- Systematic Review
Exploring the Role of Canakinumab in the Treatment of Autoinflammatory Bone Disorders: A Systematic Review
- Lisa Gamalero and
- Teresa Giani
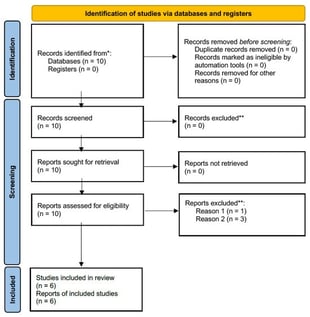
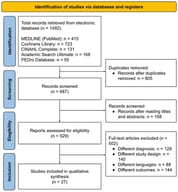
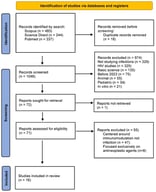
Background: Autoinflammatory bone disorders are rare, non-infectious inflammatory conditions that primarily involve the skeleton, most commonly presenting as chronic nonbacterial osteomyelitis (CNO) or chronic recurrent multifocal osteomyelitis (CRMO). Less frequently, they occur in the context of Mendelian syndromes such as Majeed syndrome, deficiency of the interleukin-1 receptor antagonist (DIRA), and pyogenic arthritis; pyoderma gangrenosum; and acne (PAPA) syndrome. Given the role of IL-1-driven innate immune dysregulation across these bone disorders, and the growing, though heterogeneous, clinical experience with IL-1 blockade, this review maps and critically appraises the available evidence on canakinumab in autoinflammatory bone disorders. Methods: We systematically searched PubMed and the Cochrane Library (English, inception–July 2025) and screened ClinicalTrials.gov. Eligible reports included any case reports/series describing canakinumab use in autoinflammatory bone disorders (CNO/CRMO, Majeed, DIRA, PAPA). Results: Six publications met the inclusion criteria (one case series, five case reports; 10 patients). Complete responses were reported in all three patients with Majeed syndrome and in two patients with sporadic CRMO associated with systemic features. Partial responses occurred in two additional sporadic CRMO cases, while no meaningful response was documented in DIRA. No interventional trials of canakinumab were identified on ClinicalTrials.gov for CNO/CRMO, Majeed, DIRA, or PAPA. Conclusions: Although the role of IL-1 in the pathogenesis of autoinflammatory bone disease provides a rationale for IL-1 blockade, evidence for canakinumab remains limited and heterogeneous, precluding definitive conclusions. Indicators of benefits appear most consistently in Majeed syndrome and in selected CRMO phenotypes.
4 February 2026