Abstract
Background: Systemic lupus erythematosus (SLE) is a chronic autoimmune disorder with significant racial and ethnic disparities in prevalence, disease severity, and outcomes. Cardiovascular complications, including pericarditis, myocarditis, valvular disease, and conduction abnormalities, contribute to increased morbidity and mortality in SLE patients. This study examines racial and ethnic disparities in cardiovascular outcomes among hospitalized SLE patients in the United States. Methods: This retrospective study utilized the National Inpatient Sample (NIS) database from 2016 to 2021 to analyze hospitalizations of adult patients (≥18 years) with a primary or secondary diagnosis of SLE. Patients were stratified into racial/ethnic groups: White, Black, Hispanic, Asian, Native American, and Other. Primary outcomes include major adverse cardiovascular events (MACEs), which are a composite of in-hospital mortality, myocardial infarction (MI), sudden cardiac death, and other SLE-related outcomes including cardiac, pulmonary, and renal involvement. Statistical analyses included multivariable logistic regression models adjusted for demographic, socioeconomic, and hospital-related factors to assess racial disparities. Results: The study included 514,750 White, 321,395 Black, and 146,600 Hispanic patients, with smaller proportions of Asian, Native American, and Other racial groups. Black patients had significantly higher odds of in-hospital mortality (OR = 1.17, 95% CI = 1.08–1.26, p < 0.001) and sudden cardiac death (OR = 1.64, 95% CI = 1.46–1.85, p < 0.001) compared to White patients. Asian patients also exhibited increased mortality risk (OR = 1.37, 95% CI = 1.14–1.63, p = 0.001) as compared to Whites. Conversely, Black (OR = 0.90, 95% CI = 0.85–0.96, p = 0.01) and Hispanic (OR = 0.87, 95% CI = 0.80–0.96, p = 0.03) patients had lower odds of MI. Racial disparities in access to care, socioeconomic status, and comorbidity burden may contribute to these differences. Conclusion: Significant racial and ethnic disparities exist in cardiovascular outcomes among hospitalized SLE patients. Black and Asian individuals face higher in-hospital all-causes mortality and sudden cardiac death risks, while Black and Hispanic patients exhibit lower MI rates. Addressing social determinants of health, improving access to specialized care, and implementing targeted interventions may reduce disparities and improve outcomes in minority populations with SLE.
1. Introduction
Systemic lupus erythematosus (SLE) is an autoimmune disorder where the immune system mistakenly attacks healthy tissues, affecting various organs such as the skin, joints, kidneys, and brain. While the exact cause is unknown, SLE is thought to result from a combination of genetic and environmental factors. It primarily affects women, especially those aged 15 to 44, and is more common in African Americans, Asians, Hispanic Americans, and African Caribbeans [1,2]. The California Lupus Surveillance Project (CLSP) found that, between 2007 and 2009, the age-standardized incidence and prevalence of SLE were higher among Black (15.5 per 100,000 person-years; 241.0 per 100,000 persons), Asian/Pacific Islander (API) (4.1 per 100,000 person-years; 90.5 per 100,000 persons), and Hispanic populations (4.2 per 100,000 person-years; 94.7 per 100,000 persons) compared to White populations (2.8 per 100,000 person-years; 55.2 per 100,000 persons) in San Francisco County. Also, previous studies indicate that, compared to Whites, Black individuals tend to experience more severe symptoms at the time of SLE diagnosis and generally have a poorer overall prognosis [3]. Although there is no universally agreed-upon set of diagnostic criteria for SLE, the 2019 classification criteria developed by the European Alliance of Associations for Rheumatology (EULAR) and the American College of Rheumatology (ACR) for research purposes help in improving the sensitivity and specificity for diagnosing SLE. These criteria include clinical signs such as fever, rash, proteinuria, arthritis, and cytopenia, as well as immunologic indicators like SLE-specific autoantibodies and low complement levels. Previous cohort studies suggest that lupus nephritis (LN) affects between 20% and 65% of individuals with SLE [4,5].
SLE commonly involves the cardiovascular system. SLE can affect all parts of the heart. Pericarditis, myocarditis, valve disorders, and conduction system issues are among the most frequent cardiac complications in patients with SLE. The pericardium is particularly commonly affected, with some postmortem studies showing a prevalence of approximately 62% [6]. A study from Wang and colleagues, which involved 258 patients (90.3% female), found that 35.7% had cardio-respiratory symptoms, with dyspnea (24.0%) and chest pain (20.5%) being the most common. Cardiac manifestations were present in 37.6% of patients, including pericardial disease (14.7%), valvular disease (7.8%), stroke/TIA (7.8%), coronary heart disease (6.6%), and heart failure hospitalizations (5.0%). Over a mean follow-up of 3 years, there were 5 deaths, 19 cardiac events, 6 cardiovascular procedures, and 44 SLE-related hospitalizations [7]. Advances in technology, particularly echocardiography, have improved the diagnosis of these conditions. Additionally, antiphospholipid antibodies have been linked to valvular disease [8].
Since previous studies indicate racial minorities have higher incidence and prevalence of SLE, and also have more severe disease, this article will delve into the cardiovascular outcomes and related disparities for different racial/ethnic groups. Some studies suggest prognosis for African American patients with lupus is considerably poorer than that of White patients [9]. Garg and colleagues suggest Black patients had a 19-times higher risk in the first 12 years following a lupus diagnosis compared to non-Black patients. Studies suggest cardiovascular disease (CVD) events were most frequent in the second and eleventh years after a lupus diagnosis. Among new cases, White patients did not experience mortality until five years post-diagnosis, while Black patients exhibited consistently higher mortality rates from the time of diagnosis [9,10,11]. Social determinants of health could be playing a role in creating racial and ethnic disparities in lupus outcomes, influencing factors such as access to healthcare, education, economic stability, and more [9].
2. Methods
2.1. Study Design and Data Source
This retrospective study utilized data from the National Inpatient Sample (NIS) database, spanning the years 2016 to 2021. The NIS, a publicly available resource provided by the Healthcare Cost and Utilization Project (HCUP) under the Agency for Healthcare Research and Quality (AHRQ), is the largest publicly available all-payer inpatient healthcare database in the United States. It includes discharge data from approximately 20% of inpatient hospitalizations across community hospitals, excluding rehabilitation and long-term acute care facilities [12]. This dataset enables the generation of national estimates for various variables, including healthcare utilization, costs, demographic profiles, and patient outcomes.
2.2. Study Population and Variables
Hospitalized patients diagnosed with SLE were identified using the International Classification of Diseases, 10th Revision (ICD-10) codes. Patients aged 18 years or older with a primary or secondary diagnosis of SLE during their hospital stay were included in the study. Patients with missing data on race were excluded from the analysis.
The patient population was stratified into the following racial groups: White, Black, Hispanic, Asian, Native American, and Other, as defined by the NIS database. Demographic variables included age, sex, insurance type, and median household income quartiles. Hospital characteristics such as bed size (small, medium, or large), location and teaching status (rural, urban non-teaching, or urban teaching), and region were also included. Comorbidities associated with SLE and its complications were identified using ICD-10 codes.
2.3. Outcomes
Key outcomes of interest included length of stay (LOS), total healthcare charges, and in-hospital all-cause mortality. Specific organ-related SLE complications, myocardial infarction (MI), and sudden cardiac death were assessed using ICD-10 codes. Mortality data were extracted using the NIS variable “died,” which denotes in-hospital deaths. Racial disparities in outcomes were evaluated by comparing each racial group with Whites as the reference population. MACEs in our study include myocardial infarction, died during hospitalization, sudden cardiac death, atrial fibrillation, and intracranial hemorrhage.
2.4. Statistical Analyses
Descriptive statistics were used to summarize baseline characteristics, including demographics, hospital characteristics, comorbidities, and outcomes, stratified by race. Continuous variables, such as LOS and total healthcare charges, were analyzed using ANOVA, while categorical variables, including mortality and complications, were analyzed using the chi-square test. Statistical significance was set at a two-tailed p-value < 0.05.
Multivariate logistic regression models were employed to calculate odds ratios (ORs) with 95% confidence intervals (CIs) for in-hospital mortality, myocardial infarction, sudden cardiac death, and organ-specific SLE complications. Whites were used as the reference group in these models. To account for potential confounding factors influencing cardiovascular outcomes, multivariate logistic regression models were constructed. Variables included for adjustment were selected a priori based on clinical relevance and established associations in prior studies. Specifically, all comorbidities listed in Table 1—including hypertension, diabetes mellitus, chronic kidney disease, chronic obstructive pulmonary disease, and others—were included as covariates. In addition, the Charlson Comorbidity Index (CCI) was incorporated as a composite measure of overall disease burden. Age, sex, and race/ethnicity were also included in all models. A uniform set of covariates was applied across analyses to ensure consistency and minimize omitted variable bias. Weighted sampling techniques inherent to the NIS dataset were applied to ensure national representativeness and accuracy of the results.

Table 1.
Racial disparities in baseline characteristics.
Rationale for Covariate Selection: The selection of covariates for multivariable logistic regression models was guided by both clinical relevance and the prior literature. Age and sex were included as fundamental demographic factors known to influence both SLE incidence and cardiovascular risk [2,3]. Key comorbidities such as hypertension, diabetes mellitus, dyslipidemia, chronic kidney disease (CKD), and chronic obstructive pulmonary disease (COPD) were selected due to their established associations with adverse cardiovascular outcomes, particularly in SLE populations [13,14]. CKD and hypertension are notably prevalent in minority populations and exacerbate SLE disease severity. Smoking and liver disease were included for their contributions to systemic inflammation and immune dysregulation. The Charlson Comorbidity Index (CCI), a validated tool for summarizing overall disease burden, was incorporated to adjust for multiple comorbidities [15]. Finally, hospital-related characteristics were included to control for potential disparities in healthcare delivery. This selection of variables was intended to enhance model robustness and minimize residual confounding.
2.5. Ethical Considerations
The study did not require Institutional Review Board (IRB) approval or informed consent because it utilized a publicly available, de-identified dataset. The primary aim of the study is to investigate racial disparities in healthcare outcomes for hospitalized patients with SLE to inform healthcare policy and promote equitable resource allocation.
3. Results
3.1. Demographic and Socioeconomic Characteristics
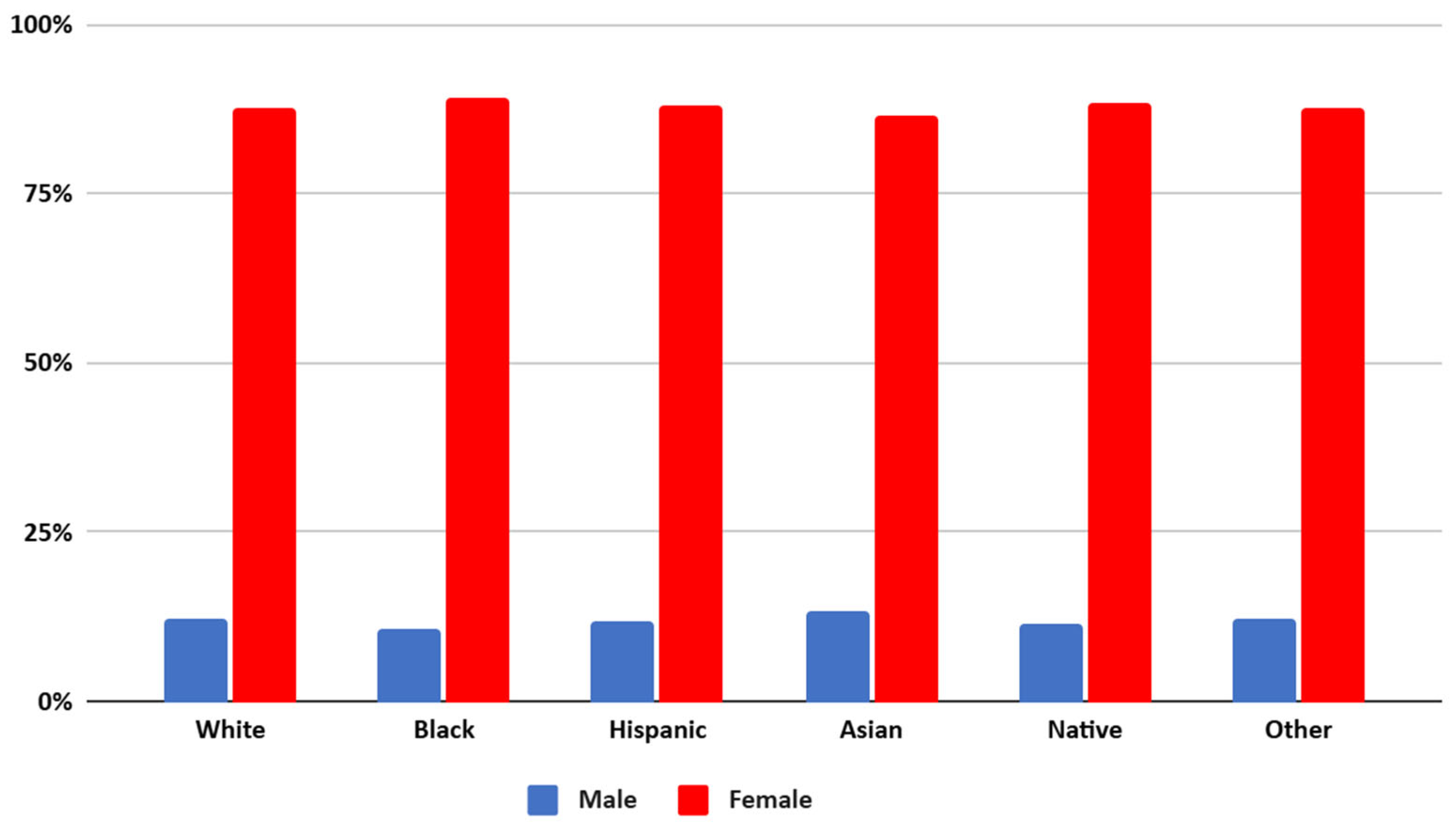
White patients represented the largest proportion of the cohort (514,750), followed by Black (321,395) and Hispanic (146,600) patients, with Asian, Native American, and Other racial groups comprising smaller subsets. The gender distribution showed higher proportions of females across all groups, ranging from 86.48% (Asian) to 89.31% (Black) (Refer Figure 1). The mean age varied significantly, with White patients having the highest mean age (58 years), while Hispanic and Black patients were the youngest on average (45–47 years).
Figure 1.
Gender distribution of patients of different racial groups with SLE in the US from NIS 2016 to 2021.
Socioeconomic factors also exhibited stark differences. A higher percentage of Black (50.14%) and Native American (47.48%) patients fell into the lowest quartile of median household income compared to White patients (25.57%). Conversely, Asian patients were more likely to belong to the highest income quartile (41.67%), reflecting socioeconomic disparities that may influence healthcare access and outcomes.
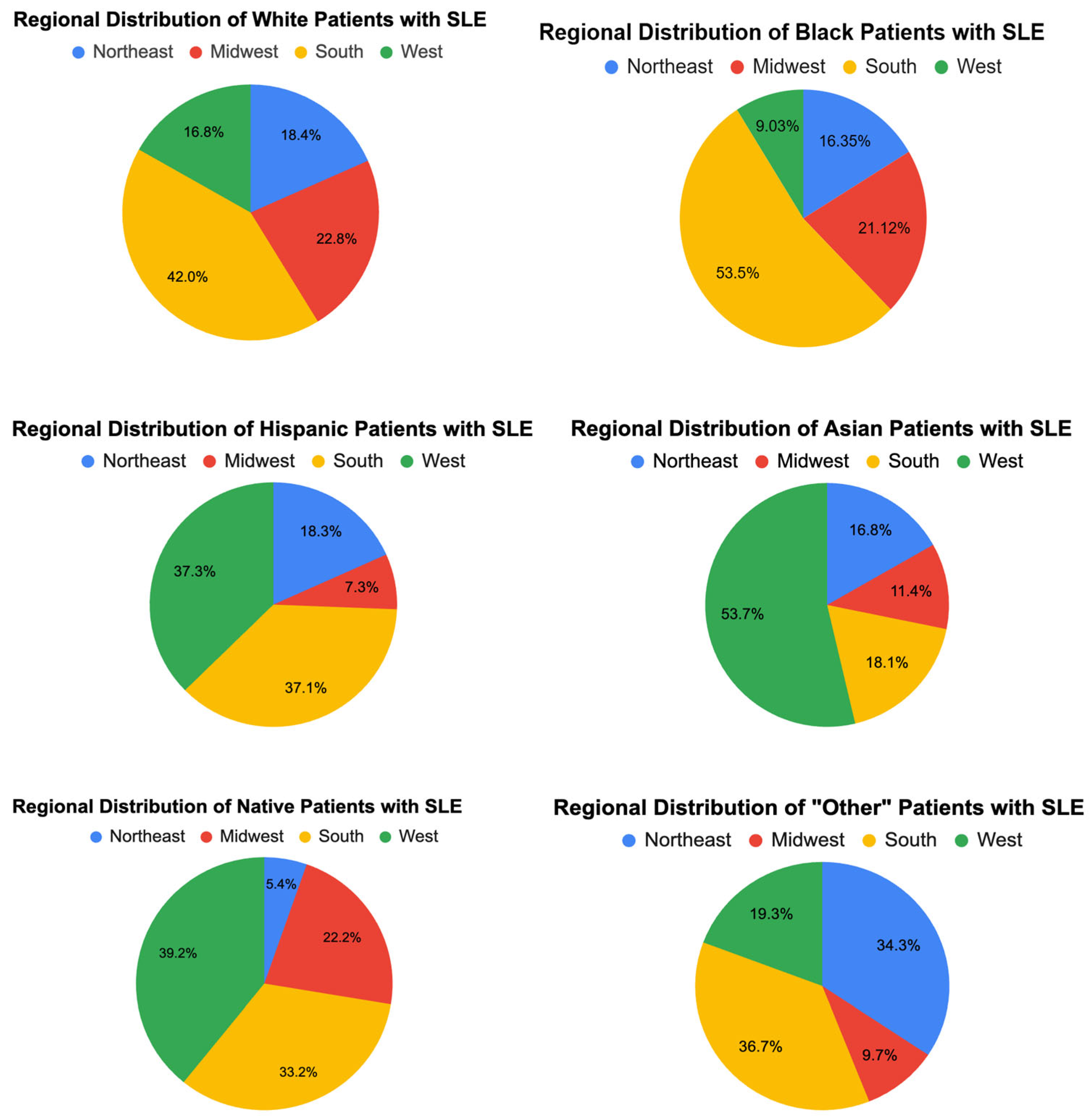
3.2. Hospital and Regional Characteristics (Figure 2)
Hospital teaching status and size showed significant variation across racial groups. Most patients (70–83%) were treated in urban teaching hospitals, with this proportion being highest for Asian (83.06%) and Hispanic (80.57%) patients. Rural hospitals accounted for a small percentage of hospitalizations overall, though Native American patients disproportionately utilized these facilities (16.39%).
Regional differences were also notable. A larger proportion of Black (53.5%) and Hispanic (37.05%) patients were treated in the South, whereas Asian patients were more likely to receive care in the West (53.71%).
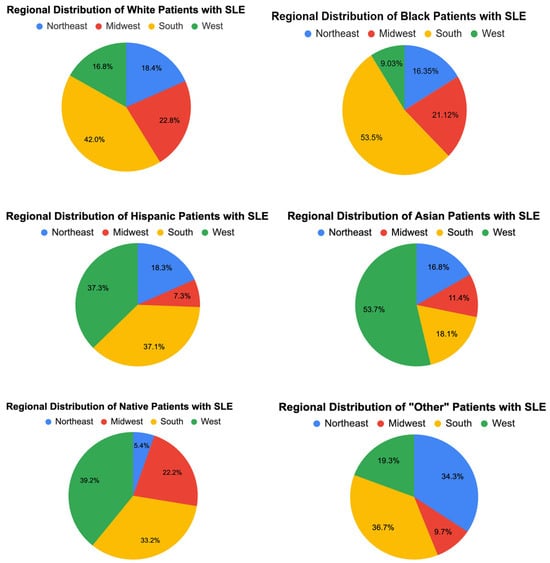
Figure 2.
Regional distribution of races in SLE patients.
Figure 2.
Regional distribution of races in SLE patients.
3.3. Comorbidities (Figure 3)
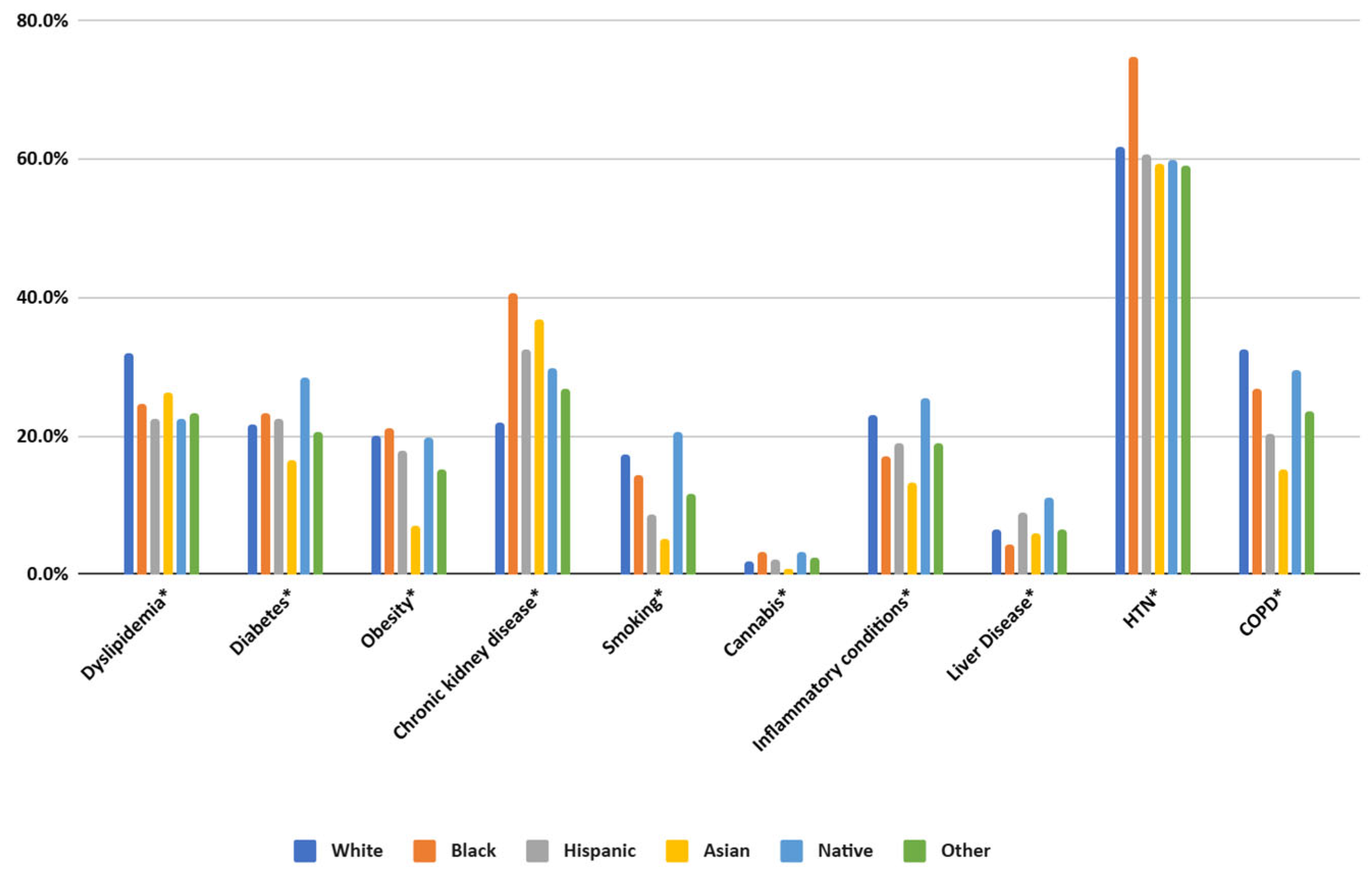
The prevalence of chronic conditions varied significantly across racial and ethnic groups. Dyslipidemia, hypertension, and diabetes were most common among White and Black patients, with hypertension particularly high among Black patients (74.61%). Chronic kidney disease was also strikingly more prevalent in Black patients (40.52%) compared to White patients (21.99%).
Some conditions exhibited unique racial patterns. Obesity was most prevalent among Black patients (21.07%), while inflammatory conditions were more common among Native Americans (25.44%). Asian patients had lower rates of smoking (5.19%) but a relatively high prevalence of dyslipidemia (26.23%).
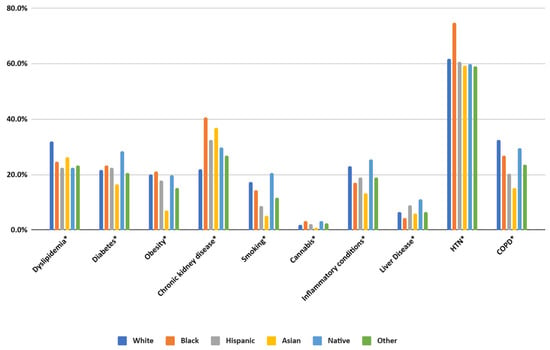
Figure 3.
Prevalence of comorbidities in patients with SLE. HTN—hypertension, COPD—chronic obstructive pulmonary disease. (* specifies p values < 0.01).
Figure 3.
Prevalence of comorbidities in patients with SLE. HTN—hypertension, COPD—chronic obstructive pulmonary disease. (* specifies p values < 0.01).
3.4. Clinical Outcomes
3.4.1. Overall Mortality and Cardiovascular Outcomes (Figure 4)
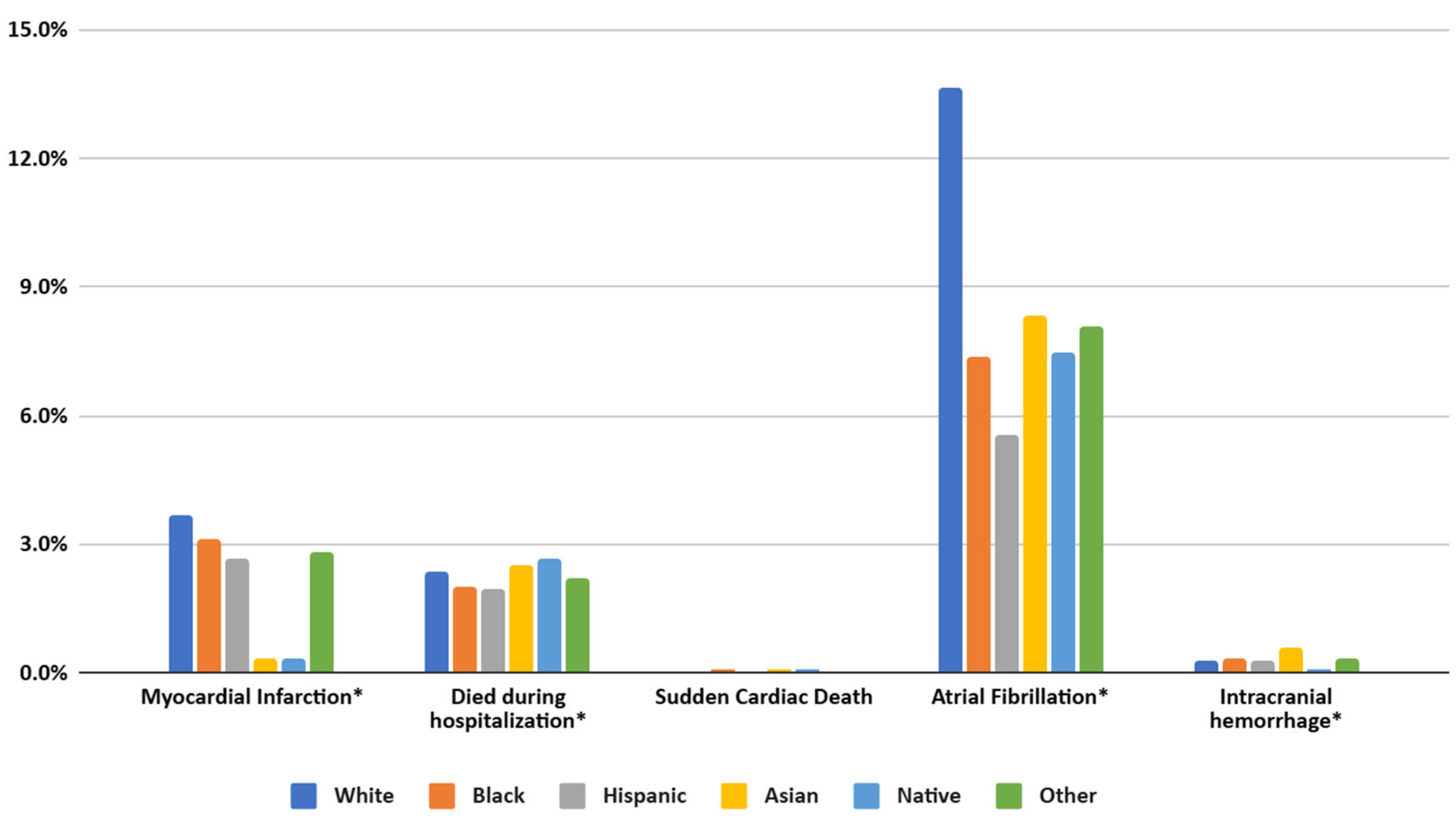
Compared to White patients, Black individuals exhibited significantly higher adjusted odds of in-hospital mortality (AOR = 1.17, 95% CI = 1.08–1.26, p < 0.001), as did Asian patients (AOR = 1.37, 95% CI = 1.14–1.63, p = 0.001). By contrast, Hispanic (AOR = 1.04, 95% CI = 0.95–1.15, p = 0.398), Native American (AOR = 1.19, 95% CI = 0.87–1.64, p = 0.281), and Other race/ethnicity (AOR = 1.20, 95% CI = 1.00–1.45, p = 0.051) showed no statistically significant difference in mortality risk relative to White. For myocardial infarction (MI), Black (AOR = 0.90, 95% CI = 0.85–0.96, p = 0.01) and Hispanic (AOR = 0.87, 95% CI = 0.80–0.96, p = 0.03) patients had lower odds compared to White, while Asian, Native, and Other groups did not differ significantly. However, the odds of experiencing a sudden cardiac event were notably higher in Black (AOR = 1.64, 95% CI = 1.46–1.85, p < 0.001) and Asian (AOR = 1.50, 95% CI = 1.11–2.01, p = 0.008) patients, but not significantly elevated for Hispanic, Native, or Other groups. Atrial fibrillation (A. Fib) was uniformly less frequent among non-White populations, including Black (AOR = 0.78, 95% CI = 0.75–0.81, p < 0.001), Hispanic (AOR = 0.64, 95% CI = 0.60–0.68, p < 0.001), Asian (AOR = 0.86, 95% CI = 0.76 – 0.98, p = 0.018), Native (AOR = 0.73, 95% CI = 0.59–0.91, p = 0.005), and Other (AOR = 0.85, 95% CI = 0.76–0.95, p = 0.004) groups. Coronary stenosis was significantly lower in Black (AOR = 0.58, 95% CI = 0.51–0.66, p < 0.001), Hispanic (AOR = 0.60, 95% CI = 0.51–0.72, p < 0.001), and Asian (AOR = 0.55, 95% CI = 0.35–0.79, p = 0.002) patients compared to White, with Native (p = 0.086) and Other (p = 0.083) showing no significant difference. Intracranial hemorrhage incidence was only higher among Asian patients (AOR = 1.80, 95% CI = 1.36–2.37, p < 0.001) and did not differ significantly for Black, Hispanic, Native, or Other groups relative to White.
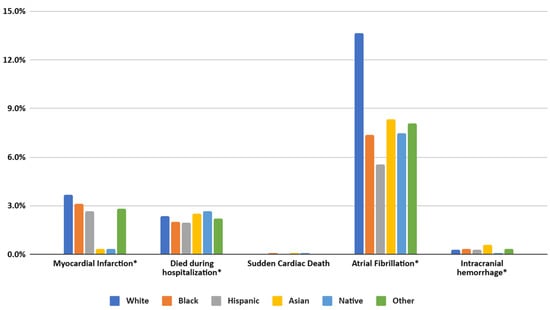
Figure 4.
Prevalence of MACEs in patients of different racial groups with SLE in the United States from the NIS database from 2016 to 2021. (* specifies p values < 0.01).
Figure 4.
Prevalence of MACEs in patients of different racial groups with SLE in the United States from the NIS database from 2016 to 2021. (* specifies p values < 0.01).
3.4.2. SLE-Specific Cardiac, Pulmonary, and Renal Manifestations
Regarding SLE-related cardiac involvement, any lupus-related endocarditis (including Libman–Sacks endocarditis) was significantly less likely among Black patients (AOR = 0.61, 95% CI = 0.47–0.78, p < 0.001), while no significant differences emerged for Hispanic, Asian, Native, or Other. In contrast, SLE pericarditis was more common across every non-White group, including Black (AOR = 2.04, 95% CI = 1.78–2.34, p < 0.001), Hispanic (AOR = 1.63, 95% CI = 1.38–1.93, p < 0.001), Asian (AOR = 2.11, 95% CI = 1.61–2.75, p < 0.001), Native (AOR = 1.74, 95% CI = 1.00–3.04, p = 0.049), and Other (AOR = 1.48, 95% CI = 1.06–2.07, p = 0.022). Pulmonary involvement (“SLE Lung”, which includes pulmonary manifestations of lupus such as lupus pneumonitis, interstitial lung disease, or other lung-related complications) was significantly higher for all non-White groups, with increased odds ranging from 1.76 in Hispanics (95% CI = 1.56–1.98, p < 0.001) to 2.06 in Asians (95% CI = 1.68–2.53, p < 0.001). Similarly, Black patients had an AOR of 1.93 (95% CI = 1.76–2.13, p < 0.001), Native patients 2.03 (95% CI = 1.35–3.04, p = 0.001), and Other 1.79 (95% CI = 1.45–2.22, p < 0.001), all of which were significantly elevated compared to White. For SLE nephropathy, both Black (AOR = 1.46, 95% CI = 1.04–2.06, p = 0.03) and Hispanic (AOR = 1.65, 95% CI = 1.10–2.49, p = 0.016) patients exhibited higher odds than White, whereas Asian (p = 0.057) and Other (p = 0.061) approached but did not achieve statistical significance. (for more details refer Table 2.)

Table 2.
Racial disparities in outcomes in patients with SLE.
3.4.3. Healthcare Utilization: Length of Stay and Total Charges
Black (coefficient = 0.30, 95% CI = 0.22–0.38, p < 0.001), Asian (0.76, 95% CI = 0.52–1.01, p < 0.001), and Other (0.57, 95% CI = 0.33–0.82, p < 0.001) patients had significantly longer hospital stays compared with White patients, whereas Hispanic (0.016, p = 0.742) and Native (0.26, p = 0.337) groups did not show a statistically meaningful difference. With respect to total health charges, Hispanic (USD + 15,679.93, 95% CI = 13,771.27–17,588.60, p < 0.001), Asian (USD + 24,054.45, 95% CI = 19,049.44–29,059.46, p < 0.001), and Other (USD + 20,149.16, 95% CI = 15,458.31–24,840.01, p < 0.001) patients incurred significantly higher hospital charges relative to White. Black (p = 0.147) and Native (p = 0.666) individuals did not have total charges that differed significantly from White.
4. Discussion
This study provides critical insights into the pervasive racial disparities in SLE-related hospital outcomes in the United States, using data from a nationally representative sample. The findings demonstrate significant differences in patient demographics, socioeconomic status, comorbidities, and hospital outcomes, highlighting the disproportionate burden of disease borne by Black and Hispanic patients. Black patients, in particular, faced elevated risks of SLE-specific complications and in-hospital mortality, emphasizing the need for targeted interventions to address systemic inequities. These results corroborate previous research and expand upon existing knowledge by offering a comprehensive analysis of how race and ethnicity intersect with healthcare disparities in SLE management.
Black patients were notably the youngest at the time of admission, with a mean age of 47.16 years, compared to 58.03 years for White patients. This disparity aligns with previous studies conducted by Lim et al. and Demas et al., indicating an earlier onset and more severe disease course of SLE among Black populations [16,17]. Younger age at hospitalization could be a marker of both increased disease activity and delayed outpatient management, suggesting disparities in early diagnosis and access to care.
A significant proportion of Black (50.1%) and Hispanic (37.0%) patients resided in the lowest income quartile, underscoring the role of socioeconomic status in healthcare disparities. As per Mccarty et al., lower income is closely associated with delayed access to medical care, reduced medication adherence, and inadequate disease monitoring [18]. Additionally, these groups were more likely to receive care at urban teaching hospitals, which, while offering specialized services, may indicate limited access to local healthcare facilities or outpatient care in underserved areas. Addressing these disparities requires systemic interventions to bridge the gap in healthcare access and affordability.
Studies have shown that Black and Hispanic patients face significant financial and access to healthcare challenges, which may contribute to their higher rates of comorbid conditions like chronic kidney disease (CKD) and hypertension, which exacerbate SLE [19,20]. These comorbidities, along with severe Charlson comorbidity scores seen among the Black population (60.8%), highlight the compounded burden these populations face. These findings align with a study conducted by Somers et al., suggesting that genetic predispositions, environmental factors, and inequities in preventive care contribute to higher rates of comorbidities in minority populations [21].
Studies have shown that higher mortality risks have been seen in Black patients and Asian patients as a result of lower socioeconomic status and a higher comorbidities ratio. Black patients exhibited a 17% higher risk of in-hospital mortality compared to White patients, while Asian patients faced an even greater relative risk (OR 1.37, p < 0.001). As per Singh et al. and Hasan et al., these disparities may reflect a combination of delayed presentations, greater disease severity at admission, and potential biases in healthcare delivery [15,22]. Conversely, Hispanic and Native American patients did not exhibit significant differences in mortality risk, though this may be influenced by the sample size and disease variability. Addressing mortality disparities requires both system-level interventions and individualized care plans.
Asian patients had the highest average length of stay (6.2 days) and incurred the highest total healthcare charges (USD 93,302). These outcomes may be related to differences in disease presentation, healthcare utilization patterns, or socioeconomic factors. Previous research by Julian et al. suggests that higher charges and longer stays often correlate with delays in receiving timely outpatient care, leading to more severe disease at admission [23].
CVD remains a major contributor to illness and death among individuals with SLE. Recent findings indicate that ischemic heart disease is the most common cause of mortality in this population, accounting for 36% of all deaths [24]. SLE is an independent risk factor for myocardial infarction, after controlling for other known CVD risks [14]. Since SLE tends to be particularly severe in certain ethnic groups, including African Americans, and inflammation plays a central role in the pathogenesis of CVD [13], as suggested in articles by Libby et al. and Scalzi et al., it is reasonable to hypothesize that the epidemiology of CVD and CVD-related mortality may differ in these racial groups. Scalzi et al. have shown that African Americans typically experience a younger age of onset for SLE compared to Caucasians, potentially leading to prolonged inflammation and disease duration, which may contribute to earlier onset of CVD or CVD-related mortality compared to age-matched non-SLE individuals and Caucasian SLE patients [25].
While research has established that SLE independently increases the risk of developing AF, the precise mechanisms underlying this association remain unclear. Mittal et al. have discussed the association of chronic inflammation with SLE, which may contribute to structural and electrical cardiovascular changes, increasing the risk of AF. Elevated inflammatory markers, such as CRP, TNF-α, IL-6, and IL-8, have been linked to AF development. Prior studies have suggested that certain treatments for SLE, including glucocorticoids such as methylprednisolone, may elevate AF risk, possibly due to steroid-induced alterations in cardiac ion channels and reduced refractory periods; however, information on steroid use was not available in our study cohort [26].
Lupus pericarditis is believed to be mediated by immune complexes and vascular proliferation is observed in histology in patients with acute lupus pericarditis [27]. Pericarditis was found to be one of the SLE comorbidities that showed the strongest association with non-White race [20]. Studies have also shown that African Americans tend to develop pericarditis at an earlier age and also tend to have greater disease severity as compared to their White counterparts [11]. As per Vina et al., potential factors have been postulated for this disparity including perceived racism in medical settings, which may contribute to worse health outcomes, and lower trust in physicians among African Americans that could have impacted the care and management of these complications [28]. Meanwhile, other studies have suggested healthcare disparities, including unequal access to specialty care, as a cause [27,28].
Lung involvement in SLE appears to be driven by several factors including the following: elevated systemic type 1 interferon (IFN) levels, circulating immune complexes, and neutrophils 9 [29]. Patients with SLE lung involvement have increased levels of proinflammatory cytokines like IFN-γ, TNF-α, IL-6, and IL-8 compared to those without lung involvement [29]. Cellular inflammation, particularly early in disease, correlates with pathological findings [29]. Neutrophil extracellular traps (NETs) play a role in lung inflammation and damage through NETosis. The interaction between initiating factors (IFNs, autoantibodies, immune complexes, etc.) and downstream responses likely drives SLE-associated lung involvement [29].
Asian patients, particularly Filipino patients, had significantly younger ages at diagnosis compared to other racial/ethnic groups (22.2 years for Filipinos vs. 27.9 years for Asians overall vs. 29.4 years for Whites) [30], which correlates with the findings of our study (46.6 for Asian vs. 58.03 for Whites). Among Asian patients, disease severity (measured by the Lupus Severity Index) was significantly higher than in White patients (7.1 vs. 6.5, p < 0.05) [30]. The younger age at diagnosis and greater disease severity observed in Asian patients could potentially translate to more frequent or severe lung involvement over time.
Intracranial hemorrhage (ICH) is a known complication of SLE and a key feature of neuropsychiatric SLE (NPSLE). Its causes are multifactorial, including thrombocytopenia, hemorrhagic infarction, hypertension, coagulation disturbances, and cerebrovascular diseases. Thrombocytopenia, in particular, has been identified as an independent risk factor for ICH in SLE patients. Its pathogenesis may be attributed to the following: antibody-mediated platelet destruction, antiphospholipid antibodies, thrombotic microangiopathy, bone marrow suppression, and megakaryocyte maturation disorders. Histopathological findings suggest thrombosis and arteritis play a role in ICH pathogenesis, with recurrent thromboses potentially causing multiple cerebral infarctions [30,31]. Sleep apnea was identified as a novel risk factor for intracranial hemorrhage, associated with both lobar and non-lobar hemorrhages across racial/ethnic groups [32].
Our study has found that non-Whites are at increased risk of ICH compared to Whites. A study conducted by Kittnet et al. also suggests similar findings that non-Whites are at increased risk of ICH as compared to Whites in the general population [33]. According to Shen at al., hypertension was a major risk factor for intracranial hemorrhage across all racial/ethnic groups, with particularly high percentages associated with treated or untreated hypertension in Black and Hispanic patients. Lack of health insurance was also seen to have a disproportionate association with intracranial hemorrhage risk in Black and Hispanic patients [32].
In a large Medicaid SLE cohort, Black and Hispanic patients showed significantly higher stroke risks compared to White patients. Black patients had a 34% increased overall stroke risk, while Hispanic patients had a 25% increase, with the disparities being most pronounced in those under 50 years. Lupus nephritis further elevated stroke risks for Blacks (44%) and Hispanics (47%). Black patients faced increased risks of both ischemic (33%) and hemorrhagic strokes (42%), whereas Hispanic patients had a markedly higher hemorrhagic stroke risk (79%) but no difference in ischemic stroke risk.
These disparities persisted even after adjusting for sociodemographic and clinical factors and may be driven by residual hypertension effects, unaddressed traditional risk factors (e.g., obesity, diabetes, hyperlipidemia) in younger patients, racial differences in SLE severity, and other unmeasured factors, suggesting complex underlying mechanisms at play [34].
Lupus nephritis is predominantly caused by a type III hypersensitivity reaction, resulting in immune complex deposition. Its pathogenesis involves a multifaceted interaction of genetic, environmental, and immunological factors. Genetic predisposition plays a critical role, with over 50 identified polymorphisms, including PDGFRA, APOL1, HAS2, and specific HLA alleles, associated with increased susceptibility. Environmental triggers include ultraviolet radiation, viral infections (e.g., Epstein–Barr virus and parvovirus B19), air pollution, and dysregulation of the gut microbiome.
Immune dysregulation is a hallmark of lupus nephritis, characterized by the formation of autoantibody-mediated immune complexes (e.g., anti-dsDNA, anti-C1q, anti-nucleosome, anti-α actinin, and anticardiolipin antibodies), complement system activation—particularly via the alternative pathway—and recruitment of inflammatory cells. Tubular epithelial cells contribute to disease progression by secreting B-cell activating factor, which promotes tertiary lymphoid structures. These processes collectively drive tissue inflammation and damage, highlighting the intricate interplay of factors underlying lupus nephritis pathogenesis [35].
Significant racial and ethnic disparities exist in the incidence and prevalence of lupus nephritis as Asians and Hispanics have significantly higher prevalence of lupus nephritis as compared to other races as mentioned in multiple cohort studies, including the California Lupus Surveillance Project, LUMINA, PROFILE, and the Southern California Lupus Registry [20]. Similarly, the GLADEL cohort found increased rates of renal involvement in Black and mestizo patients compared to White patients [20].
Genetic, environmental, and healthcare system factors contribute to disparities in lupus nephritis outcomes. CKD risk alleles are more prevalent in populations of African ancestry, with an unfavorable genetic risk gradient observed in these patients. Environmental triggers, influenced by occupational patterns, are more common among marginalized groups. Additionally, fragmented healthcare systems, unequal access to care, and underrepresentation in the healthcare workforce further exacerbate disparities in outcomes for these populations. Addressing these multifactorial disparities requires targeted interventions to improve healthcare access, foster equity, and mitigate the impact of genetic and environmental risk factors, ultimately aiming to achieve better outcomes for all patients with lupus nephritis [20].
5. Implications for Clinical Practice and Policy
Early Identification and Risk Stratification: Community-based programs in underserved areas are vital for early detection of high-risk patients, enabling timely treatment and preventing complications.
Enhanced Multidisciplinary Care Access: Integrating rheumatology with primary and specialty care improves comprehensive SLE management. Telemedicine and outreach can address access gaps in underserved populations.
Addressing Socioeconomic Barriers: Improving insurance coverage, patient education, and financial support for transportation and medications is essential to reduce disparities and disease burden.
Culturally Competent Care: Training providers to deliver culturally sensitive care, address language barriers, and reduce implicit biases fosters trust and improves outcomes for minority patients.
6. Limitations
While this study offers valuable insights, several limitations must be acknowledged. Firstly, the retrospective design inherently limits the ability to control all potential confounding variables, which may affect the validity of the findings. Secondly, the duration of observation varied among patient groups, potentially influencing the comparability of outcomes across cohorts. Thirdly, key cardiovascular risk factors, including smoking status, physical activity, medication adherence, and socioeconomic status, were not captured in the analysis. The omission of these variables may have introduced unmeasured confounding and influenced the observed associations. Another limitation is that while global p-values were reported to assess overall differences between groups, post hoc pairwise comparisons were not performed. Given the large sample size and retrospective design of the study, we prioritized minimizing the risk of type I error and overinterpretation. Future studies could consider performing detailed post hoc analyses to further delineate specific group differences.
Author Contributions
Conceptualization, F.S. and J.T.; methodology, S.P.A.; formal analysis, S.P.A. and D.M.; investigation S.P.A.; resources F.S. and J.T.; data curation, S.P.A. and F.S.; writing—original draft preparation, F.S., S.P.A., D.M., and J.T.; writing—review and editing, S.P.A. and D.M.; visualization S.P.A.; supervision, S.S.; project administration, S.S. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
This is not applicable to this study as this study utilized a national database containing de-identified data and, therefore, did not require IRB approval. No activities with humans or animals were a part of this study.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data utilized in this study are a publicly available resource under the Healthcare and Utilization Project of the Agency for Healthcare Research and Quality (AHRQ) in the United States. https://hcup-us.ahrq.gov/db/nation/nis/nisdbdocumentation.jsp. Accessed on 21 March 2025.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Justiz Vaillant, A.A.; Goyal, A.; Varacallo, M.A. Systemic Lupus Erythematosus. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2023. [Google Scholar]
- González, L.A.; Toloza, S.M.; McGwin, G., Jr.; Alarcón, G.S. Ethnicity in systemic lupus erythematosus (SLE): Its influence on susceptibility and outcomes. Lupus 2013, 22, 1214–1224. [Google Scholar] [CrossRef]
- Maningding, E.; Dall’Era, M.; Trupin, L.; Murphy, L.B.; Yazdany, J. Racial and Ethnic Differences in the Prevalence and Time to Onset of Manifestations of Systemic Lupus Erythematosus: The California Lupus Surveillance Project. Arthritis Care Res. 2020, 72, 622–629. [Google Scholar] [CrossRef] [PubMed]
- Siegel, C.H.; Sammaritano, L.R. Systemic Lupus Erythematosus: A Review. JAMA 2024, 331, 1480–1491. [Google Scholar] [CrossRef] [PubMed]
- Hocaoglu, M.; Valenzuela-Almada, M.O.; Dabit, J.Y.; Osei-Onomah, S.A.; Chevet, B.; Giblon, R.E. Incidence, Prevalence, and Mortality of Lupus Nephritis: A Population-Based Study Over Four Decades Using the Lupus Midwest Network. Arthritis Rheumatol. 2023, 75, 567–573. [Google Scholar] [CrossRef] [PubMed]
- Alghareeb, R.; Hussain, A.; Maheshwari, M.V.; Khalid, N.; Patel, P.D. Cardiovascular Complications in Systemic Lupus Erythematosus. Cureus 2022, 14, e26671. [Google Scholar] [CrossRef]
- Wang, T.K.M.; Chan, N.; Khayata, M.; Flanagan, P.; Grimm, R.A.; Griffin, B.P.; Husni, E.M.; Littlejohn, E.; Xu, B. Abstract 13131: Cardiovascular Manifestations and Outcomes in Systemic Lupus Erythematosus: The Cleveland Clinic Contemporary Experience. Circulation 2020, 142 (Suppl. 3). [Google Scholar] [CrossRef]
- Moder, K.G.; Miller, T.D.; Tazelaar, H.D. Cardiac involvement in systemic lupus erythematosus. Mayo Clin. Proc. 1999, 74, 275–284. [Google Scholar] [CrossRef]
- Advancing Health Equity in Lupus [Lupus Foundation of America]. Available online: https://www.lupus.org/health-disparities (accessed on 19 January 2025).
- Garg, S.; Bartels, C.M.; Bao, G.; Helmick, C.G.; Drenkard, C.; Lim, S.S. Timing and Predictors of Incident Cardiovascular Disease in Systemic Lupus Erythematosus: Risk Occurs Early and Highlights Racial Disparities. J. Rheumatol. 2023, 50, 84–92. [Google Scholar] [CrossRef]
- Lim, S.S.; Helmick, C.G.; Bao, G.; Hootman, J.; Bayakly, R.; Gordon, C.; Drenkard, C. Racial Disparities in Mortality Associated with Systemic Lupus Erythematosus—Fulton and DeKalb Counties, Georgia, 2002–2016. MMWR Morb. Mortal. Wkly. Rep. 2019, 68, 419–422. [Google Scholar] [CrossRef]
- Healthcare Cost and Utilization Project (HCUP). Overview of the National (Nationwide) Inpatient Sample (NIS); Agency for Healthcare Research and Quality: Rockville, MD, US. Available online: https://www.ahrq.gov/data/hcup/index.html (accessed on 21 March 2025).
- Libby, P.; Ridker, P.M.; Maseri, A. Inflammation and atherosclerosis. Circulation 2002, 105, 1135–1143. [Google Scholar] [CrossRef]
- Esdaile, J.M.; Abrahamowicz, M.; Grodzicky, T.; Li, Y.; Panaritis, C.; Berger, R.D.; Côté, R.; Grover, S.A.; Fortin, P.R.; Clarke, A.E.; et al. Traditional Framingham risk factors fail to fully account for accelerated atherosclerosis in systemic lupus erythematosus. Arthritis Rheum. 2001, 44, 2331–2337. [Google Scholar] [CrossRef]
- Singh, J.A.; Cleveland, J.D. Hospitalized Infections in Lupus: A Nationwide Study of Types of Infections, Time Trends, Health Care Utilization, and In-Hospital Mortality. Arthritis Rheumatol. 2021, 73, 617–630. [Google Scholar] [CrossRef]
- Lim, S.S.; Drenkard, C. Understanding lupus disparities through a social Determinants of Health framework. Rheum. Dis. Clin. N. Am. 2020, 46, 613–621. [Google Scholar] [CrossRef] [PubMed]
- Demas, K.L.; Costenbader, K.H. Disparities in lupus care and outcomes. Curr. Opin. Rheumatol. 2009, 21, 102–109. [Google Scholar] [CrossRef] [PubMed]
- Mccarty, D.J.; Manzi, S.; Medsger, T.A.; Ramsey-Goldman, R.; Laporte, R.E.; Kwoh, C.K. Incidence of systemic lupus erythematosus race and gender differences. Arthritis Rheum. 1995, 38, 1260–1270. [Google Scholar] [CrossRef] [PubMed]
- Pons-Estel, G.J.; Alarcón, G.S.; Scofield, L.; Reinlib, L.; Cooper, G.S. Understanding the epidemiology and progression of systemic lupus erythematosus. Semin. Arthritis Rheum. 2009, 39, 257–268. [Google Scholar] [CrossRef]
- Feldman, C.H.; Hiraki, L.T.; Liu, J.; Fischer, M.A.; Solomon, D.H.; Alarcón, G.S.; Winkelmayer, W.C.; Costenbader, K.H. Epidemiology and sociodemographics of systemic lupus erythematosus and lupus nephritis among US adults with Medicaid coverage, 2000–2004. Arthritis Rheum. 2012, 65, 753–763. [Google Scholar] [CrossRef]
- Somers, E.C.; Marder, W.; Cagnoli, P.; Lewis, E.E.; DeGuire, P.; Gordon, C.; Helmick, C.G.; Wang, L.; Wing, J.J.; Dhar, J.P.; et al. Population-Based Incidence and Prevalence of Systemic Lupus Erythematosus: The Michigan Lupus Epidemiology and Surveillance Program. Arthritis Rheumatol. 2014, 66, 369–378. [Google Scholar] [CrossRef]
- Hasan, B.; Fike, A.; Hasni, S. Health disparities in systemic lupus erythematosus—A narrative review. Clin. Rheumatol. 2022, 41, 3299–3311. [Google Scholar] [CrossRef]
- Julian, L.J.; Yelin, E.; Yazdany, J.; Panopalis, P.; Trupin, L.; Criswell, L.A.; Katz, P. Depression, medication adherence, and service utilization in systemic lupus erythematosus†. Arthritis Rheum. 2009, 61, 240–246. [Google Scholar] [CrossRef]
- Anderson, E.; Nietert, P.J.; Kamen, D.L.; Gilkeson, G.S. Ethnic disparities among patients with systemic lupus erythematosus in South Carolina. J. Rheumatol. 2008, 35, 819–825. [Google Scholar]
- Scalzi, L.V.; Hollenbeak, C.S.; Wang, L. Racial disparities in age at time of cardiovascular events and cardiovascular-related death in patients with systemic lupus erythematosus. Arthritis Rheum. 2010, 62, 2767–2775. [Google Scholar] [CrossRef]
- Mittal, S.; Siva, C. Incidence of Atrial Fibrillation and Related Outcomes among Hospitalized Patients with Systemic Lupus Erythematosus: Analysis of United States Nationwide Inpatient Sample Database 2016–2019. J. Clin. Med. 2024, 13, 1675. [Google Scholar] [CrossRef] [PubMed]
- Dein, E.; Douglas, H.; Petri, M.; Law, G.; Timlin, H. Pericarditis in Lupus. Cureus 2019, 11, e4166. [Google Scholar] [CrossRef] [PubMed]
- Vina, E.R.; Hausmann, L.R.M.; Utset, T.O.; Masi, C.M.; Liang, K.P.; Kwoh, C.K. Perceptions of racism in healthcare among patients with systemic lupus erythematosus: A cross-sectional study. Lupus Sci. Med. 2015, 2, e000110. [Google Scholar] [CrossRef] [PubMed]
- Shin, J.I.; Lee, K.H.; Park, S.; Yang, J.W.; Kim, H.J.; Song, K.; Lee, S.; Na, H.; Jang, Y.J.; Nam, J.Y.; et al. Systemic Lupus Erythematosus and Lung Involvement: A Comprehensive Review. J. Clin. Med. 2022, 11, 6714. [Google Scholar] [CrossRef]
- DeQuattro, K.; Trupin, L.; Murphy, L.B.; Rush, S.; Criswell, L.A.; Lanata, C.M.; Dall’ERa, M.; Katz, P.; Yazdany, J. High disease severity among Asian patients in a US multiethnic cohort of individuals with systemic lupus erythematosus. Arthritis Care Res. 2020, 74, 896–903. [Google Scholar] [CrossRef]
- Yin, R.; Qui, C.X.; Yin, L.J.; Zhang, Y. Multiple spontaneous intracranial hemorrhages in a patient with systemic lupus erythematosus: A case report. Int. J. Clin. Exp. Med. 2019, 12, 10900–10904. [Google Scholar]
- Shen, A.Y.; Yao, J.F.; Brar, S.S.; Jorgensen, M.B.; Chen, W. Racial/ethnic differences in the risk of intracranial hemorrhage among patients with atrial fibrillation. J. Am. Coll. Cardiol. 2007, 50, 309–315. [Google Scholar] [CrossRef]
- Kittner, S.J.; Sekar, P.; Comeau, M.E.; Anderson, C.D.; Parikh, G.Y.; Tavarez, T.; Flaherty, M.L.; Testai, F.D.; Frankel, M.R.; James, M.L.; et al. Ethnic and racial variation in intracerebral hemorrhage risk factors and risk factor burden. JAMA Netw. Open. 2021, 4, e2121921. [Google Scholar] [CrossRef]
- Barbhaiya, M.; Feldman, C.H.; Guan, H.; Chen, S.K.; Fischer, M.A.; Solomon, D.H.; Everett, B.M.; Costenbader, K.H. Racial/ethnic variation in stroke rates and risks among patients with systemic lupus erythematosus. Semin. Arthritis Rheum. 2018, 48, 840–846. [Google Scholar] [CrossRef]
- Musa, R.; Rout, P.; Qurie, A. Lupus Nephritis. [Updated 2025 Jan 16]. In StatPearls [Internet]; StatPearls Publishing: Treasure Island, FL, USA, 2025. Available online: https://www.ncbi.nlm.nih.gov/books/NBK499817/ (accessed on 21 March 2025).
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).