponno, R.B.; Kusari, S.; Spiteller, M. Recent Advances in Research on Lignans and Neolignans. Nat. Prod. Rep. 2016, 33, 1044–1092. [Google Scholar] [CrossRef]
- Xu, X.-Y.; Wang, D.-Y.; Li, Y.-P.; Deyrup, S.T.; Zhang, H.-J. Plant-Derived Lignans as Potential Antiviral Agents: A Systematic Review. Phytochem. Rev. 2022, 21, 239–289. [Google Scholar] [CrossRef]
- Cui, Q.; Du, R.; Liu, M.; Rong, L. Lignans and Their Derivatives from Plants as Antivirals. Molecules 2020, 25, 183. [Google Scholar] [CrossRef]
- Pan, J.-Y.; Chen, S.-L.; Yang, M.-H.; Wu, J.; Sinkkonen, J.; Zou, K. An Update on Lignans: Natural Products and Synthesis. Nat. Prod. Rep. 2009, 26, 1251. [Google Scholar] [CrossRef]
- Hou, C.-Y.; Tain, Y.-L.; Yu, H.-R.; Huang, L.-T. The Effects of Resveratrol in the Treatment of Metabolic Syndrome. Int. J. Mol. Sci. 2019, 20, 535. [Google Scholar] [CrossRef]
- Al-Khayri, J.M.; Mascarenhas, R.; Harish, H.M.; Gowda, Y.; Lakshmaiah, V.V.; Nagella, P.; Al-Mssallem, M.Q.; Alessa, F.M.; Almaghasla, M.I.; Rezk, A.A.-S. Stilbenes, a Versatile Class of Natural Metabolites for Inflammation—An Overview. Molecules 2023, 28, 3786. [Google Scholar] [CrossRef]
- Ribas-Agustí, A.; Martín-Belloso, O.; Soliva-Fortuny, R.; Elez-Martínez, P. Food Processing Strategies to Enhance Phenolic Compounds Bioaccessibility and Bioavailability in Plant-Based Foods. Crit. Rev. Food Sci. Nutr. 2018, 58, 2531–2548. [Google Scholar] [CrossRef]
- Shivashankara, K.S.; Acharya, S.N. Bioavailability of Dietary Polyphenols and the Cardiovascular Diseases. Open Nutraceuticals J. 2010, 3, 227–241. [Google Scholar] [CrossRef]
- Lippolis, T.; Cofano, M.; Caponio, G.R.; De Nunzio, V.; Notarnicola, M. Bioaccessibility and Bioavailability of Diet Polyphenols and Their Modulation of Gut Microbiota. Int. J. Mol. Sci. 2023, 24, 3813. [Google Scholar] [CrossRef]
- Nayak, B.; Liu, R.H.; Tang, J. Effect of Processing on Phenolic Antioxidants of Fruits, Vegetables, and Grains—A Review. Crit. Rev. Food Sci. Nutr. 2015, 55, 887–918. [Google Scholar] [CrossRef]
- Gowd, V.; Xie, L.; Sun, C.; Chen, W. Phenolic Profile of Bayberry Followed by Simulated Gastrointestinal Digestion and Gut Microbiota Fermentation and Its Antioxidant Potential in HepG2 Cells. J. Funct. Foods 2020, 70, 103987. [Google Scholar] [CrossRef]
- Mithul Aravind, S.; Wichienchot, S.; Tsao, R.; Ramakrishnan, S.; Chakkaravarthi, S. Role of Dietary Polyphenols on Gut Microbiota, Their Metabolites and Health Benefits. Food Res. Int. 2021, 142, 110189. [Google Scholar] [CrossRef]
- Inada, K.O.P.; Silva, T.B.R.; Lobo, L.A.; Domingues, R.M.C.P.; Perrone, D.; Monteiro, M. Bioaccessibility of Phenolic Compounds of Jaboticaba (Plinia jaboticaba) Peel and Seed after Simulated Gastrointestinal Digestion and Gut Microbiota Fermentation. J. Funct. Foods 2020, 67, 103851. [Google Scholar] [CrossRef]
- Kawabata, K.; Yoshioka, Y.; Terao, J. Role of Intestinal Microbiota in the Bioavailability and Physiological Functions of Dietary Polyphenols. Molecules 2019, 24, 370. [Google Scholar] [CrossRef]
- Crozier, A.; Del Rio, D.; Clifford, M.N. Bioavailability of Dietary Flavonoids and Phenolic Compounds. Mol. Aspects Med. 2010, 31, 446–467. [Google Scholar] [CrossRef]
- Peleg, H.; Naim, M.; Rouseff, R.L.; Zehavi, U. Distribution of Bound and Free Phenolic Acids in Oranges (Citrus sinensis) and Grapefruits (Citrus paradisi). J. Sci. Food Agric. 1991, 57, 417–426. [Google Scholar] [CrossRef]
- Miglio, C.; Chiavaro, E.; Visconti, A.; Fogliano, V.; Pellegrini, N. Effects of Different Cooking Methods on Nutritional and Physicochemical Characteristics of Selected Vegetables. J. Agric. Food Chem. 2008, 56, 139–147. [Google Scholar] [CrossRef]
- Andlauer, W.; Stumpf, C.; Hubert, M.; Rings, A.; Fürst, P. Fürst Influence of Cooking Process on Phenolic Marker Compounds of Vegetables. Int. J. Vitam. Nutr. Res. 2003, 73, 152–159. [Google Scholar] [CrossRef]
- Badjona, A.; Bradshaw, R.; Millman, C.; Howarth, M.; Dubey, B. Faba Bean Processing: Thermal and Non-Thermal Processing on Chemical, Antinutritional Factors, and Pharmacological Properties. Molecules 2023, 28, 5431. [Google Scholar] [CrossRef]
- Salazar-Orbea, G.L.; García-Villalba, R.; Tomás-Barberán, F.A.; Sánchez-Siles, L.M. High–Pressure Processing vs. Thermal Treatment: Effect on the Stability of Polyphenols in Strawberry and Apple Products. Foods 2021, 10, 2919. [Google Scholar] [CrossRef]
- Mohapatra, D.; Patel, A.S.; Kar, A.; Deshpande, S.S.; Tripathi, M.K. Effect of different processing conditions on proximate composition, anti-oxidants, anti-nutrients and amino acid profile of grain sorghum. Food Chem. 2019, 271, 129–135. [Google Scholar] [CrossRef]
- Yuste, S.; Macià, A.; Motilva, M.-J.; Prieto-Diez, N.; Romero, M.-P.; Pedret, A.; Solà, R.; Ludwig, I.A.; Rubió, L. Thermal and Non-Thermal Processing of Red-Fleshed Apple: How Are (Poly)Phenol Composition and Bioavailability Affected? Food Funct. 2020, 11, 10436–10447. [Google Scholar] [CrossRef]
- Llorent-Martínez, E.J.; Ortega-Vidal, J.; Ruiz-Riaguas, A.; Ortega-Barrales, P.; Fernández-de Córdova, M.L. Comparative Study of the Phytochemical and Mineral Composition of Fresh and Cooked Broccolini. Food Res. Int. 2020, 129, 108798. [Google Scholar] [CrossRef]
- Chin, L.; Therdthai, N.; Ratphitagsanti, W. Effect of Conventional and Microwave Cooking Conditions on Quality and Antioxidant Activity of Chinese Kale (Brassica alboglabra). Appl. Food Res. 2022, 2, 100079. [Google Scholar] [CrossRef]
- Salve, A.R.; LeBlanc, J.G.; Arya, S.S. Effect of Processing on Polyphenol Profile, Aflatoxin Concentration and Allergenicity of Peanuts. J. Food Sci. Technol. 2021, 58, 2714–2724. [Google Scholar] [CrossRef]
- Torres, C.A.; Sepúlveda, G.; Concha-Meyer, A.A. Effect of Processing on Quality Attributes and Phenolic Profile of Quince Dried Bar Snack. J. Sci. Food Agric. 2019, 99, 2556–2564. [Google Scholar] [CrossRef]
- Călinoiu, L.; Vodnar, D. Thermal Processing for the Release of Phenolic Compounds from Wheat and Oat Bran. Biomolecules 2019, 10, 21. [Google Scholar] [CrossRef]
- Rybak, K.; Wiktor, A.; Witrowa-Rajchert, D.; Parniakov, O.; Nowacka, M. The Effect of Traditional and Non-Thermal Treatments on the Bioactive Compounds and Sugars Content of Red Bell Pepper. Molecules 2020, 25, 4287. [Google Scholar] [CrossRef]
- Kim, M.Y.; Yoon, N.; Lee, Y.J.; Woo, K.S.; Kim, H.Y.; Lee, J.; Jeong, H.S. Influence of Thermal Processing on Free and Bound Forms of Phenolics and Antioxidant Capacity of Rice Hull (Oryza sativa L.). Prev. Nutr. Food Sci. 2020, 25, 310–318. [Google Scholar] [CrossRef]
- Olędzki, R.; Harasym, J. Assessment of the Effects of Roasting, Contact Grilling, Microwave Processing, and Steaming on the Functional Characteristics of Bell Pepper (Capsicum annuum L.). Molecules 2023, 29, 77. [Google Scholar] [CrossRef]
- Vieira, F.N.; Lourenço, S.; Fidalgo, L.G.; Santos, S.A.O.; Silvestre, A.J.D.; Jerónimo, E.; Saraiva, J.A. Long-Term Effect on Bioactive Components and Antioxidant Activity of Thermal and High-Pressure Pasteurization of Orange Juice. Molecules 2018, 23, 2706. [Google Scholar] [CrossRef]
- Yang, M.; Hou, C.-Y.; Lin, M.-C.; Chang, C.-K.; Patel, A.K.; Dong, C.-D.; Chen, Y.-A.; Wu, J.-T.; Hsieh, C.-W. Efficient Thermal Treatment of Radish (Raphanus sativus) for Enhancing Its Bioactive Compounds. J. Food Sci. Technol. 2023, 60, 1045–1053. [Google Scholar] [CrossRef]
- Narwojsz, A.; Borowska, E.J.; Polak-Śliwińska, M.; Danowska-Oziewicz, M. Effect of Different Methods of Thermal Treatment on Starch and Bioactive Compounds of Potato. Plant Foods Hum. Nutr. 2020, 75, 298–304. [Google Scholar] [CrossRef]
- Cattivelli, A.; Conte, A.; Martini, S.; Tagliazucchi, D. Influence of Cooking Methods on Onion Phenolic Compounds Bioaccessibility. Foods 2021, 10, 1023. [Google Scholar] [CrossRef]
- Alfeo, V.; Bravi, E.; Ceccaroni, D.; Sileoni, V.; Perretti, G.; Marconi, O. Effect of Baking Time and Temperature on Nutrients and Phenolic Compounds Content of Fresh Sprouts Breadlike Product. Foods 2020, 9, 1447. [Google Scholar] [CrossRef]
- Wojdyło, A.; Lech, K.; Nowicka, P.; Hernandez, F.; Figiel, A.; Carbonell-Barrachina, A.A. Influence of Different Drying Techniques on Phenolic Compounds, Antioxidant Capacity and Colour of Ziziphus Jujube Mill. Fruits. Molecules 2019, 24, 2361. [Google Scholar] [CrossRef] [PubMed]
- Losada-Echeberría, M.; Naranjo, G.; Malouche, D.; Taamalli, A.; Barrajón-Catalán, E.; Micol, V. Influence of Drying Temperature and Harvesting Season on Phenolic Content and Antioxidant and Antiproliferative Activities of Olive (Olea europaea) Leaf Extracts. Int. J. Mol. Sci. 2022, 24, 54. [Google Scholar] [CrossRef] [PubMed]
- Yan, Z.; Zhou, Z.; Jiao, Y.; Huang, J.; Yu, Z.; Zhang, D.; Chen, Y.; Ni, D. Hot-Air Drying Significantly Improves the Quality and Functional Activity of Orange Black Tea Compared with Traditional Sunlight Drying. Foods 2023, 12, 1913. [Google Scholar] [CrossRef] [PubMed]
- Mohammed, S.; Edna, M.; Siraj, K. The Effect of Traditional and Improved Solar Drying Methods on the Sensory Quality and Nutritional Composition of Fruits: A Case of Mangoes and Pineapples. Heliyon 2020, 6, e04163. [Google Scholar] [CrossRef]
- Lee, S.Y.; Ferdinand, V.; Siow, L.F. Effect of Drying Methods on Yield, Physicochemical Properties, and Total Polyphenol Content of Chamomile Extract Powder. Front. Pharmacol. 2022, 13, 1003209. [Google Scholar] [CrossRef]
- Tan, S.; Tang, J.; Shi, W.; Wang, Z.; Xiang, Y.; Deng, T.; Gao, X.; Li, W.; Shi, S. Effects of Three Drying Methods on Polyphenol Composition and Antioxidant Activities of Litchi Chinensis Sonn. Food Sci. Biotechnol. 2020, 29, 351–358. [Google Scholar] [CrossRef]
- Al Hasani, S.; Al-Attabi, Z.; Waly, M.; Al-Habsi, N.; Al-Subhi, L.; Shafiur Rahman, M. Polyphenol and Flavonoid Stability of Wild Blueberry (Sideroxylon mascatense) during Air- and Freeze-Drying and Storage Stability as a Function of Temperature. Foods 2023, 12, 871. [Google Scholar] [CrossRef]
- Monsalves, J.; Scheuermann, E. Home-Drying Operation Effect on Moisture Content, Electric Energy Consumption, Ascorbic Acid, Total Polyphenol Content, and Color of Sliced “Fuji” Apples. Int. J. Food Sci. 2023, 2023, 9996331. [Google Scholar] [CrossRef]
- Gómez-Mejía, E.; Sacristán, I.; Rosales-Conrado, N.; León-González, M.E.; Madrid, Y. Effect of Storage and Drying Treatments on Antioxidant Activity and Phenolic Composition of Lemon and Clementine Peel Extracts. Molecules 2023, 28, 1624. [Google Scholar] [CrossRef]
- Kim, J.H.; Duan, S.; Park, Y.R.; Eom, S.H. Tissue-Specific Antioxidant Activities of Germinated Seeds in Lentil Cultivars during Thermal Processing. Antioxidants 2023, 12, 670. [Google Scholar] [CrossRef] [PubMed]
- Dorra, S.T.; Farah, D.; Nesrine, H.; Wafa, A.; Youkabed, Z. Drying Behavior of Bulgur and Its Effect on Phytochemical Content. Foods 2022, 11, 1062. [Google Scholar] [CrossRef] [PubMed]
- Conti, V.; Salusti, P.; Romi, M.; Cantini, C. Effects of Drying Methods and Temperatures on the Quality of Chestnut Flours. Foods 2022, 11, 1364. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Wen, H.; Yang, N.; Li, H. Effect of Vacuum Freeze Drying and Hot Air Drying on Dried Mulberry Fruit Quality. PLoS ONE 2023, 18, e0283303. [Google Scholar] [CrossRef]
- Ballistreri, G.; Arena, E.; Fallico, B. Influence of Ripeness and Drying Process on the Polyphenols and Tocopherols of Pistacia vera L. Molecules 2009, 14, 4358–4369. [Google Scholar] [CrossRef]
- Cör Andrejč, D.; Butinar, B.; Knez, Ž.; Tomažič, K.; Knez Marevci, M. The Effect of Drying Methods and Extraction Techniques on Oleuropein Content in Olive Leaves. Plants 2022, 11, 865. [Google Scholar] [CrossRef]
- Hassan, A.M.A.; Zannou, O.; Pashazadeh, H.; Ali Redha, A.; Koca, I. Drying Date Plum (Diospyros lotus L.) Fruit: Assessing Rehydration Properties, Antioxidant Activity, and Phenolic Compounds. J. Food Sci. 2022, 87, 4394–4415. [Google Scholar] [CrossRef]
- Gębczyński, P.; Tabaszewska, M.; Kur, K.; Zbylut-Górska, M.; Słupski, J. Effect of the Drying Method and Storage Conditions on the Quality and Content of Selected Bioactive Compounds of Green Legume Vegetables. Molecules 2024, 29, 1732. [Google Scholar] [CrossRef]
- La Mantia, A.; Ianni, F.; Schoubben, A.; Cespi, M.; Lisjak, K.; Guarnaccia, D.; Sardella, R.; Blasi, P. Effect of Cocoa Roasting on Chocolate Polyphenols Evolution. Antioxidants 2023, 12, 469. [Google Scholar] [CrossRef]
- Cheng, K.; Dong, W.; Long, Y.; Zhao, J.; Hu, R.; Zhang, Y.; Zhu, K. Evaluation of the Impact of Different Drying Methods on the Phenolic Compounds, Antioxidant Activity, and in Vitro Digestion of Green Coffee Beans. Food Sci. Nutr. 2019, 7, 1084–1095. [Google Scholar] [CrossRef]
- Giuffrè, D.; Giuffrè, A.M. Fermentation Technology and Functional Foods. Front. Biosci.-Elite 2024, 16, 8. [Google Scholar] [CrossRef]
- Jahn, L.J.; Rekdal, V.M.; Sommer, M.O.A. Microbial Foods for Improving Human and Planetary Health. Cell 2023, 186, 469–478. [Google Scholar] [CrossRef] [PubMed]
- Yang, F.; Chen, C.; Ni, D.; Yang, Y.; Tian, J.; Li, Y.; Chen, S.; Ye, X.; Wang, L. Effects of Fermentation on Bioactivity and the Composition of Polyphenols Contained in Polyphenol-Rich Foods: A Review. Foods 2023, 12, 3315. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Alonso, A.; Sánchez-Paniagua López, M.; Manzanares-Palenzuela, C.L.; Redondo-Cuenca, A.; López-Ruíz, B. Edible Plant By-Products as Source of Polyphenols: Prebiotic Effect and Analytical Methods. Crit. Rev. Food Sci. Nutr. 2023, 63, 10814–10835. [Google Scholar] [CrossRef] [PubMed]
- Castellone, V.; Bancalari, E.; Rubert, J.; Gatti, M.; Neviani, E.; Bottari, B. Eating Fermented: Health Benefits of LAB-Fermented Foods. Foods 2021, 10, 2639. [Google Scholar] [CrossRef] [PubMed]
- Díaz-Mula, H.M.; Tomás-Barberán, F.A.; García-Villalba, R. Pomegranate Fruit and Juice (Cv. Mollar), Rich in Ellagitannins and Anthocyanins, Also Provide a Significant Content of a Wide Range of Proanthocyanidins. J. Agric. Food Chem. 2019, 67, 9160–9167. [Google Scholar] [CrossRef]
- Song, W.; Lagmay, V.; Jeong, B.-G.; Jung, J.; Chun, J. Changes in Physicochemical and Functional Properties of Opuntia Humifusa during Fermentation with Cellulolytic Enzyme and Lactic Acid Bacteria. LWT 2022, 159, 113192. [Google Scholar] [CrossRef]
- Kano, M.; Takayanagi, T.; Harada, K.; Sawada, S.; Ishikawa, F. Bioavailability of Isoflavones after Ingestion of Soy Beverages in Healthy Adults. J. Nutr. 2006, 136, 2291–2296. [Google Scholar] [CrossRef]
- Heo, S.J.; Kim, A.-J.; Park, M.-J.; Kang, K.; Soung, D.Y. Nutritional and Functional Properties of Fermented Mixed Grains by Solid-State Fermentation with Bacillus Amyloliquefaciens 245. Foods 2020, 9, 1693. [Google Scholar] [CrossRef]
- Jang, H.-H.; Noh, H.; Kim, H.-W.; Cho, S.-Y.; Kim, H.-J.; Lee, S.-H.; Lee, S.-H.; Gunter, M.J.; Ferrari, P.; Scalbert, A.; et al. Metabolic Tracking of Isoflavones in Soybean Products and Biosamples from Healthy Adults after Fermented Soybean Consumption. Food Chem. 2020, 330, 127317. [Google Scholar] [CrossRef]
- Ahmed, I.A.M.; Babiker, E.E.; Al-Juhaimi, F.Y.; Bekhit, A.E.-D.A. Clove Polyphenolic Compounds Improve the Microbiological Status, Lipid Stability, and Sensory Attributes of Beef Burgers during Cold Storage. Antioxidants 2022, 11, 1354. [Google Scholar] [CrossRef] [PubMed]
- De Montijo-Prieto, S.; Razola-Díaz, M.D.C.; Barbieri, F.; Tabanelli, G.; Gardini, F.; Jiménez-Valera, M.; Ruiz-Bravo, A.; Verardo, V.; Gómez-Caravaca, A.M. Impact of Lactic Acid Bacteria Fermentation on Phenolic Compounds and Antioxidant Activity of Avocado Leaf Extracts. Antioxidants 2023, 12, 298. [Google Scholar] [CrossRef] [PubMed]
- Budryn, G.; Klewicka, E.; Grzelczyk, J.; Gałązka-Czarnecka, I.; Mostowski, R. Lactic Acid Fermentation of Legume Seed Sprouts as a Method of Increasing the Content of Isoflavones and Reducing Microbial Contamination. Food Chem. 2019, 285, 478–484. [Google Scholar] [CrossRef] [PubMed]
- Gao, Q.; Song, Y.; Liang, Y.; Li, Y.; Chang, Y.; Ma, R.; Cao, X.; Wang, S. Dynamics of Physicochemical Properties, Functional Compounds and Antioxidant Capacity during Spontaneous Fermentation of Lycium Ruthenicum Murr. (Qinghai–Tibet Plateau) Natural Vinegar. Foods 2022, 11, 1344. [Google Scholar] [CrossRef]
- Pejcz, E.; Lachowicz-Wiśniewska, S.; Nowicka, P.; Wojciechowicz-Budzisz, A.; Spychaj, R.; Gil, Z. Effect of Inoculated Lactic Acid Fermentation on the Fermentable Saccharides and Polyols, Polyphenols and Antioxidant Activity Changes in Wheat Sourdough. Molecules 2021, 26, 4193. [Google Scholar] [CrossRef]
- Fan, Z.; Chen, K.; Ban, L.; Mao, Y.; Hou, C.; Li, J. Silage Fermentation: A Potential Biological Approach for the Long-Term Preservation and Recycling of Polyphenols and Terpenes in Globe Artichoke (Cynara scolymus L.) By-Products. Molecules 2020, 25, 3302. [Google Scholar] [CrossRef]
- Kayath, C.A.; Ibala Zamba, A.; Mokémiabeka, S.N.; Opa-Iloy, M.; Elenga Wilson, P.S.; Kaya-Ongoto, M.D.; Mouellet Maboulou, R.J.; Nguimbi, E. Synergic Involvements of Microorganisms in the Biomedical Increase of Polyphenols and Flavonoids during the Fermentation of Ginger Juice. Int. J. Microbiol. 2020, 2020, 8417693. [Google Scholar] [CrossRef] [PubMed]
- Gabriele, M.; Arouna, N.; Árvay, J.; Longo, V.; Pucci, L. Sourdough Fermentation Improves the Antioxidant, Antihypertensive, and Anti-Inflammatory Properties of Triticum Dicoccum. Int. J. Mol. Sci. 2023, 24, 6283. [Google Scholar] [CrossRef]
- Zhou, D.-D.; Saimaiti, A.; Luo, M.; Huang, S.-Y.; Xiong, R.-G.; Shang, A.; Gan, R.-Y.; Li, H.-B. Fermentation with Tea Residues Enhances Antioxidant Activities and Polyphenol Contents in Kombucha Beverages. Antioxidants 2022, 11, 155. [Google Scholar] [CrossRef]
- Abbasi-Parizad, P.; De Nisi, P.; Adani, F.; Pepé Sciarria, T.; Squillace, P.; Scarafoni, A.; Iametti, S.; Scaglia, B. Antioxidant and Anti-Inflammatory Activities of the Crude Extracts of Raw and Fermented Tomato Pomace and Their Correlations with Aglycate-Polyphenols. Antioxidants 2020, 9, 179. [Google Scholar] [CrossRef]
- Janiszewska-Turak, E.; Witrowa-Rajchert, D.; Rybak, K.; Rolof, J.; Pobiega, K.; Woźniak, Ł.; Gramza-Michałowska, A. The Influence of Lactic Acid Fermentation on Selected Properties of Pickled Red, Yellow, and Green Bell Peppers. Molecules 2022, 27, 8637. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Z.; Zhang, Y.; Wang, W.; Sun, S.; Wang, J.; Li, X.; Dai, F.; Jiang, Y. Changes in Physicochemical Properties, Volatile Profiles, and Antioxidant Activities of Black Apple during High-Temperature Fermentation Processing. Front. Nutr. 2022, 8, 794231. [Google Scholar] [CrossRef]
- Michalak-Tomczyk, M.; Rymuszka, A.; Kukula-Koch, W.; Szwajgier, D.; Baranowska-Wójcik, E.; Jachuła, J.; Welman-Styk, A.; Kędzierska, K. Studies on the Effects of Fermentation on the Phenolic Profile and Biological Activity of Three Cultivars of Kale. Molecules 2024, 29, 1727. [Google Scholar] [CrossRef]
- Tian, Z.-X.; Li, Y.-F.; Long, M.-X.; Liang, Q.; Chen, X.; Huang, D.-M.; Ran, Y.-Q. Effects of Six Different Microbial Strains on Polyphenol Profiles, Antioxidant Activity, and Bioaccessibility of Blueberry Pomace with Solid-State Fermentation. Front. Nutr. 2023, 10, 1282438. [Google Scholar] [CrossRef]
- Chu, R.; Uaila, E.; Ismail, T.; Lazarte, C.E. Effect of Short-Term Lactic Fermentation on Polyphenol Profile and Antioxidant Capacity in White and Red Quinoa Varieties. Foods 2024, 13, 2413. [Google Scholar] [CrossRef]
- Asensio-Grau, A.; Calvo-Lerma, J.; Heredia, A.; Andrés, A. Enhancing the Nutritional Profile and Digestibility of Lentil Flour by Solid State Fermentation with Pleurotus Ostreatus. Food Funct. 2020, 11, 7905–7912. [Google Scholar] [CrossRef]
- Miłek, M.; Mołoń, M.; Kula-Maximenko, M.; Sidor, E.; Zaguła, G.; Dżugan, M. Chemical Composition and Bioactivity of Laboratory-Fermented Bee Pollen in Comparison with Natural Bee Bread. Biomolecules 2023, 13, 1025. [Google Scholar] [CrossRef] [PubMed]
- Collins, A.; Santhakumar, A.; Latif, S.; Chinkwo, K.; Francis, N.; Blanchard, C. Impact of Processing on the Phenolic Content and Antioxidant Activity of Sorghum Bicolor L. Moench. Molecules 2024, 29, 3626. [Google Scholar] [CrossRef] [PubMed]
- Tucker, G.S. (Ed.) Food Biodeterioration and Preservation, 1st ed.; Wiley: Hoboken, NJ, USA, 2008; ISBN 978-1-4051-5417-8. [Google Scholar]
- Chan, K.-H.; Chang, C.-K.; Gavahian, M.; Yudhistira, B.; Santoso, S.P.; Cheng, K.-C.; Hsieh, C.-W. The Impact of Different Pretreatment Processes (Freezing, Ultrasound and High Pressure) on the Sensory and Functional Properties of Black Garlic (Allium sativum L.). Molecules 2022, 27, 6992. [Google Scholar] [CrossRef]
- Kamiloglu, S.; Koc Alibasoglu, E.; Acoglu Celik, B.; Celik, M.A.; Bekar, E.; Unal, T.T.; Kertis, B.; Akpinar Bayizit, A.; Yolci Omeroglu, P.; Copur, O.U. Bioaccessibility of Carotenoids and Polyphenols in Organic Butternut Squash (Cucurbita moschata): Impact of Industrial Freezing Process. Foods 2024, 13, 239. [Google Scholar] [CrossRef]
- Yanat, M.; Baysal, T. Effect of Freezing Rate and Storage Time on Quality Parameters of Strawberry Frozen in Modified and Home Type Freezer. Hrvat. Časopis Prehrambenu Tehnol. Biotehnol. Nutr. 2018, 13, 154–158. [Google Scholar] [CrossRef]
- Dalmau, M.E.; Bornhorst, G.M.; Eim, V.; Rosselló, C.; Simal, S. Effects of Freezing, Freeze Drying and Convective Drying on in Vitro Gastric Digestion of Apples. Food Chem. 2017, 215, 7–16. [Google Scholar] [CrossRef] [PubMed]
- Salazar-Orbea, G.L.; García-Villalba, R.; Bernal, M.J.; Hernández-Jiménez, A.; Egea, J.A.; Tomás-Barberán, F.A.; Sánchez-Siles, L.M. Effect of Storage Conditions on the Stability of Polyphenols of Apple and Strawberry Purees Produced at Industrial Scale by Different Processing Techniques. J. Agric. Food Chem. 2023, 71, 2541–2553. [Google Scholar] [CrossRef]
- Koyama, R.; Ishibashi, M.; Fukuda, I.; Okino, A.; Osawa, R.; Uno, Y. Pre- and Post-Harvest Conditions Affect Polyphenol Content in Strawberry (Fragaria × Ananassa). Plants 2022, 11, 2220. [Google Scholar] [CrossRef]
- Araújo-Rodrigues, H.; Santos, D.; Campos, D.A.; Guerreiro, S.; Ratinho, M.; Rodrigues, I.M.; Pintado, M.E. Impact of Processing Approach and Storage Time on Bioactive and Biological Properties of Rocket, Spinach and Watercress Byproducts. Foods 2021, 10, 2301. [Google Scholar] [CrossRef]
- Šamec, D.; Ljubej, V.; Redovniković, I.R.; Fistanić, S.; Salopek-Sondi, B. Low Temperatures Affect the Physiological Status and Phytochemical Content of Flat Leaf Kale (Brassica oleracea Var. Acephala) Sprouts. Foods 2022, 11, 264. [Google Scholar] [CrossRef] [PubMed]
- Somaratne, G.; Ferrua, M.J.; Ye, A.; Nau, F.; Floury, J.; Dupont, D.; Singh, J. Food Material Properties as Determining Factors in Nutrient Release during Human Gastric Digestion: A Review. Crit. Rev. Food Sci. Nutr. 2020, 60, 3753–3769. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Yu, W.; Wu, P.; Chen, X.D. Current In Vitro Digestion Systems for Understanding Food Digestion in Human Upper Gastrointestinal Tract. Trends Food Sci. Technol. 2020, 96, 114–126. [Google Scholar] [CrossRef]
- Parada, J.; Aguilera, J.M. Food Microstructure Affects the Bioavailability of Several Nutrients. J. Food Sci. 2007, 72, R21–R32. [Google Scholar] [CrossRef]
- Bouayed, J.; Deußer, H.; Hoffmann, L.; Bohn, T. Bioaccessible and Dialysable Polyphenols in Selected Apple Varieties following in Vitro Digestion vs. Their Native Patterns. Food Chem. 2012, 131, 1466–1472. [Google Scholar] [CrossRef]
- Alminger, M.; Aura, A.-M.; Bohn, T.; Dufour, C.; El, S.N.; Gomes, A.; Karakaya, S.; Martínez-Cuesta, M.C.; McDougall, G.J.; Requena, T.; et al. In Vitro Models for Studying Secondary Plant Metabolite Digestion and Bioaccessibility. Compr. Rev. Food Sci. Food Saf. 2014, 13, 413–436. [Google Scholar] [CrossRef] [PubMed]
- Ginsburg, I.; Koren, E.; Shalish, M.; Kanner, J.; Kohen, R. Saliva Increases the Availability of Lipophilic Polyphenols as Antioxidants and Enhances Their Retention in the Oral Cavity. Arch. Oral Biol. 2012, 57, 1327–1334. [Google Scholar] [CrossRef] [PubMed]
- Wróblewski, K.; Muhandiram, R.; Chakrabartty, A.; Bennick, A. The Molecular Interaction of Human Salivary Histatins with Polyphenolic Compounds. Eur. J. Biochem. 2001, 268, 4384–4397. [Google Scholar] [CrossRef] [PubMed]
- Domínguez-Avila, J.A.; Wall-Medrano, A.; Velderrain-Rodríguez, G.R.; Chen, C.-Y.O.; Salazar-López, N.J.; Robles-Sánchez, M.; González-Aguilar, G.A. Gastrointestinal Interactions, Absorption, Splanchnic Metabolism and Pharmacokinetics of Orally Ingested Phenolic Compounds. Food Funct. 2017, 8, 15–38. [Google Scholar] [CrossRef]
- Das, T.; Chatterjee, N.; Capanoglu, E.; Lorenzo, J.M.; Das, A.K.; Dhar, P. The Synergistic Ramification of Insoluble Dietary Fiber and Associated Non-Extractable Polyphenols on Gut Microbial Population Escorting Alleviation of Lifestyle Diseases. Food Chem. X 2023, 18, 100697. [Google Scholar] [CrossRef]
- Gayoso, L.; Claerbout, A.-S.; Calvo, M.I.; Cavero, R.Y.; Astiasarán, I.; Ansorena, D. Bioaccessibility of Rutin, Caffeic Acid and Rosmarinic Acid: Influence of the in Vitro Gastrointestinal Digestion Models. J. Funct. Foods 2016, 26, 428–438. [Google Scholar] [CrossRef]
- Faria, A.; Fernandes, I.; Norberto, S.; Mateus, N.; Calhau, C. Interplay between Anthocyanins and Gut Microbiota. J. Agric. Food Chem. 2014, 62, 6898–6902. [Google Scholar] [CrossRef]
- D’Archivio, M.; Filesi, C.; Varì, R.; Scazzocchio, B.; Masella, R. Bioavailability of the Polyphenols: Status and Controversies. Int. J. Mol. Sci. 2010, 11, 1321–1342. [Google Scholar] [CrossRef]
- Perler, B.K.; Friedman, E.S.; Wu, G.D. The Role of the Gut Microbiota in the Relationship Between Diet and Human Health. Annu. Rev. Physiol. 2023, 85, 449–468. [Google Scholar] [CrossRef]
- Moszak, M.; Szulińska, M.; Bogdański, P. You Are What You Eat—The Relationship between Diet, Microbiota, and Metabolic Disorders—A Review. Nutrients 2020, 12, 1096. [Google Scholar] [CrossRef]
- Bokulich, N.A.; Chung, J.; Battaglia, T.; Henderson, N.; Jay, M.; Li, H.; Lieber, A.D.; Wu, F.; Perez-Perez, G.I.; Chen, Y.; et al. Antibiotics, Birth Mode, and Diet Shape Microbiome Maturation during Early Life. Sci. Transl. Med. 2016, 8, 343ra82. [Google Scholar] [CrossRef] [PubMed]
- Etxeberria, U.; Fernández-Quintela, A.; Milagro, F.I.; Aguirre, L.; Martínez, J.A.; Portillo, M.P. Impact of Polyphenols and Polyphenol-Rich Dietary Sources on Gut Microbiota Composition. J. Agric. Food Chem. 2013, 61, 9517–9533. [Google Scholar] [CrossRef] [PubMed]
- Sorrenti, V.; Ali, S.; Mancin, L.; Davinelli, S.; Paoli, A.; Scapagnini, G. Cocoa Polyphenols and Gut Microbiota Interplay: Bioavailability, Prebiotic Effect, and Impact on Human Health. Nutrients 2020, 12, 1908. [Google Scholar] [CrossRef]
- Chiu, H.-F.; Venkatakrishnan, K.; Golovinskaia, O.; Wang, C.-K. Gastroprotective Effects of Polyphenols against Various Gastro-Intestinal Disorders: A Mini-Review with Special Focus on Clinical Evidence. Molecules 2021, 26, 2090. [Google Scholar] [CrossRef]
- Cardona, F.; Andrés-Lacueva, C.; Tulipani, S.; Tinahones, F.J.; Queipo-Ortuño, M.I. Benefits of Polyphenols on Gut Microbiota and Implications in Human Health. J. Nutr. Biochem. 2013, 24, 1415–1422. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Burillo, S.; Navajas-Porras, B.; López-Maldonado, A.; Hinojosa-Nogueira, D.; Pastoriza, S.; Rufián-Henares, J.Á. Green Tea and Its Relation to Human Gut Microbiome. Molecules 2021, 26, 3907. [Google Scholar] [CrossRef] [PubMed]
- Yuan, X.; Long, Y.; Ji, Z.; Gao, J.; Fu, T.; Yan, M.; Zhang, L.; Su, H.; Zhang, W.; Wen, X.; et al. Green Tea Liquid Consumption Alters the Human Intestinal and Oral Microbiome. Mol. Nutr. Food Res. 2018, 62, 1800178. [Google Scholar] [CrossRef]
- Nagao-Kitamoto, H.; Kitamoto, S.; Kuffa, P.; Kamada, N. Pathogenic Role of the Gut Microbiota in Gastrointestinal Diseases. Intest. Res. 2016, 14, 127. [Google Scholar] [CrossRef]
- Wan, M.L.Y.; Co, V.A.; El-Nezami, H. Dietary Polyphenol Impact on Gut Health and Microbiota. Crit. Rev. Food Sci. Nutr. 2021, 61, 690–711. [Google Scholar] [CrossRef]
- Caponio, G.; Noviello, M.; Calabrese, F.; Gambacorta, G.; Giannelli, G.; De Angelis, M. Effects of Grape Pomace Polyphenols and In Vitro Gastrointestinal Digestion on Antimicrobial Activity: Recovery of Bioactive Compounds. Antioxidants 2022, 11, 567. [Google Scholar] [CrossRef]
- Yang, D.; Wang, T.; Long, M.; Li, P. Quercetin: Its Main Pharmacological Activity and Potential Application in Clinical Medicine. Oxid. Med. Cell. Longev. 2020, 2020, 8825387. [Google Scholar] [CrossRef] [PubMed]
- Pandurangan, A.K.; Mohebali, N.; Mohd Esa, N.; Looi, C.Y.; Ismail, S.; Saadatdoust, Z. Gallic Acid Suppresses Inflammation in Dextran Sodium Sulfate-Induced Colitis in Mice: Possible Mechanisms. Int. Immunopharmacol. 2015, 28, 1034–1043. [Google Scholar] [CrossRef] [PubMed]
- Yang, K.; Zhang, L.; Liao, P.; Xiao, Z.; Zhang, F.; Sindaye, D.; Xin, Z.; Tan, C.; Deng, J.; Yin, Y.; et al. Impact of Gallic Acid on Gut Health: Focus on the Gut Microbiome, Immune Response, and Mechanisms of Action. Front. Immunol. 2020, 11, 580208. [Google Scholar] [CrossRef] [PubMed]
- Lima, A.C.D.; Cecatti, C.; Fidélix, M.P.; Adorno, M.A.T.; Sakamoto, I.K.; Cesar, T.B.; Sivieri, K. Effect of Daily Consumption of Orange Juice on the Levels of Blood Glucose, Lipids, and Gut Microbiota Metabolites: Controlled Clinical Trials. J. Med. Food 2019, 22, 202–210. [Google Scholar] [CrossRef]
- Nani, A.; Murtaza, B.; Sayed Khan, A.; Khan, N.A.; Hichami, A. Antioxidant and Anti-Inflammatory Potential of Polyphenols Contained in Mediterranean Diet in Obesity: Molecular Mechanisms. Molecules 2021, 26, 985. [Google Scholar] [CrossRef]
- Ohishi, T.; Fukutomi, R.; Shoji, Y.; Goto, S.; Isemura, M. The Beneficial Effects of Principal Polyphenols from Green Tea, Coffee, Wine, and Curry on Obesity. Molecules 2021, 26, 453. [Google Scholar] [CrossRef]
- Duarte, L.; Gasaly, N.; Poblete-Aro, C.; Uribe, D.; Echeverria, F.; Gotteland, M.; Garcia-Diaz, D.F. Polyphenols and Their Anti-Obesity Role Mediated by the Gut Microbiota: A Comprehensive Review. Rev. Endocr. Metab. Disord. 2021, 22, 367–388. [Google Scholar] [CrossRef]
- Naz, R.; Saqib, F.; Awadallah, S.; Wahid, M.; Latif, M.F.; Iqbal, I.; Mubarak, M.S. Food Polyphenols and Type II Diabetes Mellitus: Pharmacology and Mechanisms. Molecules 2023, 28, 3996. [Google Scholar] [CrossRef]
- Huang, D.-D.; Shi, G.; Jiang, Y.; Yao, C.; Zhu, C. A Review on the Potential of Resveratrol in Prevention and Therapy of Diabetes and Diabetic Complications. Biomed. Pharmacother. 2020, 125, 109767. [Google Scholar] [CrossRef]
- Guasch-Ferré, M.; Merino, J.; Sun, Q.; Fitó, M.; Salas-Salvadó, J. Dietary Polyphenols, Mediterranean Diet, Prediabetes, and Type 2 Diabetes: A Narrative Review of the Evidence. Oxid. Med. Cell. Longev. 2017, 2017, 6723931. [Google Scholar] [CrossRef]
- Krawczyk, M.; Burzynska-Pedziwiatr, I.; Wozniak, L.A.; Bukowiecka-Matusiak, M. Impact of Polyphenols on Inflammatory and Oxidative Stress Factors in Diabetes Mellitus: Nutritional Antioxidants and Their Application in Improving Antidiabetic Therapy. Biomolecules 2023, 13, 1402. [Google Scholar] [CrossRef] [PubMed]
- Mezhibovsky, E.; Wu, Y.; Bawagan, F.G.; Tveter, K.M.; Szeto, S.; Roopchand, D. Impact of Grape Polyphenols on Akkermansia Muciniphila and the Gut Barrier. AIMS Microbiol. 2022, 8, 544–565. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Daza, M.C.; De Vos, W.M. Polyphenols as Drivers of a Homeostatic Gut Microecology and Immuno-Metabolic Traits of Akkermansia Muciniphila: From Mouse to Man. Int. J. Mol. Sci. 2022, 24, 45. [Google Scholar] [CrossRef] [PubMed]
- Roopchand, D.E.; Carmody, R.N.; Kuhn, P.; Moskal, K.; Rojas-Silva, P.; Turnbaugh, P.J.; Raskin, I. Dietary Polyphenols Promote Growth of the Gut Bacterium Akkermansia Muciniphila and Attenuate High-Fat Diet–Induced Metabolic Syndrome. Diabetes 2015, 64, 2847–2858. [Google Scholar] [CrossRef] [PubMed]
- Mullen, W.; Stewart, A.J.; Lean, M.E.J.; Gardner, P.; Duthie, G.G.; Crozier, A. Effect of Freezing and Storage on the Phenolics, Ellagitannins, Flavonoids, and Antioxidant Capacity of Red Raspberries. J. Agric. Food Chem. 2002, 50, 5197–5201. [Google Scholar] [CrossRef]
- Witkowska, A.M.; Salem, J.-E. Pharmacological and Nutritional Modulation of Metabolome and Metagenome in Cardiometabolic Disorders. Biomolecules 2023, 13, 1340. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).