Abstract
This article details the elements used in the method verification for the simultaneous high performance liquid chromatography (HPLC) assay of Pentoxifylline, Mupirocin, Itraconazole, and Fluticasone Propionate in Humco™ Lavare Wound base. The method was proven to be linear over 50%–150% of the nominal concentration of the standards. The method was proven to be accurate over 50%–150%, with 98%–102% recovery of the actives from spiked placeboes over that range. The method was shown to be specific to the analytes listed and precise, yielding acceptable results for system reproducibility and method repeatability. The method, as written, is considered to have been verified.
1. Introduction
Compounded formulations in water-based creams containing Pentoxifylline, Mupirocin, Gentamicin Sulfate, Itraconazole, and Fluticasone Propionate are applied to wounds and abrasions to aid healing. These particular active formulations facilitate healing by containing anti-inflammatory drugs (Pentoxifylline [1] and Fluticasone Propionate [2]) along with anti-infectives (Mupirocin [3,4], Gentamicin Sulfate [5], and Itraconazole [6]) which prevent bacterial and fungal infections from occurring at the wound site. Gentamicin Sulfate is not analyzed using this method because it does not contain a UV active chromophore.
Humco™ Lavare wound base is an occlusive aqueous compounding base, containing polyethylene glycol [7] which aids in the solubilizing of actives that are added to it. Additionally, Lavare contains anti-inflammatory agents and meadowsweet extract, a natural anti-infective that encourages healing of sensitive wound tissue. Humco™ Lavare wound base has a smooth moisturizing texture and is appropriate for application of compounded medications to tender areas such as burns, ulcers, abrasions, and other dermal injuries.
This report details the method verification requirements and corresponding acceptance criteria for the analytical method used to assay Pentoxifylline, Mupirocin, Itraconazole, and Fluticasone Propionate compounded in Humco™ Lavare wound base. Following verification, the method is suitable for analyzing samples compounded in-house and samples received from other pharmacies for analytical testing [8,9,10]. The ingredients in the formulation are listed in Table 1.

Table 1.
Compounded formulation in Humco™ Lavare wound base.
| Ingredient | %w/w in formulation | Ingredient | %w/w in formulation |
|---|---|---|---|
| Pentoxifylline | 5% | Gentamicin Sulfate | 0.2% |
| Mupirocin | 5% | Fluticasone Propionate | 1% |
| Itraconazole | 3.75% | Humco™ Lavare Wound Base | Quantity sufficient (q.s.) |
2. Experimental Section
A reverse phase high performance liquid chromatography (HPLC) method was developed which uses a pH-buffered phosphate solution and acetonitrile to create a gradient to separate the components contained in the formulation. The details of the method, including HPLC instrument conditions, mobile phase preparation, and preparation of standards and samples are given. The method verification elements and acceptance criteria are also given.
2.1. Materials and Methods
2.1.1. Chromatographic Conditions
| Column: | Phenomenex® Gemini 150 × 4.6 mm C18 5µm Part # 00F-4435-E0 or equivalent (Torrance, CA, USA) |
| Guard column: | Phenomenex® SecurityGuard C18 Guard Column Part # KJ0-4282 |
| Column temperature: | 25 °C |
| Mobile Phase A: | 0.025 M Sodium phosphate dibasic, pH 3.0 with o-phosphoric acid |
| Mobile Phase B: | Acetonitrile |
| Gradient profile: | See Table 2 |

Table 2.
Mobile phase gradient profile.
| Time (min) | %A | %B |
|---|---|---|
| 0 | 90 | 10 |
| 5 | 90 | 10 |
| 25 | 20 | 80 |
| 30 | 90 | 10 |
| 35 | 90 | 10 |
| Flow rate: | 1.0 mL/min |
| Injection volume: | 10 µL |
| Wavelength: | 220 nm |
| Seal/needle rinse: | 50/50 Acetonitrile/water |
| Run time: | 37 min |
| Typical retention times: | See Table 3 |

Table 3.
Typical retention times of actives.
| Active | Approximate retention time (min) | Active | Approximate retention time (min) |
|---|---|---|---|
| Pentoxifylline | 12.2–12.9 | Fluticasone Propionate | 23.3–24.3 |
| Mupirocin | 17.0–18.0 | Itraconazole | 25.0–25.8 |
2.1.2. Materials and Equipment
| Pentoxifylline: | United States Pharmacopeia or reference standard grade |
| Fluticasone Propionate: | United States Pharmacopeia or reference standard grade |
| Mupirocin: | United States Pharmacopeia or reference standard grade |
| Itraconazole: | United States Pharmacopeia or reference standard grade |
| Sodium phosphate dibasic: | Reagent grade |
| HPLC grade water: | HPLC grade |
| Acetonitrile: | HPLC grade |
| o-Phosphoric acid, 85%: | HPLC grade |
| Tetrahydrofuran: | HPLC grade |
| Humco™ Lavare wound base: | In-house supply |
| Syringe filter: | Thermo target 2 0.2 µm 30 mm Nylon media Syringe Filter Part #F2500-2 or equivalent |
2.1.3. Mobile Phase A Preparation
A 1000 mL portion of purified water and 3.0 g sodium phosphate dibasic were combined and mixed well. The pH of the solution was adjusted to 3.0 ± 0.1 with o-phosphoric acid 85%.
2.1.4. Diluent (50/50 Water/Acetonitrile)
A 500 mL portion of HPLC grade water and 500 mL of acetonitrile were combined and mixed well. Volumes were scaled as necessary.
2.1.5. Standard Preparation
For the Stock Standard, actives were accurately weighed, to the nearest 0.1 mg. A quantity of 100 mg of Pentoxifylline, 100 mg of Mupirocin, 20 mg of Fluticasone Propionate, and 75 mg of Itraconazole was weighed and transferred into a 100 mL volumetric flask. A 10 mL volume of tetrahydrofuran (THF) was added to the flask followed by 25 mL of acetonitrile. The contents were sonicated about 2 min until all components dissolved. The flask was diluted to volume with diluent. This was the Stock Standard solution.
For the Working Standard Solution, an aliquot of 10 mL of the Stock Standard solution was pipetted into a 50 mL volumetric flask. This solution was diluted to volume with diluent. This was the Working Standard solution.
2.1.6. Sample Preparation
For the Stock Sample solution, about 2 g of the sample was weighed into a 100 mL volumetric flask. About 10 mL of THF was added to the flask followed by 25 mL of acetonitrile, and the sample was allowed to fully disperse with sonication. The flask was then diluted to volume with diluent. This was the Stock Sample solution.
For the Working Sample solution a 10 mL aliquot of the Stock Sample solution was pipetted into a 50 mL volumetric flask and diluted to volume with diluent. This was the Working Sample solution. Approximately 3 mL of the sample was filtered using a 0.2 µm Nylon syringe filter into an appropriate HPLC vial for analysis.
2.2. Method Verification Elements
The following analytical method verification sections detail the documentation required to verify the performance characteristics of the procedure and ensure that it meets the requirements for the intended analytical applications. The acceptance criteria was the successful completion of each section. The verification included specificity, linearity, accuracy, and precision (system precision or reproducibility and method precision or repeatability), and range [8,9,10].
2.2.1. Specificity
The specificity is the ability to assess unequivocally the analyte of interest in the presence of components that may be expected to be present, such as matrix components (preservatives or placebo peaks) or peaks in the blank. The Blank preparation and the Placebo preparation (Lavare wound base) were examined to ensure that no interference occurred at the retention time of any of the actives in the chromatograms.
2.2.2. Linearity
The linearity of an analytical procedure is its ability to elicit test results that are directly proportional to the concentration of the analyte in samples over a specified range. The analytical method was shown to be linear over the range of 50%–150% of the nominal standard concentration, with the plot of concentration vs. analyte peak area for each analyte having a correlation coefficient (r2) of ≥0.99.
Limit of quantitation (LOQ) and limit of detection (LOD) for each of the four actives was determined by successive dilution of the Working Standard solution and applying the signal-to-noise ratio test to the resulting chromatograms. LOQ is the concentration at which the signal to noise ratio was about 10:1, while LOD is the concentration at which the signal to noise ratio was about 3:1.
2.2.3. Accuracy
The accuracy of an analytical procedure is the closeness of test results obtained by that procedure to the true value. The accuracy of this method was verified by determining the recovery of a known amount of each analyte added to the sample matrix (a Spiked Placebo). The % recovery of each analyte from the placebo spiked at 50%–150% of the nominal standard concentration was determined to be 98%–102%. Additionally, the % RSD among sets of samples at each concentration was shown to be ≤2%.
2.2.4. Precision
The precision of an analytical procedure is the degree of agreement among individual test results when the procedure is applied repeatedly to multiple samplings of a homogeneous sample. This is further broken down into system precision and method precision.
System Precision (Reproducibility)
The system precision or reproducibility evaluated the ability of the method to analyze a single preparation by injecting the sample six times. The peak area % RSD of each analyte among the six injections was ≤2%.
Method Precision (Repeatability)
The method precision or repeatability evaluated the ability of the method to analyze multiple preparations of sample. This was determined by assaying three individual preparations injected in triplicate. The peak area % RSD of each analyte for the three individual preparations must not be more than 2%.
2.2.5. Range
The range for an analytical procedure is established over the concentrations of the analytes where acceptable precision, accuracy, and linearity has been demonstrated. The range of the analytical method was established by examining the precision, accuracy, and linearity studies.
3. Results and Discussion
3.1. System Suitability
System suitability of the method was proven using the parameters that are used by compendia to prove that the data generated is valid. The relative standard deviation of the peak area responses of Pentoxifylline, Mupirocin, Itraconazole, and Fluticasone Propionate for the first five consecutive injections and for all injections of the Working Standard solution was ≤2%. The relative standard deviation of the retention times of Pentoxifylline, Mupirocin, Itraconazole, and Fluticasone Propionate for all Working Standard injections was ≤2%. Theoretical plates for Pentoxifylline, Mupirocin, Itraconazole, and Fluticasone Propionate in the Working Standard solution were ≥2000. The tailing factor for Pentoxifylline, Mupirocin, Itraconazole, and Fluticasone Propionate in the Working Standard solution was ≤2.0. The resolution between each of the components of Pentoxifylline, Mupirocin, Itraconazole, and Fluticasone Propionate in the Working Standard solution was >1.5. No interference in the blank or placebo preparation (≥0.3%) was observed at the retention time of Pentoxifylline, Mupirocin, Itraconazole, and Fluticasone Propionate.
The system suitability met all acceptance criteria, therefore, the system was suitable to analyze the samples for further method verification elements.
Table 4 gives the System Suitability results and specifications obtained during the method verification.

Table 4.
System Suitability of the HPLC: n/a = not applicable.
| Active | Peak area % RSD (n = 6) | Peak area % RSD (overall) | Average overall retention time (min) | Retention time % RSD (overall) | Average theoretical plates | Average tailing | Average resolution |
|---|---|---|---|---|---|---|---|
| Pentoxifylline | 1% | 1% | 12.5 | 0.3 | 197,467 | 1.1 | n/a |
| Mupirocin | 1% | 1% | 17.6 | 0.2 | 334,115 | 1.1 | 3.9 |
| Fluticasone Propionate | 1% | 1% | 23.7 | 0.1 | 415,238 | 1.1 | 22.2 |
| Itraconazole | 1% | 1% | 25.4 | 0.1 | 348,768 | 1.1 | 10.2 |
| Specification | ≤2% | ≤2% | n/a | ≤2% | ≥2000 | ≤2.0 | >1.5 |
3.2. Specificity Results
3.2.1. Examining the Blank
The sample blank was assayed to verify that there are no significant peaks with similar retention times as Pentoxifylline, Mupirocin, Fluticasone Propionate, or Itraconazole. The Blank chromatogram exhibits no peaks (other than a small solvent front peak) beyond normal noise. There is a baseline ramp up to 27 min that is present, but this is a function of the gradient elution and not a true peak. No significant peaks (≥0.3% of the analytes of interest), beyond the noise level were noted in the sample blank near the retention times of the analytes of interest.
3.2.2. Examining the Sample Matrix (Placebo)
The sample matrix without the active ingredient (also known as a placebo)—Humco™ Lavare wound base in this case—was assayed to verify that there are no significant peaks with similar retention times as Pentoxifylline, Mupirocin, Fluticasone Propionate, or Itraconazole. There was an identified peak (Placebo 1) in the Placebo chromatogram, representing components of the botanical extract contained in the product. This peak does not interfere with analysis of the actives as it does not occur at the actives’ retention times. As such, no significant peaks (≥0.3% of the analytes of interest) were noted in the Placebo sample matrix near the retention time of the analytes of interest. Unidentified small peaks in the Standard and Sample chromatograms are less than 0.3% of the peak area of the Fluticasone Propionate peak and are process impurities of Mupirocin that are present in the raw material.
Blank, Placebo, Working Standard, and Sample chromatograms are given in Figure 1, Figure 2, Figure 3 and Figure 4.
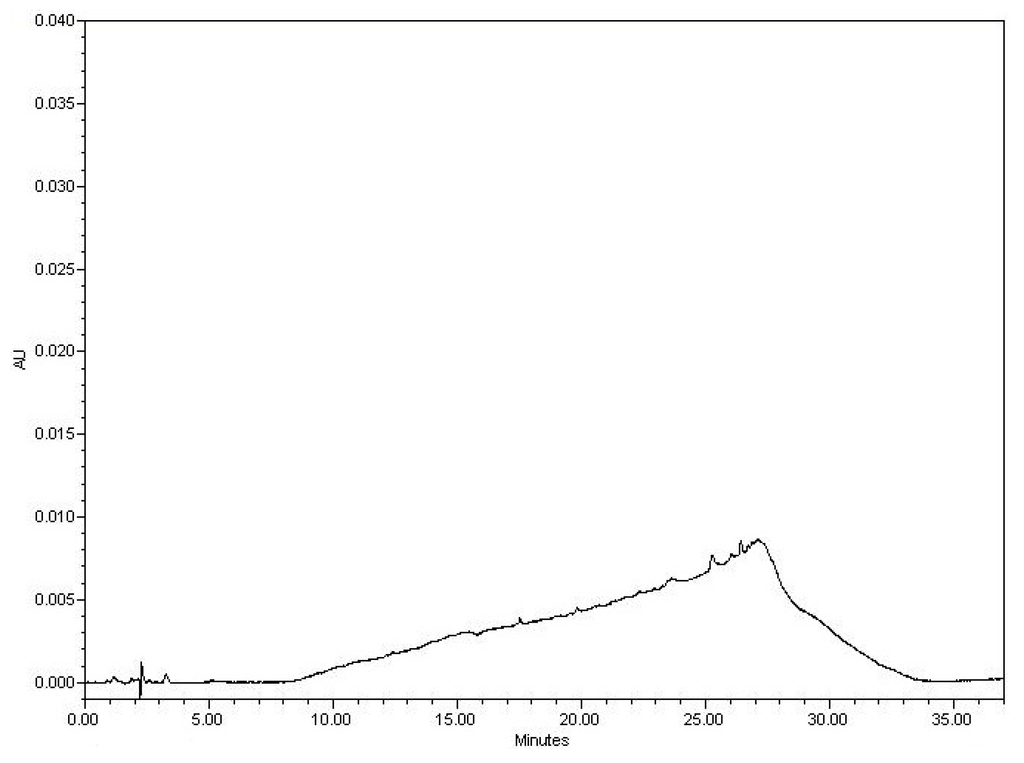
Figure 1.
Blank chromatogram.
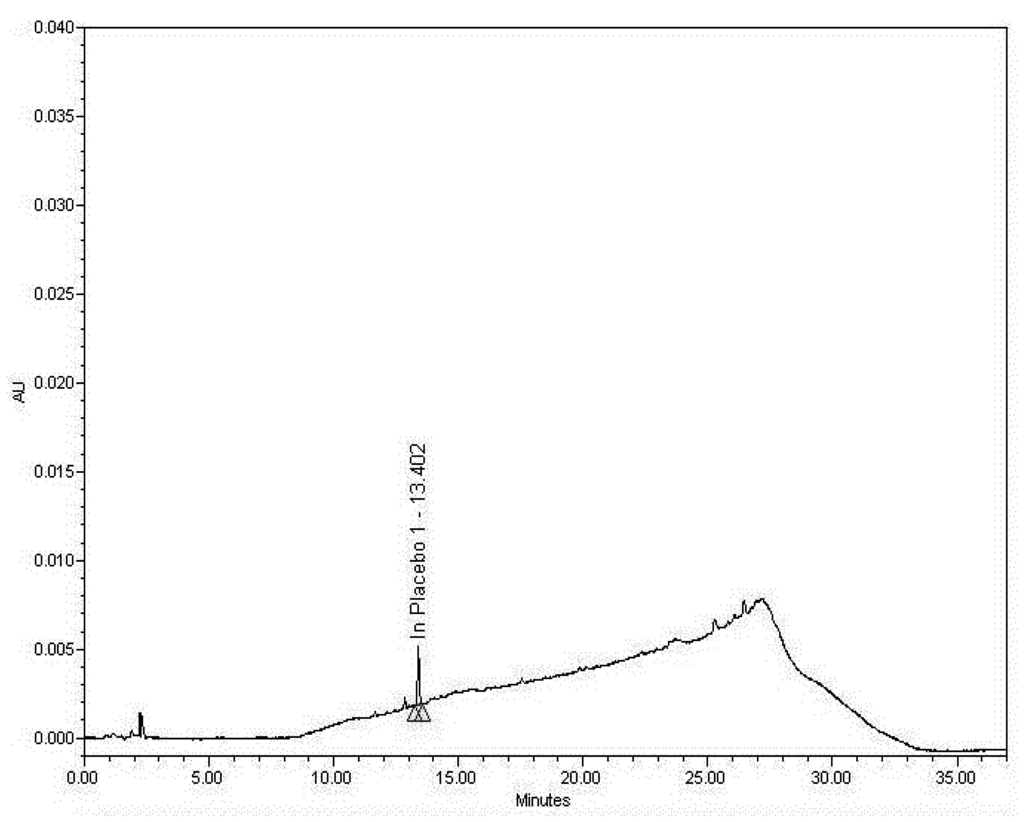
Figure 2.
Placebo (Humco™ Lavare wound base) chromatogram.
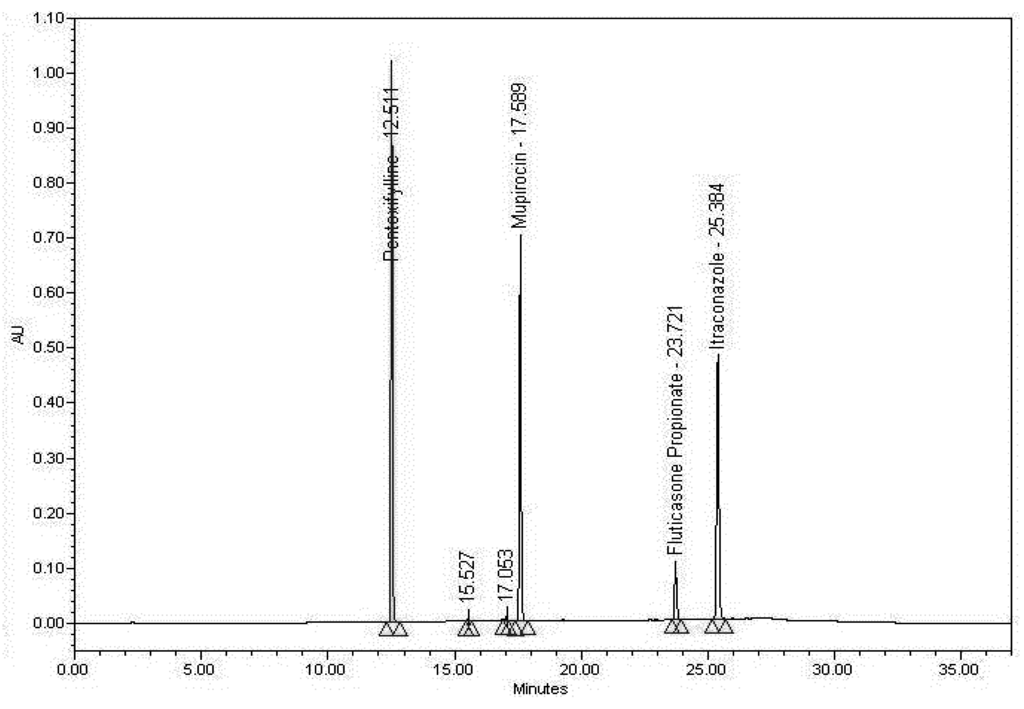
Figure 3.
Working Standard solution chromatogram.
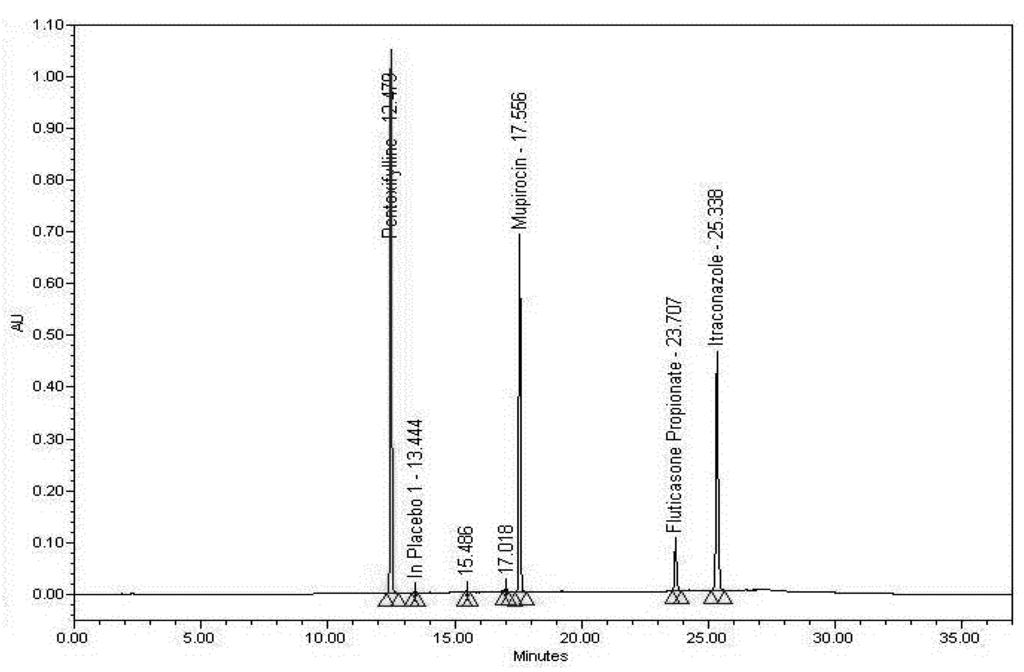
Figure 4.
Sample preparation chromatogram.
3.2.3. Specificity Discussion
There was no interference in the blank or Placebo (Lavare wound base) chromatograms at the retention times of Pentoxifylline, Mupirocin, Fluticasone Propionate, or Itraconazole. Therefore, the acceptance criteria for Specificity of Pentoxifylline, Mupirocin, Fluticasone Propionate, and Itraconazole are met.
3.3. Linearity Results
Linearity for Pentoxifylline, Mupirocin, Itraconazole, and Fluticasone Propionate was conducted over a range of 50%–150% of the nominal analytes in the prepared sample concentration. Five concentrations were tested within the range of 50%–150%.
3.3.1. Experimental
The Stock Standard solution of Pentoxifylline, Mupirocin, Itraconazole, and Fluticasone Propionate was prepared. The linearity was accomplished by making dilutions from the Stock Standard solution. For example, for the 50% level, 5 mL of the Stock Standard was diluted in a 50 mL volumetric flask; for the 80% level, 8 mL of the Stock Standard was diluted in a 50 mL volumetric flask; for the 100% level, 10 mL of the Stock Standard was diluted in a 50 mL volumetric flask; for the 120% level, 12 mL of the Stock Standard was diluted in a 50 mL volumetric flask; and for the 150% level, 15 mL of the Stock Standard was diluted in a 50 mL volumetric flask. Serial dilutions of the Working Standard were made to determine the approximate LOQ and LOD of the four actives.
Table 5 gives the Stock Standard weights for each compound and the concentrations (mg/mL) for each of the linearity levels.

Table 5.
Concentrations of each active used in the linearity and accuracy evaluations.
| Compound | Standard weight (mg) | 50% Conc. (mg/mL) | 80% Conc. (mg/mL) | 100% Conc. (mg/mL) | 120% Conc. (mg/mL) | 150% Conc. (mg/mL) |
|---|---|---|---|---|---|---|
| Pentoxifylline | 100.7 | 0.1007 | 0.1611 | 0.2014 | 0.2417 | 0.3021 |
| Mupirocin | 100.4 | 0.1004 | 0.1606 | 0.2008 | 0.2410 | 0.3012 |
| Fluticasone Propionate | 21.9 | 0.0219 | 0.0350 | 0.0438 | 0.0526 | 0.0657 |
| Itraconazole | 75.6 | 0.0756 | 0.1210 | 0.1512 | 0.1814 | 0.2268 |
3.3.2. Linearity of Actives
3.3.3. Linearity Discussion
The correlation coefficient (r2) from the plotted area response versus concentration for Pentoxifylline is 0.9991 which is ≥0.99, for Mupirocin is 0.9996 which is ≥0.99, for Fluticasone Propionate is 0.9997 which is ≥0.99, and for Itraconazole is 0.9996 which is ≥0.99. The data used for calculation of the linearity is represented in Table 6.
The average percent recovery for Pentoxifylline is 98.5%–100.9% which is 98%–102%, for Mupirocin is 98.9%–100.6% which is 98%–102%, for Fluticasone Propionate is 99.6%–100.7% which is 98%–102%, and for Itraconazole is 99.0%–100.5% which is 98%–102% of the amount prepared for the 50%–150% level.
All acceptance criteria for the linearity of Pentoxifylline, Mupirocin, Fluticasone Propionate, and Itraconazole are met.

Table 6.
The linearity level, theoretical and actual concentrations, and % recovery as well as the r2-value (correlation coefficient) for each active.
| Active | Linearity % | Theoretical conc. (mg/mL) | Actual conc. (mg/mL) | % Recovery | r2 |
|---|---|---|---|---|---|
| Pentoxifylline | 50 | 0.1007 | 0.0992 | 98.5% | 0.9991 |
| 80 | 0.1611 | 0.1613 | 100.1% | ||
| 100 | 0.2014 | 0.2030 | 100.8% | ||
| 120 | 0.2417 | 0.2438 | 100.9% | ||
| 150 | 0.3021 | 0.2998 | 99.2% | ||
| Mupirocin | 50 | 0.1004 | 0.0993 | 98.9% | 0.9996 |
| 80 | 0.1606 | 0.1616 | 100.6% | ||
| 100 | 0.2008 | 0.2014 | 100.3% | ||
| 120 | 0.2410 | 0.2415 | 100.2% | ||
| 150 | 0.3012 | 0.3003 | 99.7% | ||
| Fluticasone Propionate | 50 | 0.0219 | 0.0218 | 99.6% | 0.9997 |
| 80 | 0.0350 | 0.0353 | 100.7% | ||
| 100 | 0.0438 | 0.0437 | 99.9% | ||
| 120 | 0.0526 | 0.0524 | 99.7% | ||
| 150 | 0.0657 | 0.0658 | 100.1% | ||
| Itraconazole | 50 | 0.0756 | 0.0748 | 99.0% | 0.9996 |
| 80 | 0.1210 | 0.1215 | 100.5% | ||
| 100 | 0.1512 | 0.1517 | 100.3% | ||
| 120 | 0.1814 | 0.1819 | 100.3% | ||
| 150 | 0.2268 | 0.2260 | 99.7% |

Table 7.
Limit of quantitation (LOQ) and limit of detection (LOD) concentrations for each active (determined by S/N ratio).
| Active | LOQ (mg/mL) | LOD (mg/mL) |
|---|---|---|
| Pentoxifylline | 0.00043 | 0.00018 |
| Mupirocin | 0.00040 | 0.00012 |
| Fluticasone Propionate | 0.00088 | 0.00029 |
| Itraconazole | 0.00030 | 0.00011 |
3.4. Accuracy Results
The accuracy of the method was proven by using spiked placebo solutions that were prepared by spiking in the appropriate amount of the analytes of interest into the sample matrix and assayed using a standard. The spiked placebo preparation spiked with each of the analytes of interest (Pentoxifylline, Mupirocin, Fluticasone Propionate, and Itraconazole) over a range of 50%–150% of the nominal standard concentration. These solutions were assayed, and the data was compared with the amount prepared versus the amount recovered.
3.4.1. Accuracy of Actives
3.4.2. Accuracy Discussion
The recovery for the Pentoxifylline, Mupirocin, Fluticasone Propionate, and Itraconazole was within the acceptance criteria of 98%–102%. The % RSD among the accuracy preparations was ≤2% RSD, meeting the acceptance criteria.
The accuracy of Pentoxifylline, Mupirocin, Fluticasone Propionate, and Itraconazole meets the acceptance criteria.
3.5. Precision Results
The system precision (reproducibility) and method precision (repeatability) were evaluated using preparations of the spiked placebo. The system precision evaluated the ability of the method to analyze a single sample preparation by injecting the sample six times. The method precision evaluated the ability of the method to analyze multiple preparations of sample. This was determined by assaying three individual preparations.

Table 8.
Accuracy level, theoretical and actual concentrations of each active, % recovery of each active, and the % RSD of triplicate injections at each accuracy level for each active.
| Active | Accuracy% | Theoretical conc. (mg/mL) | Actual conc. (mg/mL) | % recovery | % RSD |
|---|---|---|---|---|---|
| Pentoxifylline | 50 | 0.1007 | 0.0989 | 98.2% | 0% |
| 80 | 0.1611 | 0.1615 | 100.3% | 1% | |
| 100 | 0.2041 | 0.2034 | 99.7% | 1% | |
| 120 | 0.2417 | 0.2434 | 100.7% | 1% | |
| 150 | 0.3021 | 0.2998 | 99.2% | 1% | |
| Mupirocin | 50 | 0.1004 | 0.0997 | 99.3% | 0% |
| 80 | 0.1606 | 0.1614 | 100.4% | 0% | |
| 100 | 0.2008 | 0.2008 | 100.0% | 1% | |
| 120 | 0.2410 | 0.2418 | 100.3% | 1% | |
| 150 | 0.3012 | 0.3004 | 99.7% | 1% | |
| Fluticasone Propionate | 50 | 0.0219 | 0.0216 | 98.4% | 0% |
| 80 | 0.0350 | 0.0354 | 100.9% | 2% | |
| 100 | 0.0438 | 0.0440 | 100.5% | 2% | |
| 120 | 0.0526 | 0.0526 | 100.1% | 1% | |
| 150 | 0.0657 | 0.0654 | 99.6% | 1% | |
| Itraconazole | 50 | 0.0756 | 0.0749 | 99.1% | 0% |
| 80 | 0.1210 | 0.1211 | 100.2% | 1% | |
| 100 | 0.1512 | 0.1517 | 100.4% | 1% | |
| 120 | 0.1814 | 0.1825 | 100.6% | 1% | |
| 150 | 0.2268 | 0.2258 | 99.5% | 1% |
3.5.1. System Precision Results
System precision (reproducibility) results are given in Table 9.

Table 9.
System precision results (% RSD) for 6 injections of a single sample preparation.
| Active | Peak Area % RSD; (n = 6 injections) |
|---|---|
| Pentoxifylline | 1% |
| Mupirocin | 1% |
| Fluticasone Propionate | 1% |
| Itraconazole | 1% |
3.5.2. System Precision Discussion
The %RSD for the Pentoxifylline, Mupirocin, Fluticasone Propionate, and Itraconazole peak areas for the six replicate injections is ≤2%. The system precision acceptance criteria are met.
3.5.3. Method Precision Results
Method Precision (Repeatability) results are given in Table 10.

Table 10.
Method precision results (% RSD) for 3 individual preparations of a sample.
| Active | Peak area % RSD; (n = 3 preparations) |
|---|---|
| Pentoxifylline | 1% |
| Mupirocin | 1% |
| Fluticasone Propionate | 1% |
| Itraconazole | 1% |
3.5.4. Method Precision Discussion
The %RSD for the Pentoxifylline, Mupirocin, Fluticasone Propionate, and Itraconazole peak areas for the three preparations is ≤2%. The method precision acceptance criteria are met.
3.6. Range Results and Discussion
The results of the precision, accuracy, and linearity each pass the respective specifications over the 50%–150% nominal range for each analyte specified in this study. The range for the method is concluded to be 50%–150% the nominal standard concentration of each analyte in the study.
4. Conclusions
The method verification elements of linearity, accuracy, specificity, precision, and range [8,9,10] met each of the respective elements’ acceptance criteria, therefore, the analytical method is considered to be verified for its intended purposes as defined previously.
Acknowledgments
The author wishes to thank the Humco Holding Group Board of Directors for approving funding for the work performed in this study.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Trental, Pentoxil (pentoxifylline) dosing, indications, interactions, and adverse effects. Available online: http://www.webmd.com/drugs/2/drug-3696/trental-oral/details (accessed on 13 September 2015).
- National Institutes of Health: Fluticasone Propionate Nasal Spray. Available online: http://www.nlm.nih.gov/medlineplus/druginfo/meds/a695002.html (accessed on 3 November 2015).
- Fuller, A.T.; Mellows, G.; Woolford, M.; Banks, G.T.; Barrow, K.D.; Chain, E.B. Pseudomonic acid: An antibiotic produced by Pseudomonas fluorescens. Nature 1911, 234, 416–417. [Google Scholar] [CrossRef]
- Hughes, J.; Mellows, G. Inhibition of isoleucyl-transfer ribonucleic acid synthetase in Echerichia coli by pseudomonic acid. Biochem. J. 1978, 176, 305–318. [Google Scholar] [CrossRef] [PubMed]
- Weinstein, M.J.; Luedemann, G.M.; Oden, E.M.; Wagman, G.H.; Rosselet, J.P.; Marquez, J.A.; Coniglio, C.T.; Charney, W.; Herzog, H.L.; Black, J. Gentamicin, 1a New Antimicrobial Complex from Micromonospora. J. Med. Chem. 1963, 6, 463–464. [Google Scholar] [CrossRef] [PubMed]
- Janssen Pharmaceuticals Sporanox (Itraconazole Capsules) Package Insert. Available online: http://www.janssen.com/us/sites/www_janssen_com_usa/files/products-documents/039808-150915_sporanox_pi_code_update_only_12.pdf (accessed on 15 August 2015).
- Remington, J.P.; Allen, L.V. Remington: The Science and Practice of Pharmacy; Pharmaceutical Press: London, UK, 2013. [Google Scholar]
- <1225> Validation of Compendial Procedures; United States Pharmacopoeia. Available online: http://www.ofnisystems.com/wp-content/uploads/2013/12/USP36_1225.pdf (accessed on 15 August 2015).
- Validation of Analytical Procedures: Text and Methodology. Available online: http://www.ich.org/products/guidelines/quality/quality-single/article/validation-of-analytical-procedures-text-and-methodology.html (accessed on 1 August 2015).
- Guidance for Industry: Analytical Procedures and Methods Validation for Drugs and Biologics; Food and Drug Administration Center for Drug Evaluation Research: Silver Spring, MD, USA, 2015.
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
