Circulating miRNAs and Vascular Injury Markers Associate with Cardiovascular Function in Older Patients Reaching End-Stage Kidney Disease
Abstract
1. Introduction
2. Materials and Methods
2.1. Patient Cohort
2.2. Renal Care
2.3. Magnetic Resonance Imaging (MRI)
2.4. Cardiovascular Structure and Function
2.5. Angiopoietin-2 II (Ang-2) and Asymmetric Dimethylarginine (ADMA)
2.6. Circulating Angiogenic miRNAs
2.7. Statistical Analysis
3. Results
3.1. Patient Cohort Description
3.2. Associations between Vascular Injury Markers and Cardiovascular Disease
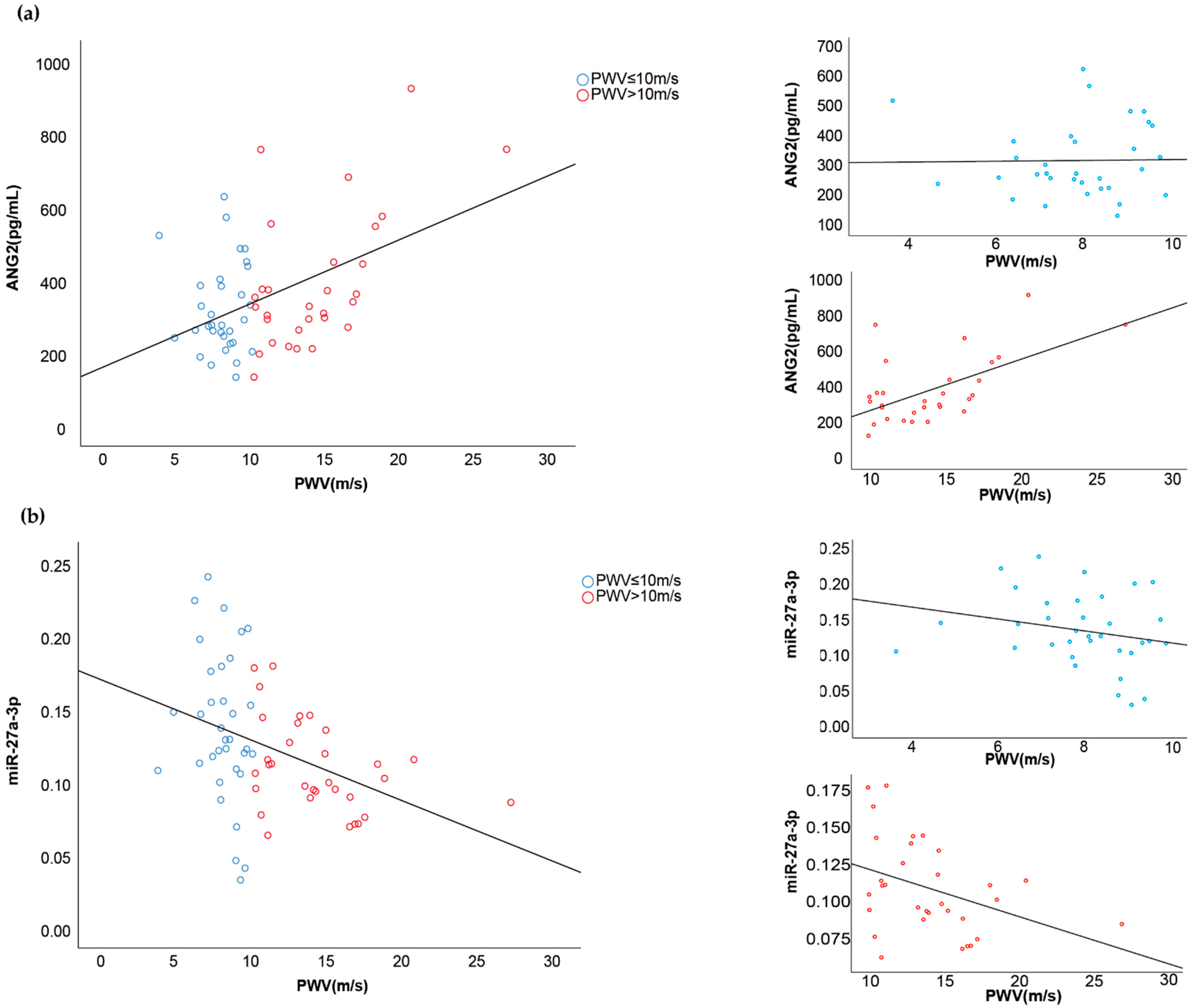
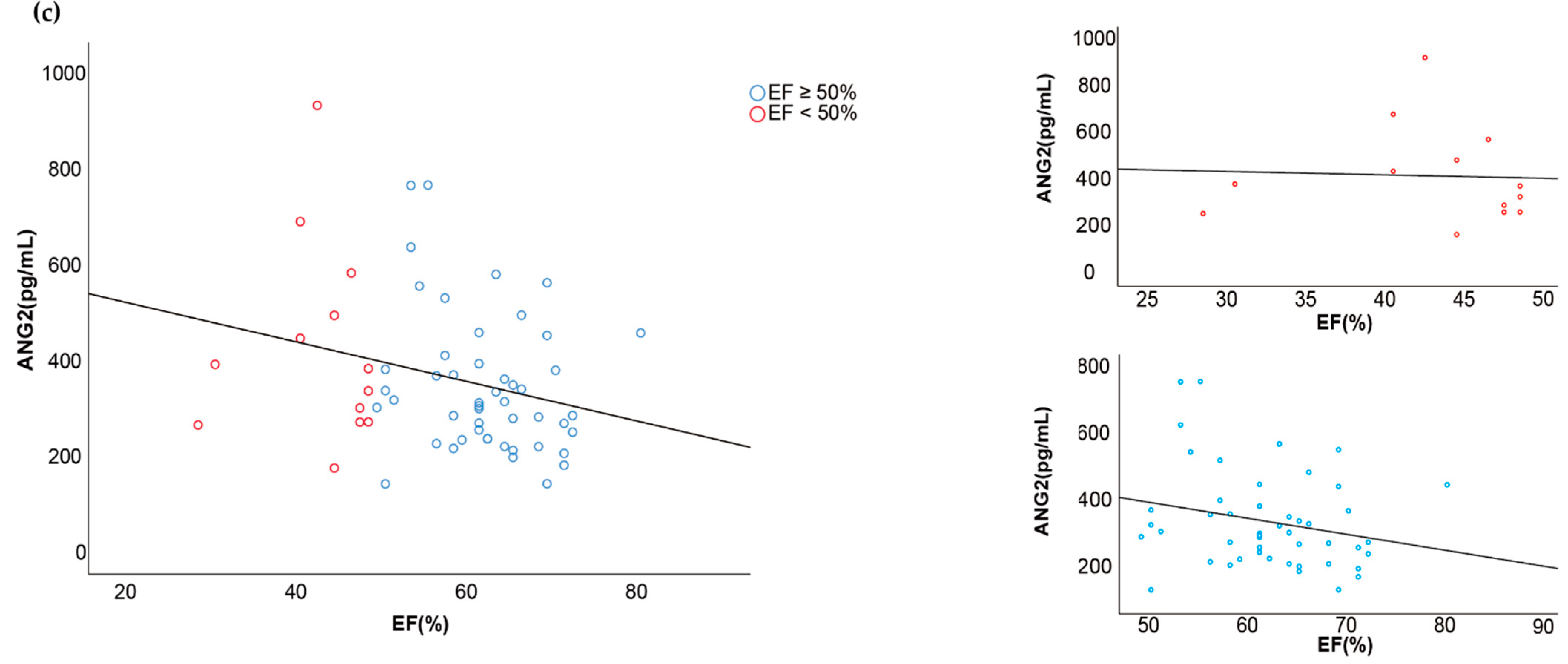
3.3. Associations between Vascular Injury Markers and Cardiovascular Function
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- GBD Chronic Kidney Disease Collaboration. Global, regional, and national burden of chronic kidney disease, 1990–2017: A systematic analysis for the Global Burden of Disease Study 2017. Lancet 2020, 395, 709–733. [Google Scholar] [CrossRef]
- Geluk, C.A.; Tio, R.A.; Tijssen, J.G.; van Dijk, R.B.; Dijk, W.A.; Hillege, H.L.; de Jong, P.E.; van Gilst, W.H.; Zijlstra, F. Clinical characteristics, cardiac events and coronary angiographic findings in the prospective PREVEND cohort: An observational study. Neth. Heart J. Mon. J. Neth. Soc. Cardiol. Neth. Heart Found. 2007, 15, 133–141. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Kalantar-Zadeh, K.; Jafar, T.H.; Nitsch, D.; Neuen, B.L.; Perkovic, V. Chronic kidney disease. Lancet 2021, 398, 786–802. [Google Scholar] [CrossRef]
- Kurella, M.; Chertow, G.M.; Luan, J.; Yaffe, K. Cognitive impairment in chronic kidney disease. J. Am. Geriatr. Soc. 2004, 52, 1863–1869. [Google Scholar] [CrossRef] [PubMed]
- Pippias, M.; Jager, K.J.; Kramer, A.; Leivestad, T.; Sanchez, M.B.; Caskey, F.J.; Collart, F.; Couchoud, C.; Dekker, F.W.; Finne, P.; et al. The changing trends and outcomes in renal replacement therapy: Data from the ERA-EDTA Registry. Nephrol. Dial. Transpl. 2016, 31, 831–841. [Google Scholar] [CrossRef] [PubMed]
- Drew, D.A.; Weiner, D.E.; Tighiouart, H.; Duncan, S.; Gupta, A.; Scott, T.; Sarnak, M.J. Cognitive decline and its risk factors in prevalent hemodialysis patients. Am. J. Kidney Dis. 2017, 69, 780–787. [Google Scholar] [CrossRef]
- Zijlstra, L.E.; Trompet, S.; Jukema, J.W.; Kroft, L.J.; de Bresser, J.; van Osch, M.J.; Hammer, S.; Witjes, M.-N.; van Buren, M.; Mooijaart, S.P. Association of cardiovascular structure and function with cerebrovascular changes and cognitive function in older patients with end-stage renal disease. Aging 2020, 12, 1496. [Google Scholar] [CrossRef] [PubMed]
- Levey, A.S.; Coresh, J. Chronic kidney disease. Lancet 2012, 379, 165–180. [Google Scholar] [CrossRef]
- Grams, M.E.; Yang, W.; Rebholz, C.M.; Wang, X.; Porter, A.C.; Inker, L.A.; Horwitz, E.; Sondheimer, J.H.; Hamm, L.L.; He, J. Risks of adverse events in advanced CKD: The chronic renal insufficiency cohort (CRIC) study. Am. J. Kidney Dis. 2017, 70, 337–346. [Google Scholar] [CrossRef] [PubMed]
- Ikram, M.A.; Vernooij, M.W.; Hofman, A.; Niessen, W.J.; van der Lugt, A.; Breteler, M.M.B. Kidney function is related to cerebral small vessel disease. Stroke 2008, 39, 55–61. [Google Scholar] [CrossRef] [PubMed]
- Foley, R.N.; Parfrey, P.S.; Sarnak, M.J. Epidemiology of cardiovascular disease in chronic renal disease. J. Am. Soc. Nephrol. 1998, 9, S16–S23. [Google Scholar] [CrossRef]
- Go, A.S.; Chertow, G.M.; Fan, D.; McCulloch, C.E.; Hsu, C.Y. Chronic kidney disease and the risks of death, cardiovascular events, and hospitalization. N. Engl. J. Med. 2004, 351, 1296–1305. [Google Scholar] [CrossRef]
- Chronic Kidney Disease Prognosis Consortium. Association of estimated glomerular filtration rate and albuminuria with all-cause and cardiovascular mortality in general population cohorts: A collaborative meta-analysis. Lancet 2010, 375, 2073–2081. [Google Scholar] [CrossRef]
- Bozbas, H.; Pirat, B.; Demirtas, S.; Simşek, V.; Yildirir, A.; Sade, E.; Sayin, B.; Sezer, S.; Karakayali, H.; Muderrisoglu, H. Evaluation of coronary microvascular function in patients with end-stage renal disease, and renal allograft recipients. Atherosclerosis 2009, 202, 498–504. [Google Scholar] [CrossRef] [PubMed]
- Gradaus, F.; Ivens, K.; Peters, A.J.; Heering, P.; Schoebel, F.C.; Grabensee, B.; Strauer, B.E. Angiographic progression of coronary artery disease in patients with end-stage renal disease. Nephrol. Dial. Transplant. 2001, 16, 1198–1202. [Google Scholar] [CrossRef] [PubMed]
- Kahn, M.R.; Robbins, M.J.; Kim, M.C.; Fuster, V. Management of cardiovascular disease in patients with kidney disease. Nat. Rev. Cardiol. 2013, 10, 261–273. [Google Scholar] [CrossRef] [PubMed]
- Popolo, A.; Autore, G.; Pinto, A.; Marzocco, S. Oxidative stress in patients with cardiovascular disease and chronic renal failure. Free Radic. Res. 2013, 47, 346–356. [Google Scholar] [CrossRef] [PubMed]
- Ooi, Q.L.; Tow, F.K.N.-F.H.; Deva, R.; Alias, M.A.; Kawasaki, R.; Wong, T.Y.; Mohamad, N.; Colville, D.; Hutchinson, A.; Savige, J. The microvasculature in chronic kidney disease. Clin. J. Am. Soc. Nephrol. 2011, 6, 1872–1878. [Google Scholar] [CrossRef]
- Dickstein, K.; Cohen-Solal, A.; Filippatos, G.; McMurray, J.J.; Ponikowski, P.; Poole-Wilson, P.A.; Stromberg, A.; van Veldhuisen, D.J.; Atar, D.; Hoes, A.W.; et al. ESC guidelines for the diagnosis and treatment of acute and chronic heart failure 2008: The Task Force for the diagnosis and treatment of acute and chronic heart failure 2008 of the European Society of Cardiology. Developed in collaboration with the Heart Failure Association of the ESC (HFA) and endorsed by the European Society of Intensive Care Medicine (ESICM). Eur. J. Heart Fail. 2008, 10, 933–989. [Google Scholar] [CrossRef] [PubMed]
- de Roos, A.; van der Grond, J.; Mitchell, G.; Westenberg, J. Magnetic resonance imaging of cardiovascular function and the brain: Is dementia a cardiovascular-driven disease? Circulation 2017, 135, 2178–2195. [Google Scholar] [CrossRef]
- Mitchell, G.F.; van Buchem, M.A.; Sigurdsson, S.; Gotal, J.D.; Jonsdottir, M.K.; Kjartansson, Ó.; Garcia, M.; Aspelund, T.; Harris, T.B.; Gudnason, V. Arterial stiffness, pressure and flow pulsatility and brain structure and function: The Age, Gene/Environment Susceptibility–Reykjavik study. Brain 2011, 134, 3398–3407. [Google Scholar] [CrossRef]
- Bijkerk, R.; van Solingen, C.; de Boer, H.C.; van der Pol, P.; Khairoun, M.; de Bruin, R.G.; van Oeveren-Rietdijk, A.M.; Lievers, E.; Schlagwein, N.; van Gijlswijk, D.J.; et al. Hematopoietic microRNA-126 protects against renal ischemia/reperfusion injury by promoting vascular integrity. J. Am. Soc. Nephrol. 2014, 25, 1710–1722. [Google Scholar] [CrossRef] [PubMed]
- Anand, S.; Majeti, B.K.; Acevedo, L.M.; Murphy, E.A.; Mukthavaram, R.; Scheppke, L.; Huang, M.; Shields, D.J.; Lindquist, J.N.; Lapinski, P.E.; et al. MicroRNA-132-mediated loss of p120RasGAP activates the endothelium to facilitate pathological angiogenesis. Nat. Med. 2010, 16, 909–914. [Google Scholar] [CrossRef] [PubMed]
- Creemers, E.E.; Tijsen, A.J.; Pinto, Y.M. Circulating microRNAs: Novel biomarkers and extracellular communicators in cardiovascular disease? Circ. Res. 2012, 110, 483–495. [Google Scholar] [CrossRef] [PubMed]
- Kato, M.; Castro, N.E.; Natarajan, R. MicroRNAs: Potential mediators and biomarkers of diabetic complications. Free Radic. Biol. Med. 2013, 64, 85–94. [Google Scholar] [CrossRef] [PubMed]
- Khairoun, M.; de Koning, E.J.; van den Berg, B.M.; Lievers, E.; de Boer, H.C.; Schaapherder, A.F.; Mallat, M.J.; Rotmans, J.I.; van der Boog, P.J.; van Zonneveld, A.J.; et al. Microvascular damage in type 1 diabetic patients is reversed in the first year after simultaneous pancreas-kidney transplantation. Am. J. Transplant. 2013, 13, 1272–1281. [Google Scholar] [CrossRef]
- Bartel, D.P. MicroRNAs: Target recognition and regulatory functions. Cell 2009, 136, 215–233. [Google Scholar] [CrossRef]
- Guay, C.; Regazzi, R. Circulating microRNAs as novel biomarkers for diabetes mellitus. Nat. Rev. Endocrinol. 2013, 9, 513–521. [Google Scholar] [CrossRef] [PubMed]
- Bijkerk, R.; Florijn, B.W.; Khairoun, M.; Duijs, J.; Ocak, G.; de Vries, A.P.J.; Schaapherder, A.F.; Mallat, M.J.K.; de Fijter, J.W.; Rabelink, T.J.; et al. Acute Rejection After Kidney Transplantation Associates With Circulating MicroRNAs and Vascular Injury. Transplant. Direct 2017, 3, e174. [Google Scholar] [CrossRef] [PubMed]
- Bijkerk, R.; Duijs, J.M.; Khairoun, M.; Ter Horst, C.J.; van der Pol, P.; Mallat, M.J.; Rotmans, J.I.; de Vries, A.P.; de Koning, E.J.; de Fijter, J.W.; et al. Circulating microRNAs associate with diabetic nephropathy and systemic microvascular damage and normalize after simultaneous pancreas-kidney transplantation. Am. J. Transplant. 2015, 15, 1081–1090. [Google Scholar] [CrossRef] [PubMed]
- Bijkerk, R.; Kallenberg, M.H.; Zijlstra, L.E.; van den Berg, B.M.; de Bresser, J.; Hammer, S.; Bron, E.E.; Achterberg, H.; van Buchem, M.A.; Berkhout-Byrne, N.C.; et al. Circulating angiopoietin-2 and angiogenic microRNAs associate with cerebral small vessel disease and cognitive decline in older patients reaching end stage renal disease. Nephrol. Dial. Transplant. 2020, gfaa370. [Google Scholar] [CrossRef]
- Wang, G.-K.; Zhu, J.-Q.; Zhang, J.-T.; Li, Q.; Li, Y.; He, J.; Qin, Y.-W.; Jing, Q. Circulating microRNA: A novel potential biomarker for early diagnosis of acute myocardial infarction in humans. Eur. Heart J. 2010, 31, 659–666. [Google Scholar] [CrossRef] [PubMed]
- Tijsen, A.J.; Creemers, E.E.; Moerland, P.D.; de Windt, L.J.; van der Wal, A.C.; Kok, W.E.; Pinto, Y.M. MiR423-5p as a circulating biomarker for heart failure. Circ. Res. 2010, 106, 1035–1039. [Google Scholar] [CrossRef] [PubMed]
- Taubel, J.; Hauke, W.; Rump, S.; Viereck, J.; Batkai, S.; Poetzsch, J.; Rode, L.; Weigt, H.; Genschel, C.; Lorch, U.; et al. Novel antisense therapy targeting microRNA-132 in patients with heart failure: Results of a first-in-human Phase 1b randomized, double-blind, placebo-controlled study. Eur. Heart J. 2021, 42, 178–188. [Google Scholar] [CrossRef]
- Wu, S.Y.; Rupaimoole, R.; Shen, F.; Pradeep, S.; Pecot, C.V.; Ivan, C.; Nagaraja, A.S.; Gharpure, K.M.; Pham, E.; Hatakeyama, H.; et al. A miR-192-EGR1-HOXB9 regulatory network controls the angiogenic switch in cancer. Nat. Commun. 2016, 7, 11169. [Google Scholar] [CrossRef] [PubMed]
- Widlansky, M.E.; Jensen, D.M.; Wang, J.; Liu, Y.; Geurts, A.M.; Kriegel, A.J.; Liu, P.; Ying, R.; Zhang, G.; Casati, M.; et al. miR-29 contributes to normal endothelial function and can restore it in cardiometabolic disorders. EMBO Mol. Med. 2018, 10, e8046. [Google Scholar] [CrossRef] [PubMed]
- Berkhout-Byrne, N.; Kallenberg, M.H.; Gaasbeek, A.; Rabelink, T.J.; Hammer, S.; van Buchem, M.A.; van Osch, M.J.; Kroft, L.J.; Boom, H.; Mooijaart, S.P.; et al. The Cognitive decline in Older Patients with End stage renal disease (COPE) study–rationale and design. Curr. Med Res. Opin. 2017, 33, 2057–2064. [Google Scholar] [CrossRef] [PubMed]
- van den Brand, J.A.; van Boekel, G.A.; Willems, H.L.; Kiemeney, L.A.; den Heijer, M.; Wetzels, J.F.M. Introduction of the CKD-EPI equation to estimate glomerular filtration rate in a Caucasian population. Nephrol. Dial. Transplant. 2011, 26, 3176–3181. [Google Scholar] [CrossRef]
- Levey, A.S.; Bosch, J.P.; Lewis, J.B.; Greene, T.; Rogers, N.; Roth, D. A more accurate method to estimate glomerular filtration rate from serum creatinine: A new prediction equation. Ann. Intern. Med. 1999, 130, 461–470. [Google Scholar] [CrossRef]
- Du Bois, D.; Du Bois, E.F. A formula to estimate the approximate surface area if height and weight be known. Nutrition 1989, 5, 303–313. [Google Scholar] [PubMed]
- Williams, B.; Mancia, G.; Spiering, W.; Rosei, E.A.; Azizi, M.; Burnier, M.; Clement, D.L.; Coca, A.; De Simone, G.; Dominiczak, A.; et al. 2018 ESC/ESH Guidelines for the management of arterial hypertension: The Task Force for the management of arterial hypertension of the European Society of Cardiology (ESC) and the European Society of Hypertension (ESH). G. Ital. Cardiol. 2018, 39, 3021–3104. [Google Scholar] [CrossRef] [PubMed]
- Ponikowski, P.; Voors, A.A.; Anker, S.D.; Bueno, H.; Cleland, J.G.; Coats, A.J.; Falk, V.; González-Juanatey, J.R.; Harjola, V.-P.; Jankowska, E.A.; et al. 2016 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure. Kardiol. Pol. 2016, 74, 1037–1147. [Google Scholar] [CrossRef] [PubMed]
- Grey, E.; Bratteli, C.; Glasser, S.P.; Alinder, C.; Finkelstein, S.M.; Lindgren, B.R.; Cohn, J.N. Reduced small artery but not large artery elasticity is an independent risk marker for cardiovascular events. Am. J. Hypertens. 2003, 16, 265–269. [Google Scholar] [CrossRef]
- Nguyen, K.T.; Donoghue, L.; Giridharan, G.A.; Naber, J.P.; Vincent, D.; Fukamachi, K.; Kotru, A.; Sethu, P. Acute Response of Human Aortic Endothelial Cells to Loss of Pulsatility as Seen during Cardiopulmonary Bypass. Cells Tissues Organs 2021, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Chang, F.C.; Chiang, W.C.; Tsai, M.H.; Chou, Y.H.; Pan, S.Y.; Chang, Y.T.; Yeh, P.Y.; Chen, Y.T.; Chiang, C.K.; Chen, Y.M.; et al. Angiopoietin-2-induced arterial stiffness in CKD. J. Am. Soc. Nephrol. 2014, 25, 1198–1209. [Google Scholar] [CrossRef]
- Sun, Y.; Xiao, Y.; Sun, H.; Zhao, Z.; Zhu, J.; Zhang, L.; Dong, J.; Han, T.; Jing, Q.; Zhou, J.; et al. miR-27a regulates vascular remodeling by targeting endothelial cells’ apoptosis and interaction with vascular smooth muscle cells in aortic dissection. Theranostics 2019, 9, 7961–7975. [Google Scholar] [CrossRef]
- Choe, N.; Kwon, D.H.; Ryu, J.; Shin, S.; Cho, H.J.; Joung, H.; Eom, G.H.; Ahn, Y.; Park, W.J.; Nam, K.I.; et al. miR-27a-3p Targets ATF3 to Reduce Calcium Deposition in Vascular Smooth Muscle Cells. Mol. Ther. Nucleic Acids 2020, 22, 627–639. [Google Scholar] [CrossRef] [PubMed]
- Pacurari, M.; Tchounwou, P.B. Role of microRNAs in renin-angiotensin-aldosterone system-mediated cardiovascular inflammation and remodeling. Int. J. Inflamm. 2015, 2015, 101527. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.-J.; Xu, R.; Yu, H.-M.; Chang, Q.; Zhong, J.-C. The ACE2/apelin signaling, microRNAs, and hypertension. Int. J. Hypertens. 2015, 2015, 896861. [Google Scholar] [CrossRef]
- Otani, A.; Takagi, H.; Oh, H.; Koyama, S.; Honda, Y. Angiotensin II induces expression of the Tie2 receptor ligand, angiopoietin-2, in bovine retinal endothelial cells. Diabetes 2001, 50, 867–875. [Google Scholar] [CrossRef]
- Chen, W.-J.; Yin, K.; Zhao, G.-J.; Fu, Y.-C.; Tang, C.-K. The magic and mystery of microRNA-27 in atherosclerosis. Atherosclerosis 2012, 222, 314–323. [Google Scholar] [CrossRef] [PubMed]
- Li, T.; Cao, H.; Zhuang, J.; Wan, J.; Guan, M.; Yu, B.; Li, X.; Zhang, W. Identification of miR-130a, miR-27b and miR-210 as serum biomarkers for atherosclerosis obliterans. Clin. Chim. Acta 2011, 412, 66–70. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.-H.; Choi, S.; Jung, J.-H.; Lee, N. Effects of atrial fibrillation on arterial stiffness in patients with hypertension. Angiology 2008, 59, 459–463. [Google Scholar] [CrossRef] [PubMed]
- Chu, C.-Y.; Lin, T.-H.; Hsu, P.-C.; Lee, W.-H.; Lee, H.-H.; Chiu, C.-A.; Su, H.-M.; Lee, C.-S.; Yen, H.-W.; Voon, W.-C. Heart rate significantly influences the relationship between atrial fibrillation and arterial stiffness. Int. J. Med Sci. 2013, 10, 1295. [Google Scholar] [CrossRef] [PubMed]
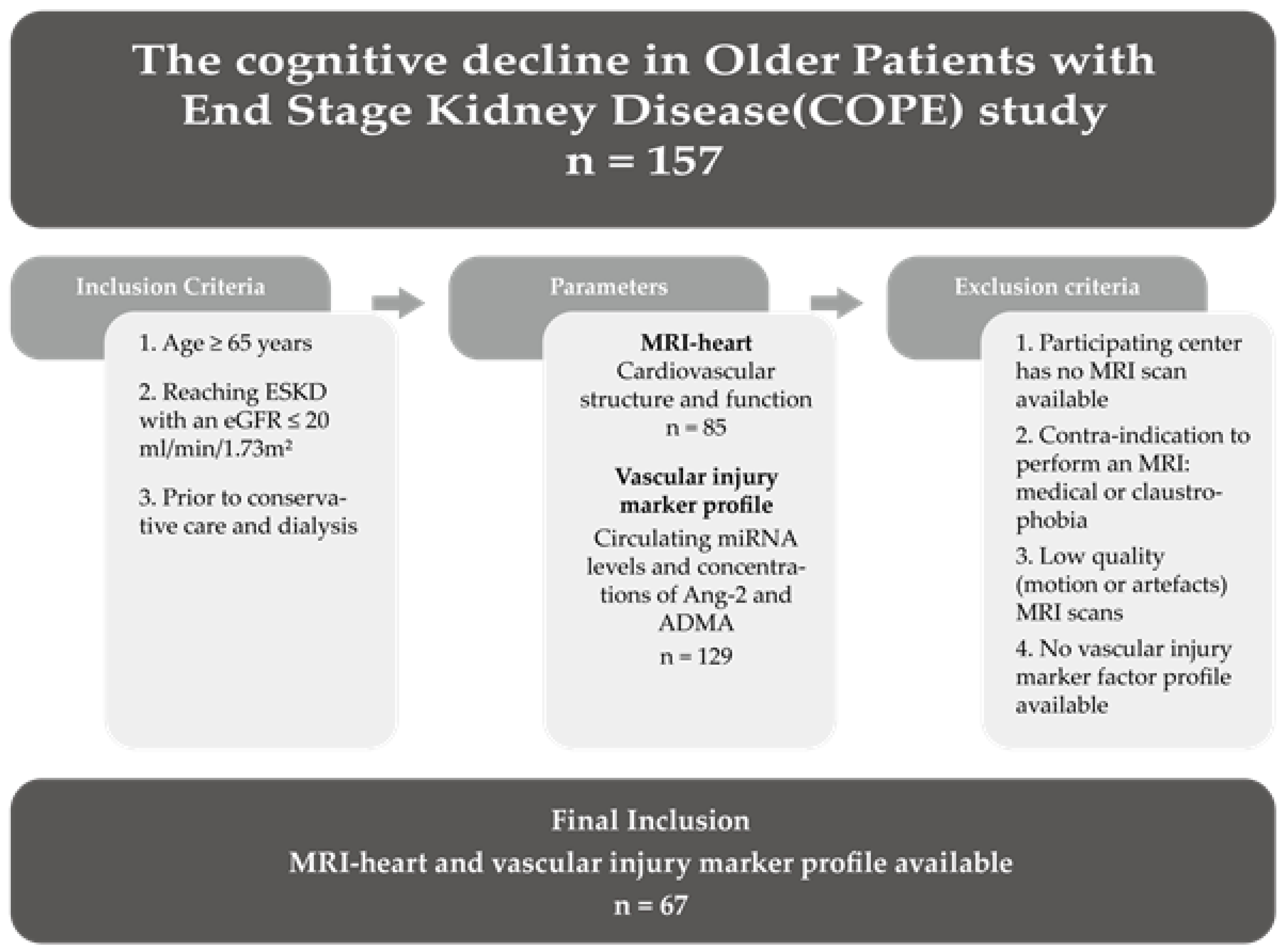
| Male gender, n (%) | 44 (65.7) |
| Age, years; mean ± SD | 75.1 ± 6.6 |
| Body mass index (BMI); mean ± SD | 27.9 ± 3.83 |
| Race, Caucasian, n (%) | 58 (86.6) |
| Higher educational level 1, n (%) | 25 (37.4) |
| Primary kidney disease, n (%) | |
| Non-vascular cause | 25 (37.3) |
| Vascular cause | 41 (61.2) |
| Comorbidity, n (%) | |
| Diabetes mellitus | 28 (41.8) |
| Peripheral vascular disease | 10 (14.9) |
| Cerebral vascular accident | 17 (25.4) |
| Heart failure | 4 (6.0) |
| Coronary heart disease | 15 (22.4) |
| Left ventricle hypertrophy | 8 (11.9) |
| Atrial fibrillation | 14 (20.9) |
| Alcohol consumption | 39 (58.2) |
| History of smoking | 39 (72.2) |
| Medication use, n (%) | |
| Polypharmacy (the use of ≥5 medications) | 62 (92.5) |
| Antihypertensive medication | 62 (92.5) |
| Diuretics | 38 (56.7) |
| Cholesterol-lowering | 49 (73.1) |
| Anti-coagulants | 50 (74.6) |
| Objective measures, mean ± SD | |
| Blood pressure (mmHg) | |
| Systolic | 155.7 ± 21.7 |
| Diastolic | 81.6 ± 11.3 |
| eGFR (mL/min/1.73 m2) | 16.0 ± 4.0 |
| Urea (mmol/L) | 21.3 ± 6.6 |
| Albumin (mg/24 h) 2 | 803 ± 956 |
| Troponin-T (µg/L) | 0.071 ± 0.117 |
| NT-proBNP (ng/L) | 792 ± 1155 |
| Cholesterol | 4.5 ± 1.1 |
| HDL (mmol/L) | 1.22 ± 0.39 |
| LDL-cholesterol (mmol/L) | 2.49 ± 0.80 |
| Cardiovascular function, measured by MRI, median [IQR] | |
| Pulse wave velocity (m/s) | |
| Ejection fraction (%) | 9.8 [8.0–13.7] |
| Cardiac index (L/min/m2) | 61 [51–66] |
| 2.5 [2.1–3.0] |
| Non-Vascular Cause Primary Kidney Disease (n = 25) | Vascular Cause Primary Kidney Disease (n = 42) | p Value | |||
| mean | SD | mean | SD | ||
| ADMA | 0.726 | 0.190 | 0.716 | 0.203 | 0.837 |
| ANG2 | 321.502 | 145.797 | 366.568 | 166.078 | 0.287 |
| miR-126 | 0.950 | 0.277 | 0.962 | 0.293 | 0.866 |
| miR-132 | 0.010 | 0.006 | 0.012 | 0.008 | 0.285 |
| miR-192 | 0.009 | 0.014 | 0.008 | 0.008 | 0.848 |
| miR-223 | 3.867 | 1.859 | 3.764 | 1.077 | 0.802 |
| miR-27a | 0.134 | 0.036 | 0.115 | 0.046 | 0.074 |
| miR-29a | 0.027 | 0.036 | 0.027 | 0.031 | 0.981 |
| miR-326 | 0.005 | 0.003 | 0.008 | 0.008 | 0.148 |
| ESKD without HF (n = 63) | ESKD with HF (n = 4) | p Value | |||
| mean | SD | mean | SD | ||
| ADMA | 0.712 | 0.195 | 0.824 | 0.198 | 0.273 |
| ANG2 | 344.666 | 157.669 | 490.254 | 179.785 | 0.081 |
| miR-126 | 0.952 | 0.286 | 0.995 | 0.292 | 0.772 |
| miR-132 | 0.011 | 0.007 | 0.009 | 0.005 | 0.682 |
| miR-192 | 0.008 | 0.011 | 0.009 | 0.006 | 0.901 |
| miR-223 | 3.790 | 1.365 | 4.278 | 2.163 | 0.505 |
| miR-27a | 0.122 | 0.043 | 0.156 | 0.056 | 0.133 |
| miR-29a | 0.028 | 0.033 | 0.018 | 0.015 | 0.576 |
| miR-326 | 0.006 | 0.006 | 0.012 | 0.015 | 0.532 |
| ESKD without CHD (n = 52) | ESKD with CHD (n = 15) | p Value | |||
| mean | SD | mean | SD | ||
| ADMA | 0.691 | 0.174 | 0.816 | 0.237 | 0.027 * |
| ANG2 | 350.111 | 161.698 | 367.596 | 166.662 | 0.725 |
| miR-126 | 0.947 | 0.302 | 0.980 | 0.218 | 0.692 |
| miR-132 | 0.012 | 0.008 | 0.008 | 0.004 | 0.086 |
| miR-192 | 0.009 | 0.011 | 0.007 | 0.010 | 0.684 |
| miR-223 | 3.644 | 1.412 | 4.424 | 1.246 | 0.058 |
| miR-27a | 0.118 | 0.043 | 0.145 | 0.045 | 0.044 * |
| miR-29a | 0.030 | 0.035 | 0.017 | 0.014 | 0.178 |
| miR-326 | 0.007 | 0.006 | 0.007 | 0.008 | 0.958 |
| ESKD without LVH (n = 59) | ESKD with LVH (n = 8) | p Value | |||
| mean | SD | mean | SD | ||
| ADMA | 0.728 | 0.200 | 0.651 | 0.155 | 0.299 |
| ANG2 | 359.281 | 168.427 | 305.318 | 58.771 | 0.442 |
| miR-126 | 0.935 | 0.273 | 1.098 | 0.340 | 0.128 |
| miR-132 | 0.011 | 0.008 | 0.011 | 0.005 | 0.961 |
| miR-192 | 0.007 | 0.006 | 0.016 | 0.025 | 0.331 |
| miR-223 | 3.858 | 1.425 | 3.525 | 1.303 | 0.534 |
| miR-27a | 0.126 | 0.046 | 0.107 | 0.023 | 0.25 |
| miR-29a | 0.028 | 0.034 | 0.022 | 0.019 | 0.618 |
| miR-326 | 0.007 | 0.007 | 0.004 | 0.002 | 0.27 |
| ESKD without AF (n = 53) | ESKD with AF (n = 14) | p Value | |||
| mean | SD | mean | SD | ||
| ADMA | 0.704 | 0.190 | 0.775 | 0.212 | 0.233 |
| ANG2 | 329.794 | 125.840 | 437.253 | 235.965 | 0.122 |
| miR-126 | 0.959 | 0.293 | 0.937 | 0.254 | 0.797 |
| miR-132 | 0.011 | 0.008 | 0.010 | 0.005 | 0.624 |
| miR-192 | 0.009 | 0.012 | 0.007 | 0.006 | 0.594 |
| miR-223 | 3.681 | 1.265 | 4.339 | 1.807 | 0.121 |
| miR-27a | 0.117 | 0.040 | 0.149 | 0.053 | 0.016 * |
| miR-29a | 0.025 | 0.028 | 0.035 | 0.046 | 0.321 |
| miR-326 | 0.006 | 0.006 | 0.010 | 0.009 | 0.089 # |
| Better Cardiovascular Function | Worse Cardiovascular Function | ||||
|---|---|---|---|---|---|
| Pulse Wave Velocity ≤ 10 m/s (n = 35) | Pulse Wave Velocity > 10 m/s (n = 32) | Correlation | t-Test | ||
| p Value | R | p Value | |||
| ADMA | 0.73 ± 0.20 | 0.787 | 0.034 | 0.547 | |
| Ang-2 | 319.6 ± 123.7 | 390.8 ± 189.4 | 0.000 * | 0.451 | 0.083 # |
| miR-126 | 0.947 ± 0.324 | 0.963 ± 0.237 | 0.849 | −0.013 | 0.818 |
| miR-132 | 0.011 ± 0.008 | 0.011 ± 0.006 | 0.169 | 0.190 | 0.970 |
| miR-192 | 0.009 ± 0.014 | 0.007 ± 0.006 | 0.999 | 0.000 | 0.561 |
| miR-223 | 3.850 ± 1.442 | 3.784 ± 1.388 | 0.217 | −0.149 | 0.849 |
| miR-27a | 0.137 ± 0.051 | 0.110 ± 0.031 | 0.001 * | −0.389 | 0.012 * |
| miR-29a | 0.027 ± 0.032 | 0.027 ± 0.033 | 0.645 | 0.112 | 0.991 |
| miR-326 | 0.008 ± 0.008 | 0.006 ± 0.004 | 0.798 | 0.006 | 0.241 |
| Ejection Fraction ≥ 50% (n = 53) | Ejection Fraction < 50% (n = 14) | ||||
| ADMA | 0.72 ± 0.20 | 0.70 ± 0.16 | 0.530 | 0.076 | 0.721 |
| Ang-2 | 337.3 ± 144.9 | 417.1 ± 208.1 | 0.035 * | −0.250 | 0.114 |
| miR-126 | 0.944 ± 0.294 | 0.996 ± 0.245 | 0.463 | −0.098 | 0.555 |
| miR-132 | 0.011 ± 0.007 | 0.011 ± 0.008 | 0.857 | −0.027 | 0.813 |
| miR-192 | 0.008 ± 0.012 | 0.008 ± 0.005 | 0.941 | −0.047 | 0.946 |
| miR-223 | 3.766 ± 1.406 | 4.036 ± 1.442 | 0.431 | −0.152 | 0.539 |
| miR-27a | 0.125 ± 0.043 | 0.120 ± 0.054 | 0.779 | 0.000 | 0.754 |
| miR-29a | 0.029 ± 0.035 | 0.024± 0.014 | 0.243 | 0.110 | 0.665 |
| miR-326 | 0.006 ± 0.006 | 0.010 ± 0.009 | 0.079 # | −0.216 | 0.061 # |
| Cardiac Index > 2.2 L/min/m2 (n = 45) | Cardiac Index ≤ 2.2 L/min/m2 (n = 21) | ||||
| ADMA | 0.74 ± 0.22 | 0.68 ± 0.14 | 0.269 | 0.143 | 0.257 |
| Ang-2 | 339.2 ± 166.7 | 379.8 ± 153.9 | 0.817 | −0.046 | 0.364 |
| miR-126 | 0.930 ± 0.264 | 1.018 ± 0.322 | 0.479 | −0.091 | 0.246 |
| miR-132 | 0.011 ± 0.007 | 0.011 ± 0.007 | 0.548 | −0.073 | 0.802 |
| miR-192 | 0.009 ± 0.012 | 0.007 ± 0.007 | 0.987 | 0.010 | 0.555 |
| miR-223 | 3.750 ± 1.312 | 4.018 ± 1.621 | 0.960 | 0.024 | 0.476 |
| miR-27a | 0.125 ± 0.043 | 0.121 ± 0.049 | 0.972 | 0.019 | 0.751 |
| miR-29a | 0.026 ± 0.030 | 0.030 ± 0.039 | 0.940 | 0.027 | 0.658 |
| miR-326 | 0.007 ± 0.008 | 0.006 ± 0.004 | 0.931 | −0.009 | 0.301 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, Q.; Nooren, S.J.L.; Zijlstra, L.E.; Westenberg, J.J.M.; Kroft, L.J.M.; Jukema, J.W.; Berkhout-Byrne, N.C.; Rabelink, T.J.; van Zonneveld, A.J.; van Buren, M.; et al. Circulating miRNAs and Vascular Injury Markers Associate with Cardiovascular Function in Older Patients Reaching End-Stage Kidney Disease. Non-Coding RNA 2022, 8, 2. https://doi.org/10.3390/ncrna8010002
Zhao Q, Nooren SJL, Zijlstra LE, Westenberg JJM, Kroft LJM, Jukema JW, Berkhout-Byrne NC, Rabelink TJ, van Zonneveld AJ, van Buren M, et al. Circulating miRNAs and Vascular Injury Markers Associate with Cardiovascular Function in Older Patients Reaching End-Stage Kidney Disease. Non-Coding RNA. 2022; 8(1):2. https://doi.org/10.3390/ncrna8010002
Chicago/Turabian StyleZhao, Qiao, Sabine J. L. Nooren, Laurien E. Zijlstra, Jos J. M. Westenberg, Lucia J. M. Kroft, J. Wouter Jukema, Noeleen C. Berkhout-Byrne, Ton J. Rabelink, Anton Jan van Zonneveld, Marjolijn van Buren, and et al. 2022. "Circulating miRNAs and Vascular Injury Markers Associate with Cardiovascular Function in Older Patients Reaching End-Stage Kidney Disease" Non-Coding RNA 8, no. 1: 2. https://doi.org/10.3390/ncrna8010002
APA StyleZhao, Q., Nooren, S. J. L., Zijlstra, L. E., Westenberg, J. J. M., Kroft, L. J. M., Jukema, J. W., Berkhout-Byrne, N. C., Rabelink, T. J., van Zonneveld, A. J., van Buren, M., Mooijaart, S. P., & Bijkerk, R. (2022). Circulating miRNAs and Vascular Injury Markers Associate with Cardiovascular Function in Older Patients Reaching End-Stage Kidney Disease. Non-Coding RNA, 8(1), 2. https://doi.org/10.3390/ncrna8010002







