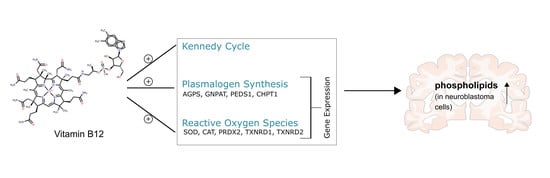
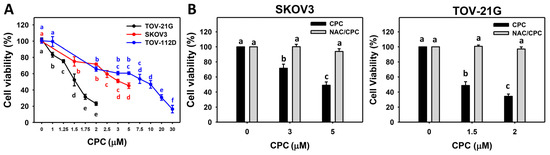
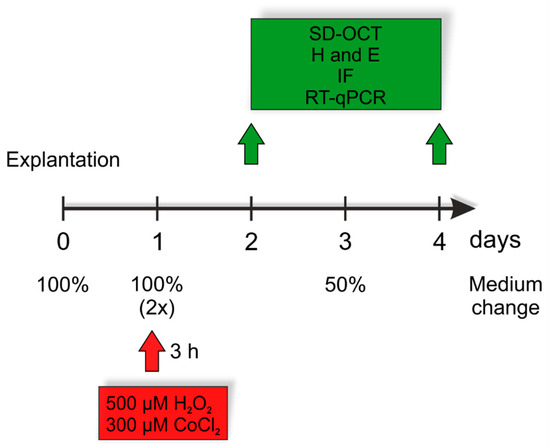
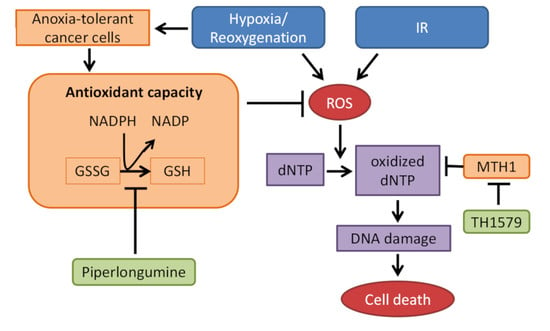
Oxidative Stress in Human Health and Disease
A topical collection in Cells (ISSN 2073-4409).
Viewed by 86788Editors
Interests: neurodegeneration/-regeneration; neuroinflammation; amyotrophic lateral sclerosis; schizophrenia
Topical Collection Information
Dear Colleagues,
Oxygen is essential for human life. The complete oxidation of nutrients for biological energy supply is one of the most important prerequisites for the formation of higher life forms. However, since adaptation to oxygen as an energy source, the cells that benefit from oxidative respiration are also stressed by the reactive oxygen species (ROS). Oxygen radicals are constantly produced in aerobic organisms and are involved in hormone synthesis, signaling pathways, transcriptional regulation, among others. Therefore, it can be assumed that ROS in limited concentrations are part of a healthy metabolism. Healthy cells balance the formation and elimination of ROS, creating and maintaining ROS-homeostasis. When the concentration of free radicals exceeds a critical level, and the homeostasis is disturbed oxidative stress occurs. Upon oxidative stress, free radicals of oxygen can cause damage to cells, cell organelles, and components, such as lipids, proteins, and DNA. This damage, if not repaired, can lead to cell death. Oxidative changes play a role in the pathogenesis of many diseases, such as diabetes, cardiovascular and neurodegenerative diseases, but also autoimmune processes and cancer, to name a few. To counteract this potential threat of oxidative stress, eukaryotic cells have developed protective systems that attempt to prevent the generation of free radicals in parallel with the evolutionary adaptation to the oxygen-rich atmosphere through the uptake of the oxidative metabolizing mitochondria. These oxidation protection systems have in common that they can prevent or delay oxidative damage and thus help to maintain the balance between the malignant or healthy stage in various diseases. Thus, the oxidative system plays a major role in both the physiology and pathology of various diseases.
This Topical Collection entitled “Oxidative Stress in Human Health and Disease” welcomes manuscripts (original papers as well as comprehensive reviews) providing insights into mechanisms of oxidative stress development leading to pathophysiology as well as physiological mechanisms to overcome these harmful events.
We look forward to receiving your contributions.
Prof. Dr. Carsten Theiss
Dr. Veronika Matschke
Collection Editors
Manuscript Submission Information
Manuscripts should be submitted online at www.mdpi.com by registering and logging in to this website. Once you are registered, click here to go to the submission form. Manuscripts can be submitted until the deadline. All submissions that pass pre-check are peer-reviewed. Accepted papers will be published continuously in the journal (as soon as accepted) and will be listed together on the collection website. Research articles, review articles as well as short communications are invited. For planned papers, a title and short abstract (about 100 words) can be sent to the Editorial Office for announcement on this website.
Submitted manuscripts should not have been published previously, nor be under consideration for publication elsewhere (except conference proceedings papers). All manuscripts are thoroughly refereed through a single-blind peer-review process. A guide for authors and other relevant information for submission of manuscripts is available on the Instructions for Authors page. Cells is an international peer-reviewed open access semimonthly journal published by MDPI.
Please visit the Instructions for Authors page before submitting a manuscript. The Article Processing Charge (APC) for publication in this open access journal is 2700 CHF (Swiss Francs). Submitted papers should be well formatted and use good English. Authors may use MDPI's English editing service prior to publication or during author revisions.
Keywords
- ROS metabolism
- oxidative stress
- reductive stress
- neurodegenerative disease
- cancer
- inflammation
- psychotic disorders
- antioxidant defense mechanisms
- mitochondria