- Article
Uncertainty of Blood Alcohol Concentration (BAC) Results as Related to Instrumental Conditions: Optimization and Robustness of BAC Analysis Headspace Parameters
- Haleigh A. Boswell and
- Frank L. Dorman
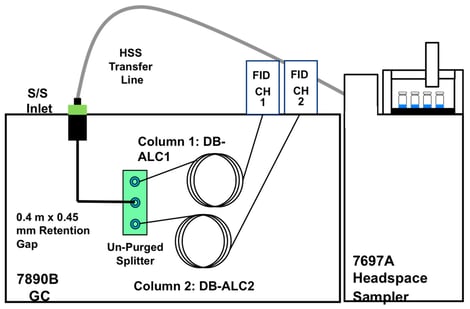
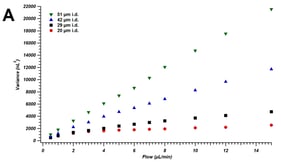
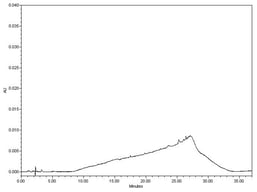
Analysis of blood alcohol concentration is a routine analysis performed in many forensic laboratories. This analysis commonly utilizes static headspace sampling, followed by gas chromatography combined with flame ionization detection (GC-FID). Studies have shown several “optimal” methods for instrumental operating conditions, which are intended to yield accurate and precise data. Given that different instruments, sampling methods, application specific columns and parameters are often utilized, it is much less common to find information on the robustness of these reported conditions. A major problem can arise when these “optimal” conditions may not also be robust, thus producing data with higher than desired uncertainty or potentially inaccurate results. The goal of this research was to incorporate the principles of quality by design (QBD) in the adjustment and determination of BAC (blood alcohol concentration) instrumental headspace parameters, thereby ensuring that minor instrumental variations, which occur as a matter of normal work, do not appreciably affect the final results of this analysis. This study discusses both the QBD principles as well as the results of the experiments, which allow for determination of more favorable instrumental headspace conditions. Additionally, method detection limits will also be reported in order to determine a reporting threshold and the degree of uncertainty at the common threshold value of 0.08 g/dL. Furthermore, the comparison of two internal standards, n-propanol and t-butanol, will be investigated. The study showed that an altered parameter of 85 °C headspace oven temperature and 15 psi headspace vial pressurization produces the lowest percent relative standard deviation of 1.3% when t-butanol is implemented as an internal standard, at least for one very common platform. The study also showed that an altered parameter of 100 °C headspace oven temperature and 15-psi headspace vial pressurization produces the lowest MDL of 0.00002 g/dL when n-propanol is implemented as an internal standard. These altered headspace parameters have the potential to produce more precise and accurate BAC determination.
11 December 2015