Abstract
The use of a novel micro pressurized liquid extraction (µPLE) method for the isolation of 16 priority pollutant polycyclic aromatic hydrocarbons (PAHs) from various solid samples is explored. The technique employs rapid heating in a single static extraction mode to remove analytes in a matter of seconds from 5–10 mg samples using only 125 µL of solvent. For example, results show that 30 s extractions with toluene at 200 °C produce respective PAH recovery ranges of 90%–130% and 88%–114% from samples of soil and smoked chicken. Comparatively, solids containing significant amounts of biochar were more challenging to extract from. For instance, when using a pure biochar sample matrix, recoveries for the 16 PAHs range from only 33%–66% after 60 s of extraction with toluene at 200 °C. Overall, these extraction results agree very well with those reported when using conventional methods on similar samples. Therefore, the findings indicate that µPLE can potentially provide an alternative sample preparation method for PAHs that is both very rapid and requires little solvent.
1. Introduction
Sample preparation can often be a very laborious and costly task that inhibits the speed and ease of modern chemical analysis. This is especially true for liquid-solid extractions. For example, Soxhlet extraction is an effective tool that remains a common approach for solid sample preparation despite its relatively long procedures and large solvent requirements [1,2,3,4]. Accordingly, innovations in this area are on-going in order to improve the analysis of solids in general [1,2]. One of the most beneficial technologies that has emerged to address this issue is pressurized liquid extraction (PLE). PLE is a technique that uses solvents at elevated pressures and temperatures to extract analytes from solid matrices. As a result, PLE extractions are normally faster and use less solvent than Soxhlet methods, but still often provide similar extraction efficiency [3,5]. Even so, the typical demands of a routine high sample throughput setting can still be better addressed with further reductions in the time and solvent required in PLE.
The miniaturization of analytical systems is one potential approach that may help to overcome such challenges [6]. For example, a few miniaturized PLE approaches have been described, where modifications were made to accommodate smaller sample sizes and employ lower solvent volumes in the process [7,8,9]. While beneficial in this regard, however, such methods still produced extraction times that were very similar to conventional PLE. Further, these techniques normally employ conventional instrumentation aspects such as pumping and valves that can make them more complex to maintain/operate and more difficult to widely implement. Thus, while miniaturization seems a potentially useful approach to explore in this area, further advances that can help address these issues are still of great benefit to investigate.
We have recently introduced a new micro pressurized liquid extraction (μPLE) method that is able to efficiently extract analytes from target matrices in a single rapid static extraction step [10]. Specifically, the method uses reduced sample sizes (5–10 mg) and solvent volumes (125 µL) to prepare solid samples for analysis in a matter of seconds. Further, to date the technique has been applied to the extraction of target analytes from various samples such as pharmaceutical tablets, green tea leaves and dried blood spots [10].
Polycyclic aromatic hydrocarbons (PAHs) are an important class of analytes that have been studied in a variety of matrices. PAHs are ubiquitous carcinogenic pollutants whose toxicity arises from their metabolism in the body to reactive compounds that form adducts to DNA [11]. They essentially enter the environment through incomplete combustion of organic material [11], and their sources may be natural or anthropogenic. For instance, fossil fuel combustion is a major source of environmental PAH contamination, while smaller scale activities like grilling/smoking food can also contribute to human exposure [12]. Accordingly, the bioaccumulation of PAHs in food from environmental contamination is also a major concern [13]. In particular, since they are highly hydrophobic and adhere strongly to carbonaceous materials [14], this has prompted close monitoring and regulation of PAH distribution [11,15].
Many studies concerning the extraction of PAHs from diverse matrices have been conducted using conventional methods, such as Soxhlet [16,17,18], ultrasound-assisted solvent extraction [19,20], PLE [21,22,23], and others [24,25,26]. Since these methods can greatly benefit from further improvements in the time and solvent they require, it would therefore be interesting to examine if μPLE could facilitate the preparation of such solid samples for analysis. Here, we report the use of μPLE for the extraction of 16 US EPA priority pollutant PAHs from various relevant solid matrices. These samples include foodstuffs, soil, and biochar-containing solids. Comparisons with results reported for conventional methods on similar samples are also made where possible to help place the findings in context.
2. Experimental Section
2.1. μPLE Apparatus
The μPLE system has been described previously [10]. A schematic of the apparatus and experimental procedure is shown in Figure 1. Briefly, stainless steel extraction vessels measuring 55 mm long × 2.1 mm i.d. × 2.9 mm o.d. were employed. The resistively heated extraction apparatus is composed of an open-ended glass tube that acts as an extraction vessel holder. Nickel-chromium wire is coiled around the entire holder with approximately 1 mm spacing between each wind, and is resistively heated by a variable transformer (120 V supply, 10 A max; model 3PN 1010, Staco, Dayton, USA) to control extraction temperatures. The housing exterior is secured and insulated by a thin layer of ceramic adhesive (Cotronics Corp, Brooklyn, USA).
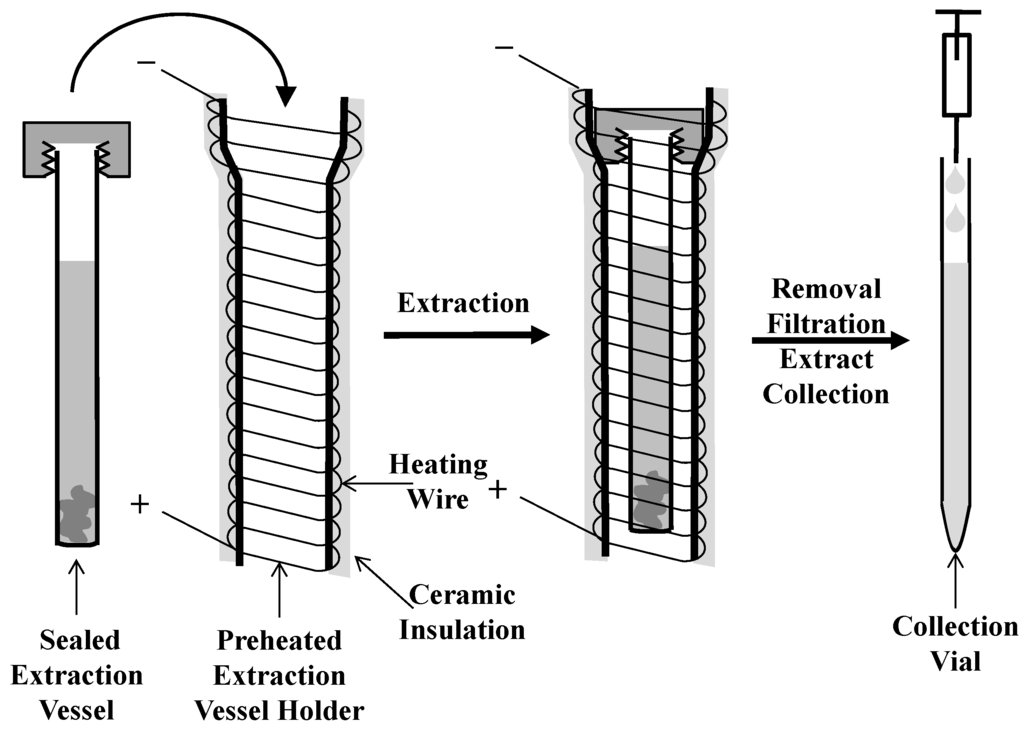
Figure 1.
Schematic diagram of the µPLE apparatus and extraction procedure.
Figure 1.
Schematic diagram of the µPLE apparatus and extraction procedure.
2.2. Extraction and Analysis Procedures
In each experiment, a 5–10 mg portion of matrix solid was weighed into the extraction vessel and spiked with a 50:50 acetone/hexane aliquot containing 800 ng of each of the 16 PAHs. This was then left to thoroughly dry for 45 min at room temperature before adding 125 µL of extraction solvent (toluene unless stated otherwise), which also contained 2 µg of a tetradecane internal standard. The vessel was then sealed and placed in the preheated extraction vessel holder for a set amount of extraction time. Upon completion, vessels were removed and immediately cooled in a beaker of room temperature water. The contents were mixed by inverting the vessel once and then the extracts were removed with a 250 µL syringe and filtered through an Acrodisc® syringe filter with a polyethersulfone membrane (13 mm diameter, 0.45 µm porosity; VWR International, Radnor, PA, USA). The filtrate was then collected in a glass vial for chromatographic analysis.
Analyses of all PAH samples and standards were done with an HP 5890 Series II (Hewlett-Packard Co. USA) GC-FID system. Separations were performed on an SPB-5 (5%-phenyl, 95%-methylpolysiloxane) megabore column (30 m × 0.53 mm i.d. × 0.5 µm film thickness; Supelco Bellefonte, USA) using splitless injection.
The optimized temperature program developed for the approximately 50 min separation of the 16 PAH analytes was as follows: 2 min at 100 °C, then 10 °C/min to 190 °C (hold 8 min), then 20 °C/min to 260 °C (hold 10 min), then 10 °C/min to 290 °C (hold 15 min). High purity nitrogen (Praxair, Calgary, AB, Canada) was used as a carrier gas at about 4 mL/min. High purity hydrogen (Praxair) and medical-grade air (Praxair) were used for the detector flame at respective flow rates of 30 and 300 mL/min. The injector/detector temperature was normally 300 °C. Data acquisition was performed using Peak Simple software (SRI Instruments, Torrance, CA, USA).
2.3. Quantification of Extracts
Extract quantification was achieved through calibration with PAH standards. For each sample, a standard containing all of the PAHs was prepared and analyzed to establish response factors for all of the analytes relative to the tetradecane internal standard, similar to that reported in other PAH extraction experiments [3]. These factors were then applied to the same ratios from the extracted sample in order to quantify the mass of each PAH present in the extract. This was then also done for an unspiked matrix sample. Extraction recovery values were subsequently calculated as follows:
while each PAH could be individually analyzed in this manner, it should be noted that Benzo[B]fluoranthene and Benzo[k]fluoranthene had to be quantified together, due to insufficient baseline resolution between the two peaks. Figure 2 demonstrates this with a portion of a typical chromatogram, which shows that each of these peaks significantly overlaps the other. While different conditions were attempted, better separation of the two could not be achieved. In future experiments, it may be useful in this regard to employ a narrower bore column (i.e., 250 µm i.d.) in attempts to gain better resolving power, although this is not always successful [9].
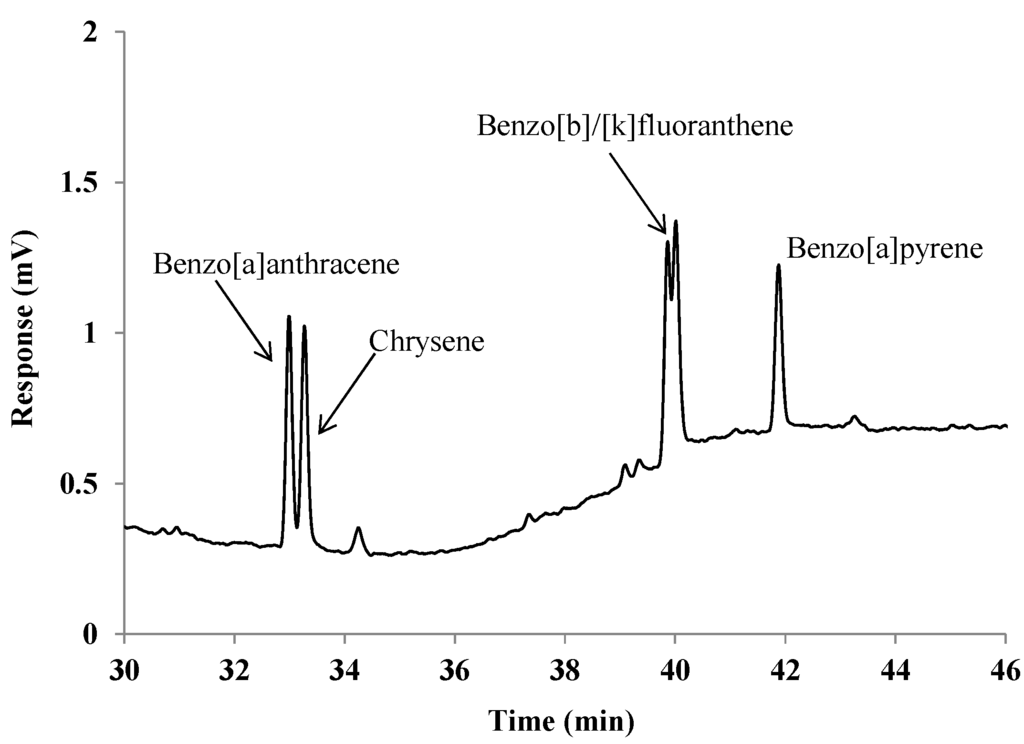
Figure 2.
A portion of a GC-FID chromatogram of a standard PAH solution (6.4 ng each), which demonstrates the unresolved peaks of Benzo[b]fluoranthene and Benzo[k]fluoranthene, denoted as Benzo [b]/[k] fluoranthene here and in the text.
Figure 2.
A portion of a GC-FID chromatogram of a standard PAH solution (6.4 ng each), which demonstrates the unresolved peaks of Benzo[b]fluoranthene and Benzo[k]fluoranthene, denoted as Benzo [b]/[k] fluoranthene here and in the text.
2.4. Matrix Preparation
Ottawa sand and soil were used directly as obtained. For chicken breast samples one whole breast was steamed for two hours using a common electric stove element at medium heat. The thoroughly cooked meat was then placed on foil in a commercial smoker unit and smoked at 175 °C for 40 min. The outer smoked portions of the meat were then used as samples during experiments. For charred toast, the surface of one slice of bread was evenly charred on both sides using a propane torch. Next, the bread was further toasted in a commercial toaster for 2 min. The charred toast was then ground with a mortar and pestle as finely as possible and stored in a sealed container until used. For biochar-amended soil, a sample of soil was mixed thoroughly with finely ground biochar (coconut charcoal source) to form a homogeneous mixture composed of 2.4% biochar by weight as previously described [16]. Coarsely ground biochar was also used directly as a sample matrix.
2.5. Materials
A mixture of 16 US EPA priority pollutant PAHs (naphthalene, acenaphthene, acenaphthylene, fluorene, phenanthrene, anthracene, fluoranthene, pyrene, benzo[a]anthracene, chrysene, benzo[b]fluoranthene, benzo[k]fluoranthene, benzo[a]pyrene, indeno[1,2,3-cd]pyrene, dibenz[a,h]anthracene and benzo[ghi]perylene) was purchased at a concentration of 2000 ng/μL in a 1 mL stock solution of 1:1 methylene chloride/benzene (VWR, Mississauga, ON, Canada). This was diluted 5 fold with 1:1 hexanes/acetone to yield a 400 ng/µL solution of all analytes. Tetradecane (99%) was obtained from Aldrich (Oakville, ON, Canada). Toluene (99.5%), hexanes (98.5%), and acetone (99.5%) were obtained from EMD chemicals (Mississauga, ON, Canada). Ottawa sand (20–30 mesh) was obtained from Fisher Scientific (Fair Lawn, NJ, USA). Coconut charcoal (8–12 mesh) was obtained from BDH chemicals (Toronto, ON, CAN). Soil was bought at a local garden store. Chicken breast and white bread were bought at a local grocer.
3. Results and Discussion
3.1. Ottawa Sand
Initial experiments were aimed at probing the capability of the µPLE technique to extract the PAHs under study. For this, an Ottawa sand matrix was chosen because it provided a reasonably homogeneous solid that PAHs moderately adhere to. Further, such extractions from Ottawa sand have also been previously reported using conventional PLE [27]. Therefore, this allowed the general extractability of the PAHs to be initially assessed.
The results of these experiments are presented in Table 1. As seen, quantitative recovery of the PAHs is attained, which range in individual values from 97%–116% (spiking level 800 ng each). This yields an average recovery of 109% with an average % RSD value of 8. Overall, these findings also agree quite well with conventional PLE extractions from Ottawa sand. For instance, earlier work has shown that conventional PLE recovered 28%–109% (spiking level 2000 ng each) of these PAHs, with an average value of 96% and an average % RSD value of 12 [27]. Further in that work, when the results of the three largest PAHs are disregarded, the minimum recovery value increases to near 87%. In fact, after system deactivation, near 82% of even those heavier PAHs were recovered [27]. The results also indicate that the µPLE extractions from Ottawa sand are also relatively quite fast and efficient. For example, quantitative µPLE extractions of the PAHs were attained here in only 15 s at 200 °C using 125 µL of 1:1 hexane/acetone as the extraction solvent. By comparison, conventional PLE required about 7 min of extraction time and 30 mL of organic solvent per sample [27]. Therefore, given that the recovery and error associated with the µPLE extractions are quite comparable to those realized with conventional PLE, it appears that µPLE is reasonably capable of extracting the PAHs from this relatively simple matrix.

Table 1.
Extraction recovery of PAHs from Ottawa sand by μPLE *.
| Analyte | % Recovery | % RSD |
|---|---|---|
| Naphthalene | 112 | 9 |
| Acenaphthene | 114 | 6 |
| Acenaphthylene | 115 | 5 |
| Fluorene | 116 | 4 |
| Phenanthrene | 114 | 5 |
| Anthracene | 113 | 2 |
| Fluoranthene | 110 | 2 |
| Pyrene | 103 | 4 |
| Benzo[a]anthracene | 113 | 7 |
| Chrysene | 112 | 10 |
| Benzo[b]/[k] fluoranthene | 114 | 12 |
| Benzo[a]pyrene | 104 | 11 |
| Indeno[1,2,3-cd]pyrene | 97 | 11 |
| Dibenz[a,h]anthracene | 104 | 15 |
| Benzo[g,h,i]perylene | 100 | 14 |
| Average | 109 | 8 |
* Conditions are 8.2 ± 0.9 mg samples extracted at 200 °C for 15 s using 1:1 hexane/acetone as an extraction solvent (n = 3).
3.2. Soil
Considering the above results with Ottawa sand, it was next interesting to examine if µPLE could extract the PAHs from soil samples. PAH contamination in soils is ubiquitous, and the sorption of these analytes to soil matrices is modulated by significant carbon content [28]. As such, soil is widely studied with PAH extractions and can present a more strongly binding matrix to recover these analytes from.
Initially, the same parameters used above for Ottawa sand (i.e., 200 °C using 1:1 hexane/acetone as the solvent) were investigated but it was found that not all of the analyte recoveries were quantitative. For example, after 1 min of extraction time under these conditions, the average recovery of the high molecular weight PAHs (i.e., the bottom eight analytes in Table 1; Benzo[a]Anthracene to Benzo[g,h,i]perylene) was only 65%. In efforts to address this, increasing extraction temperatures up to 250 °C and longer extraction times up to 4 min were next examined. However, it was found that neither measure showed any significant improvements in the PAH extraction recoveries. Accordingly, changing the extraction solvent was next attempted. Other solvents tested included methylene chloride and toluene. Ultimately it was found that using pure toluene as the extraction solvent had the most pronounced effect of all the solvents investigated and resulted in greatly improved extraction recoveries. As such, toluene was used as the extraction solvent in all further studies here. Table 2 presents these results for the soil samples and as can be seen, when using toluene as the extraction solvent, PAH recoveries of 90%–130% (spiking level 800 ng each) were obtained. This yielded an average extraction recovery of 103% with an average %RSD value of 8. Further, these results were obtained using 125 µL of toluene at 200 °C in just 30 s. These findings also compare well with soil extractions done previously by conventional PLE, which showed that after 10 min of extraction using 50 mL of organic solvent per sample, 75%–103% (spiking level 2000 ng each) of PAHs were recovered, with an average recovery of 88% and an average %RSD value of 10 [29]. Therefore, µPLE shows reasonably good performance in recovering the PAHs from soil samples.

Table 2.
Extraction recovery of PAHs from soil, chicken and charred toast by µPLE *.
| Matrix | Soil | Chicken Breast | Charred Toast | |||
|---|---|---|---|---|---|---|
| Analyte | % Recovery | % RSD | % Recovery | % RSD | % Recovery | % RSD |
| Naphthalene | 92 | 8 | 114 | 4 | 93 | 6 |
| Acenaphthene | 90 | 3 | 103 | 3 | 87 | 4 |
| Acenaphthylene | 93 | 4 | 97 | 7 | 92 | 1 |
| Fluorene | 92 | 4 | 96 | 4 | 88 | 3 |
| Phenanthrene | 92 | 6 | 93 | 6 | 87 | 2 |
| Anthracene | 95 | 6 | 104 | 3 | 87 | 3 |
| Fluoranthene | 93 | 6 | 91 | 11 | 82 | 2 |
| Pyrene | 109 | 20 | 88 | 13 | 83 | 5 |
| Benzo[a]anthracene | 113 | 9 | 93 | 18 | 81 | 7 |
| Chrysene | 113 | 11 | 94 | 16 | 90 | 5 |
| Benzo[b]/[k]fluoranthene | 119 | 9 | 100 | 16 | 78 | 4 |
| Benzo[a]pyrene | 116 | 9 | 110 | 17 | 78 | 3 |
| Indeno[1,2,3-cd]pyrene | 130 a | - | 108 | 13 | 80 | 3 |
| Dibenz[a,h]anthracene | 122 a | - | 110 | 18 | 79 | 7 |
| Benzo[g,h,i]perylene | 127 | 12 | 107 | 10 | 82 | 3 |
| Average | 103 | 8 | 101 | 11 | 84 | 4 |
* Conditions are 8.4 ± 0.3 mg (soil), 8.3 ± 0.6 mg (chicken breast), 8.5 ± 0.4 mg (charred toast) samples extracted at 200 °C for 30 s using toluene as an extraction solvent (n = 3). a: Based on a single trial due to chromatographic interference in the others.
3.3. Food
Since different food matrices are another common sample class that is often examined with PAH extractions, two examples of these were next probed. The first was a meat sample in the form of a smoked fully cooked chicken breast. Using toluene as the optimal solvent, it was again determined that good extraction efficiency could be obtained for PAHs from this matrix when using µPLE. Table 2 presents the results, which show that µPLE recovered 88%–114% (spiking level 800 ng each) of the PAHs, yielding an average recovery of 101% with an average %RSD value of 11. This was again attained after 30 s of extraction at 200 °C using 125 µL of solvent. These findings also agree well with conventional PLE extractions of PAHs from meat samples that produced recoveries ranging from about 60%–100% (spiking level 1000 ng each), with an average recovery near 80% and an average %RSD value of 7 [30]. This is useful since the latter also required 15 min of extraction time and 20 mL of organic solvent per sample [30].
The second food sample examined was charred toast. This was interesting not only as a different food type, but also because samples containing various levels of charred material (e.g., from burning during cooking) are commonly investigated in PAH extractions due to their potentially strong matrix effects. The results are shown in Table 2. As seen, using 125 µL of toluene at 200 °C for 30 s resulted in 78%–93% (spiking level 800 ng each) of the PAHs being recovered from the charred toast matrix by µPLE. As a result, this yielded an average recovery of 84% with an average %RSD value of 4. Therefore, while these extractions were quite reproducible, the recoveries for most of these PAHs were notably lower than those obtained with the other matrices here. This is likely a result of the presence of char in the matrix, which can lower the extraction efficiency of PAHs. For instance, although no reports of using conventional PLE with this type of sample have appeared, even those that performed exhaustive Soxhlet extractions over 16 h with 150 mL of organic solvent still only recovered 81%–95% (spiking level 250 ng each) of the PAHs from charred toast, with an average recovery of 89% and an average %RSD value of 8 [31]. Therefore, it seems that the presence of char in the matrix here may also similarly somewhat impede the extraction efficiency realized by µPLE.
3.4. Biochar-Amended Soil
Biochar is a product of biomass pyrolysis and is widely applied to soils to modulate porosity, water retention, and other aspects of soil fertility [32]. Further, it has been shown that the sorption affinity of PAHs for soil significantly increases when small proportions of biochar are present within the sample [33]. Therefore, this was an interesting matrix to next explore with µPLE since it is commonly examined in PAH extractions and it provided a useful opportunity to confirm the impact of biochar on µPLE extraction efficiency.
For this, the same soil used for the experiments of Table 2 was blended with 2.4% w/w of biochar and then used as a sample matrix for PAH extractions by µPLE. Using similar conditions as before with the pure soil sample (i.e., 125 µL of toluene at 200 °C for 60 s) the biochar-amended soil was next examined. The results are shown in Table 3. As can be seen, under these conditions 51%–106% (spiking level 800 ng each) of the PAHs were recovered, yielding an average recovery of 84% and an average %RSD value of 6. These values are significantly lower than those of the pure soil sample given in Table 2, even though the extraction time is twice as long (i.e., 60 vs. 30 s). Therefore, the presence of biochar appears to notably lower the extraction efficiency of the PAHs. Closer examination also reveals that most of the lower recoveries occurred for the higher molecular weight PAHs. For example, the average recovery for the last eight PAHs in Table 3 (i.e., from Benzo[a]anthracene to Benzo[g,h,i]perylene) is 66%, whereas the first eight produced an average recovery of 100%. This clearly reflects the strong sorption of the heavier analytes to the biochar.
In attempts to address this, longer µPLE extraction times were pursued but they did not produce significant improvements in the PAH recoveries. As well, multiple extraction cycles on the same biochar-amended soil sample (i.e., 3 × 30 second extractions at 200 °C, with 125 µL of fresh toluene exchanged after each run upon cooling and opening the vessel) were also performed but the results did not significantly change. Therefore, it appears that even a relatively small quantity of biochar can impede the PAH extraction efficiency realized with µPLE. However, this is also commonly observed in conventional PAH extractions. For instance, although the use of conventional PLE on such samples has not been reported, 36 h Soxhlet extractions with 160 mL of organic solvent recovered 49%–136% (endogenous levels) of PAHs from a biochar-amended soil sample, yielding an average recovery of 99% with an average %RSD value of 50 [16].

Table 3.
Extraction recovery of PAHs from biochar-amended soil and biochar by µPLE *.
| Matrix | Biochar-amended soil | Biochar | ||
|---|---|---|---|---|
| Analyte | % Recovery | % RSD | % Recovery | % RSD |
| Naphthalene | 102 | 5 | 61 | 7 |
| Acenaphthene | 104 | 3 | 60 | 13 |
| Acenaphthylene | 106 | 6 | 66 | 11 |
| Fluorene | 101 | 7 | 66 | 18 |
| Phenanthrene | 101 | 4 | 65 | 18 |
| Anthracene | 97 | 4 | 65 | 14 |
| Fluoranthene | 92 | 3 | 59 | 17 |
| Pyrene | 98 | 8 | 58 | 20 |
| Benzo[a]anthracene | 77 | 3 | 55 | 17 |
| Chrysene | 79 | 2 | 58 | 14 |
| Benzo[b]/[k]fluoranthene | 76 | 6 | 47 | 17 |
| Benzo[a]pyrene | 73 | 10 | 45 | 15 |
| Indeno[1,2,3-cd]pyrene | 51 | 8 | 33 | 25 |
| Dibenz[a,h]anthracene | 53 | 10 | 35 | 17 |
| Benzo[g,h,i]perylene | 53 | 5 | 38 | 28 |
| Average | 84 | 6 | 54 | 17 |
* Conditions are 8.3 ± 0.7 mg (char-amended soil), 9.4 ± 0.3 mg (Biochar) extracted at 200 °C for 1 min using toluene as an extraction solvent (n = 3).
3.5. Biochar
Since only a relatively small proportion of biochar mixed with soil produced a notable decrease in extraction recovery, it was of interest to see how a much larger presence of biochar would also impact results. Char-like matrices have been proposed to sorb species, such as PAHs, very strongly due to both the chemical similarity between matrix and analyte, as well as the surface structure of the matrix [34]. Char typically has a very high surface area, with deep narrow pores that are accessible to spiked analytes and can hinder extraction [34]. Therefore, in a final set of experiments, PAHs were extracted from pure biochar using µPLE. For this, the same conditions as above were again used (i.e., 125 µL of toluene at 200 °C for 60 s). Table 3 shows the results and as can be seen, 33%–66% (spiking level 800 ng each) of the PAHs were recovered under these conditions, with an average recovery of 54% and an average %RSD value of 17. Therefore, pure biochar further reduces the PAH recoveries realized. As well, once again, this is particularly true for the high molecular weight analytes. While this is somewhat expected, it is interesting to investigate since some methods are unable to extract PAHs in any significant quantity from pure biochar samples. For example, a report using conventional PLE for biochar extractions showed that none of the PAHs heavier than chrysene could be recovered at all [35]. Further by comparison, 36 h of Soxhlet extraction using pure biochar and 200 mL of organic solvent per sample resulted in 16%–64% (spiking level 1000–2000 ng each) of the PAHs being recovered, yielding an average recovery of 42% with an average %RSD value of 18 [35].
3.6. Comparisons with Other Methods
In order to better understand the µPLE results obtained here, it is useful to place them in overall context with those obtained by conventional extraction methods on similar samples. Table 4 presents this with a summary of the results obtained by µPLE in this study and those reported previously using conventional methods. As can be seen from the data, the µPLE extraction recovery results compare very well to those obtained from conventional extraction techniques for similar samples. For instance, for the non-char containing samples, both µPLE and conventional PLE produce reasonably good analyte recoveries. Further, for those containing char, the recoveries were lower for µPLE and similarly so for conventional Soxhlet extractions. This problem is in fact commonly encountered in the analysis of PAHs from biochar-containing samples by conventional PLE and Soxhlet [35] and is normally attributed to the very strong sorption of PAHs to biochar [16,33].
It should also be pointed out that some of the results shown produced analyte recovery values larger than 100%. Specifically, of the six different sample types extracted by µPLE, 4 produced results that were greater than 100%. This was also true for three out of the six comparative studies shown in Table 4 that used conventional PLE and Soxhlet on similar samples. However, such values are often obtained in extraction studies and reported within acceptable recovery ranges [27]. Therefore, no major differences should be anticipated with µPLE in this regard.
It is further worth noting that comparisons were drawn by showing the combined average recovery and % RSD values in addition to the range of individual analyte recoveries obtained. This is similar to other studies [27,35] and is used here only to help facilitate a general comparison of methods from different perspectives and to gain a reasonable understanding of the potential of µPLE.
More importantly, however, the extraction time and the solvent requirements for µPLE are significantly smaller than those of the other conventional methods. For example, while µPLE consistently uses only 125 µL of extraction solvent for the samples studied here, the other techniques listed in Table 4 use solvent volumes ranging from 20–160 mL. Further, while µPLE required only 15–60 s for extracting the PAHs, other conventional methods require extraction times ranging from 7 min to 36 h for this.
It should be noted that examples of conventional PLE usage for these sample types were most relevant for comparison but could not be found for all matrices investigated. In the absence of this, even more conventional Soxhlet examples were chosen. Nonetheless, other methods perhaps more efficient than Soxhlet could also be compared here. For instance, a previously reported ultrasound-assisted method for extracting PAHs from charred toast required 30 min and 30 mL of hexane per sample to recover on average 64% (spiking level 24–1680 ng each) of the high molecular weight PAHs (Benzo[a]Anthracene to Benzo[g,h,i]perylene) [19]. Therefore, it appears that µPLE is capable of extracting PAHs from the samples investigated with very similar efficiency to conventional extraction methods in terms of analyte recovery and reproducibility. However, µPLE requires much less extraction time and solvent in the process.
Incidentally, these µPLE results also compare well to other miniaturized PLE systems [7,8,9]. For example, these approaches normally employed 10–30 min extraction times and 100 µL to 8 mL of organic solvents for extracting PAHs from sediments, and other hydrophobic environmental contaminants from feedstuffs.

Table 4.
Comparison of PAH extractions by µPLE and other methods.
| Sample | µPLE | Conventional Methods | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Time (s) | Solvent Volume (µL) | Recovery Range (%) | AVG ± RSD (%) | Method | Reference | Time | Solvent Volume (mL) | Recovery Range (%) | AVG ± RSD (%) | |
| Ottawa sand | 15 | 125 | 97–116 | 109 ± 8 | PLE | 27 | 7 min | 30 | 28–109 | 96 ± 12 |
| Soil | 30 | 125 | 90–130 | 103 ± 8 | PLE | 29 | 10 min | 50 | 75–103 | 88 ± 10 |
| Chicken breast | 30 | 125 | 88–114 | 101 ± 11 | PLE | 30 | 15 min | 20 | 60–100 | 80 ± 7 |
| Charred toast | 30 | 125 | 78–93 | 84 ± 4 | Soxhlet | 31 | 16 h | 150 | 81–95 | 89 ± 8 |
| Ultrasound | 19 | 30 min | 30 | 44–81 | 64 ± 3 | |||||
| Biochar-amended soil | 60 | 125 | 51–106 | 84 ± 6 | Soxhlet | 16 | 36 h | 160 | 49–136 | 99 ± 50 |
| Biochar | 60 | 125 | 33–66 | 54 ± 17 | Soxhlet | 35 | 36 h | 160 | 16–64 | 42 ± 18 |
4. Conclusions
The use of µPLE to extract PAHs from a variety of solid matrices was demonstrated. The method uses very small quantities of solvent in a static extraction mode to remove analytes in a matter of seconds from samples. The method was found to extract the 16 EPA priority pollutant PAHs from various solid samples using only a fraction of the time and solvent required by conventional methods for similar samples and with similar results. For example, µPLE required 15 to 60 s extraction time and 125 µL of solvent, whereas conventional PLE normally required up to 15 min and 50 mL of solvent. Accordingly, the µPLE system described here may provide a simple, robust alternative for the preparation of PAH-containing samples that is both inexpensive and easily adaptable to many laboratories.
Acknowledgments
The authors are grateful to the Natural Sciences and Engineering Research Council of Canada for a Discovery Grant in support of this project.
Author Contributions
All authors contributed equally to the work.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Luque de Castro, M.D.; Priego-Capote, F. Soxhlet extraction: Past and present panacea. J. Chromatogr. A 2010, 1217, 2383–2389. [Google Scholar] [CrossRef] [PubMed]
- Luque de Castro, M.D.; García-Ayuso, L.E. Soxhlet extraction of solid materials: An outdated technique with a promising innovative future. Anal. Chim. Acta 1998, 369, 1–10. [Google Scholar] [CrossRef]
- Hawthorne, S.B.; Grabanski, C.B.; Martin, E.; Miller, D.J. Comparisons of Soxhlet extraction, pressurized liquid extraction, supercritical fluid extraction and subcritical water extraction for environmental solids: recovery, selectivity and effects on sample matrix. J. Chromatogr. A 2000, 892, 421–433. [Google Scholar] [CrossRef] [PubMed]
- Sporring, S.; Bøwadt, S.; Svensmark, B.; Björklund, E. Comprehensive comparison of classic Soxhlet extraction with Soxtec extraction, ultrasonication extraction, supercritical fluid extraction, microwave assisted extraction and accelerated solvent extraction for the determination of polychlorinated biphenyls in soil. J. Chromatogr. A 2005, 1090, 1–9. [Google Scholar] [PubMed]
- Nieto, A.; Borrull, F.; Pocurull, E.; Marcé, R.M. Pressurized liquid extraction: A useful technique to extract pharmaceuticals and personal-care products from sewage sludge. Trac-Trend. Anal. Chem. 2010, 29, 752–764. [Google Scholar] [CrossRef]
- Pena-Pereira, F.; Duarte, R.M.B.O.; Duarte, A.C. Considerations on the application of miniaturized sample preparation approaches for the analysis of organic compounds in environmental matrices. Cent. Eur. J. Chem. 2012, 10, 433–449. [Google Scholar] [CrossRef]
- Ramos, L.; Vreuls, J.J.; Brinkman, U.A.T. Miniaturised pressurised liquid extraction of polycyclic aromatic hydrocarbons from soil and sediment with subsequent large-volume injection-gas chromatography. J. Chromatogr. A 2000, 891, 275–286. [Google Scholar] [CrossRef]
- Pena-Abaurrea, M.; Ramos, J.J.; Gonzalez, M.J.; Ramos, L. Miniaturized selective pressurized liquid extraction of polychlorinated biphenyls and polybrominated diphenyl ethers from feedstuffs. J. Chromatogr. A 2013, 1273, 18–25. [Google Scholar] [CrossRef] [PubMed]
- Hyötyläinen, T.; Andersson, T.; Hartonen, K.; Kuosmanen, K.; Riekkola, M.-L. Pressurized hot water extraction coupled on-line with LC-GC: Determination of polyaromatic hydrocarbons in sediment. Anal. Chem. 2000, 72, 3070–3076. [Google Scholar] [CrossRef] [PubMed]
- Alkhateeb, F.L.; Thurbide, K.B. A novel micro pressurized liquid extraction method for very rapid solid sample preparation. Anal. Methods 2015, 7, 1509–1516. [Google Scholar] [CrossRef]
- Purcaro, G.; Moret, S.; Conte, L.S. Overview on polycyclic aromatic hydrocarbons: Occurrence, legislation and innovative determination in foods. Talanta 2013, 105, 292–305. [Google Scholar] [CrossRef] [PubMed]
- Viegas, O.; Novo, P.; Pinho, O.; Ferreira, I.M.P.L.V.O. A comparison of the extraction procedures and quantification methods for the chromatographic determination of polycyclic aromatic hydrocarbons in charcoal grilled meat and fish. Talanta 2012, 88, 677–683. [Google Scholar] [CrossRef] [PubMed]
- Lü, H.; Cai, Q.-Y.; Jones, K.C.; Zeng, Q.-Y.; Katsoyiannis, A. Levels of organic pollutants in vegetables and human exposure through diet: A review. Crit. Rev. Environ. Sci. Technol. 2014, 44, 1–33. [Google Scholar] [CrossRef]
- Brändli, R.C.; Hartnik, T.; Henriksen, T.; Cornelissen, G. Sorption of native polyaromatic hydrocarbons (PAH) to black carbon and amended activated carbon in soil. Chemosphere 2008, 73, 1805–1810. [Google Scholar] [CrossRef] [PubMed]
- Boström, C.-E.; Gerde, P.; Hanberg, A.; Jernström, B.; Johansson, C.; Kyrklund, T.; Rannug, A.; Törnqvist, M.; Victorin, K.; Westerholm, R. Cancer risk assessment, indicators, and guidelines for polycyclic aromatic hydrocarbons in the ambient air. Environ. Health Persp. 2002, 110, 451–488. [Google Scholar] [CrossRef]
- Fabbri, D.; Rombolà, A.G.; Torri, C.; Spokas, K.A. Determination of polycyclic aromatic hydrocarbons in biochar and biochar amended soil. J. Anal. Appl. Pyrol. 2013, 103, 60–67. [Google Scholar] [CrossRef]
- Barreca, S.; Mazzola, A.; Orecchio, S.; Tuzzolino, N. Polychlorinated biphenyls in sediments from Sicilian coastal area (Scoglitti) using automated soxhlet, GC-MS, and principal component analysis. Polycycl. Aromat. Comp. 2014, 34, 237–262. [Google Scholar] [CrossRef]
- León, V.M.; Moreno-González, R.; González, E.; Martínez, F.; García, V.; Campillo, J.A. Interspecific comparison of polycyclic aromatic hydrocarbons and persistent organochlorines bioaccumulation in bivalves from a Mediterranean coastal lagoon. Sci. Total Environ. 2013, 463–464, 975–987. [Google Scholar] [CrossRef] [PubMed]
- Rey-Salgueiro, L.; García-Falcón, M.S.; Martínez-Carballo, E.; Simal-Gándara, J. Effects of toasting procedures on the levels of polycyclic aromatic hydrocarbons in toasted bread. Food Chem. 2008, 108, 607–615. [Google Scholar] [CrossRef] [PubMed]
- Viegas, O.; Yebra-Pimentel, I.; Martinez-Carballo, E.; Simal-Gandara, J.; Ferreira, I.M.P.L.V.O. Effect of beer marinades on formation of polycyclic aromatic hydrocarbons in charcoal-grilled pork. J. Agric. Food Chem. 2014, 62, 2638–2643. [Google Scholar] [CrossRef] [PubMed]
- Irvine, G.M.; Blais, J.M.; Doyle, J.R.; Kimpe, L.E.; White, P.A. Cancer risk to First Nations’ people from exposure to polycyclic aromatic hydrocarbons near in-situ bitumen extraction in Cold Lake, Alberta. Environ. Health 2014, 13, 7. [Google Scholar] [CrossRef] [PubMed]
- Lorenzi, D.; Cave, M.; Dean, J.R. An investigation into the occurrence and distribution of polycyclic aromatic hydrocarbons in two soil size fractions at a former industrial site in NE England, UK using in situ PFE–GC–MS. Environ. Geochem. Health 2010, 32, 553–565. [Google Scholar] [CrossRef] [PubMed]
- Lund, M.; Duedahl-Olesen, L.; Christensen, J.H. Extraction of polycyclic aromatic hydrocarbons from smoked fish using pressurized liquid extraction with integrated fat removal. Talanta 2009, 79, 10–15. [Google Scholar] [CrossRef] [PubMed]
- Aguinaga, N.; Campillo, N.; Viñas, P.; Hernández-Córdoba, M. Determination of 16 polycyclic aromatic hydrocarbons in milk and related products using solid-phase microextraction coupled to gas chromatography-mass spectrometry. Anal. Chim. Acta 2007, 596, 285–290. [Google Scholar] [CrossRef] [PubMed]
- Cacho, J.I.; Campillo, N.; Viñas, P.; Hernández-Córdoba, M. Use of headspace sorptive extraction coupled to gas chromatography-mass spectrometry for the analysis of volatile polycyclic aromatic hydrocarbons in herbal infusions. J. Chromatogr. A 2014, 1356, 38–44. [Google Scholar] [CrossRef] [PubMed]
- Campíns-Falcó, P.; Verdú-Andrés, J.; Sevillano-Cabeza, A.; Molins-Legua, C.; Herráez-Hernández, R. New micromethod combining miniaturized matrix solid-phase dispersion and in-tube in-valve solid-phase microextraction for estimating polycyclic aromatic hydrocarbons in bivalves. J. Chromatogr. A 2008, 1211, 13–21. [Google Scholar] [CrossRef] [PubMed]
- Haskins, S.D.; Duval, J.M.; Kelly, D.G.; Lundgreen-Nielsen, S.L.; Weir, R.D. Pressurized fluid extraction of polycyclic aromatic hydrocarbons using silanized extraction vessels. Microchim. Acta 2012, 178, 187–193. [Google Scholar] [CrossRef]
- Wilcke, W. Polycyclic aromatic hydrocarbons (PAHs) in soil—A review. J. Plant Nutr. Soil Sci. 2000, 163, 229–248. [Google Scholar] [CrossRef]
- Zhang, Q.; Liang, T.; Wang, L.; Cao, H. Determination of polycyclic aromatic hydrocarbons from soil samples using selective pressurized liquid extraction. Anal. Methods 2012, 4, 2441–2446. [Google Scholar] [CrossRef]
- Wang, G.; Lee, A.S.; Lewis, M.; Kamath, B.; Archer, R.K. Accelerated solvent extraction and gas chromatography/mass spectrometry for determination of polycyclic aromatic hydrocarbons in smoked food samples. J. Agric. Food Chem. 1999, 47, 1062–1066. [Google Scholar] [CrossRef] [PubMed]
- Al-Rashdan, A.; Helaleh, M.I.H.; Nisar, A.; Ibtisam, A.; Al-Ballam, Z. Determination of the levels of polycyclic aromatic hydrocarbons in toasted bread using gas chromatography mass spectrometry. Int. J. Anal. Chem. 2010, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Manyà, J.J. Pyrolysis for biochar purposes: A review to establish current knowledge gaps and research needs. Environ. Sci. Technol. 2012, 46, 7939–7954. [Google Scholar] [CrossRef] [PubMed]
- Chen, B.; Yuan, M. Enhanced sorption of polycyclic aromatic hydrocarbons by soil amended with biochar. J. Soil. Sediment. 2011, 11, 62–71. [Google Scholar] [CrossRef]
- Jonker, M.T.O.; Koelmans, A.A. Sorption of polycyclic aromatic hydrocarbons and polychlorinated biphenyls to soot and soot-like materials in the aqueous environment: Mechanistic considerations. Environ. Sci. Technol. 2002, 36, 3725–3734. [Google Scholar] [CrossRef] [PubMed]
- Hilber, I.; Blum, F.; Leifeld, J.; Schmidt, H.-P.; Bucheli, T.D. Quantitative determination of PAHs in biochar: A prerequisite to ensure its quality and safe application. J. Agric. Food Chem. 2012, 60, 3042–3050. [Google Scholar] [CrossRef] [PubMed]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
