Exclusive Review Papers in 'Stem Cells'
A topical collection in Cells (ISSN 2073-4409). This collection belongs to the section "Stem Cells".
Viewed by 27558Editor
Topical Collection Information
Dear Colleagues,
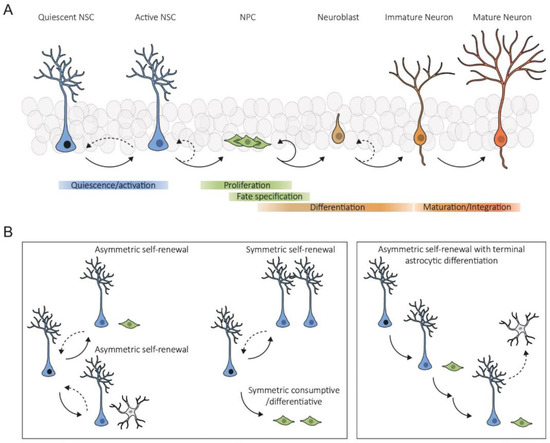
Under the section "Stem Cells ", a Topical Collection that aims to publish high-quality review articles within the field of stem cells will be set up. Stem cells form the basis for the emergence of complex, multicellular organisms and enable their maintenance, regeneration, and reproduction. Two central properties of stem cells are important for this: (I) their ability to self-renew and (II) their potential to differentiate into other cell types. To safeguard these properties, stem cells are protected by a microenvironment, the stem cell niche, which controls the quiescence, proliferation, and differentiation of stem cells. It is important to emphasize that the stem cell niches are affected by environmental influences such as excessive food supply, high oxygen tension, and elevated mechanical stress, but also by chronic inflammation and ageing. A loss of niche integrity leads to a reduction in the stem cell pool through undirected cell differentiation and thus endangers the long-term survival of the affected organism. The identification of the stem cell compartments of the body and a profound understanding of their respective niche are thus essential to understand organismal functions and to develop therapeutic procedures for patients to overcome the limited regenerative ability of chronically diseased organs and to prevent cancer progression.
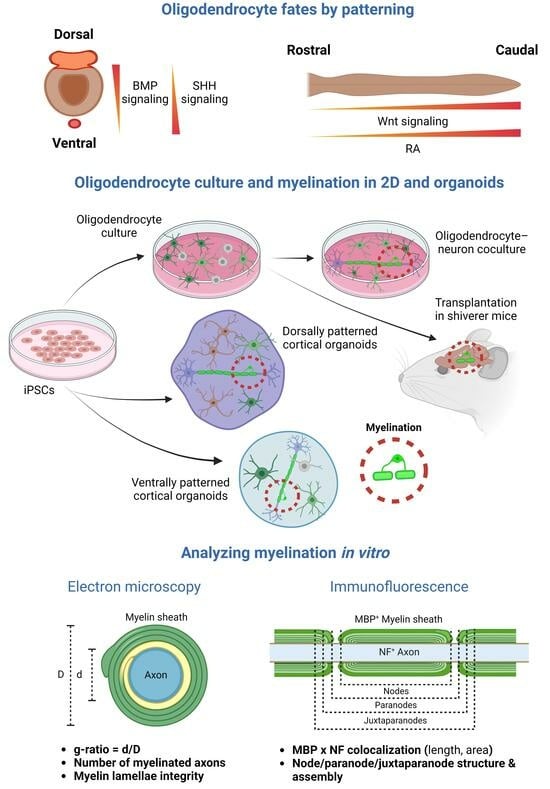
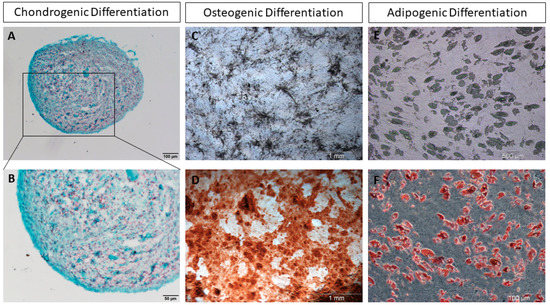
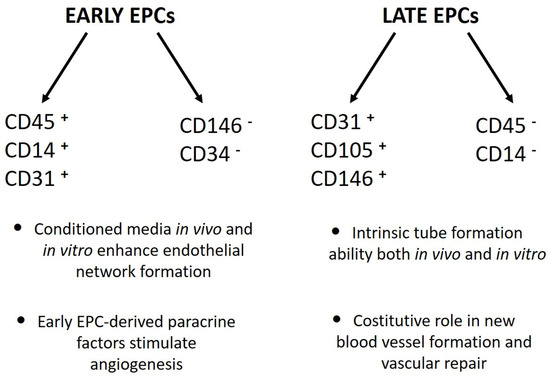
Stem cells are central in regenerative medicine. Hematopoietic stem cells have long been used in the treatment of patients, but mesenchymal stem/stromal cells and the processes they mediate will also play a significant role in the future. In addition to these adult stem cells, induced pluripotent stem cells (iPSCs) generated by the reprogramming of somatic cells are a promising basis for disease modelling and personalized medicine. Despite great progress, there are still gaps in the understanding of molecular mechanisms for the effective, directed differentiation of stem cells as well as the maturation and maintenance of the cells and tissues they give rise to. A promising approach is the usage of organoids for stabilizing differentiated cells derived from stem cells. Today, we are only at the beginning of the broad use of stem cells in medicine, with more than just biological and technical questions that still to be answered. In parallel to the increase in knowledge in the field of stem cell research, there should also be an ethical discussion about the benefits and risks of stem cell use.
The Topical Collection “Stem cells” is intended to contribute to the compilation of current knowledge on stem cells and to make it available to a broad readership. Distinguished researchers from all over the world working in all disciplines of current stem cell research, starting from basic research through tissue engineering to the clinical use of stem cells and ethical aspects, are therefore invited to contribute to this review series. Potential contributors/invited authors are kindly requested to send a tentative title and a short abstract to our Editorial Office (cells@mdpi.com) for pre-evaluation. Please note that selected full papers will still be subjected to a thorough and rigorous peer review. All papers will be published on an ongoing basis with full open access. We are looking forward to receiving your interesting contributions.
Dr. Claus Kordes
Collection Editor
Manuscript Submission Information
Manuscripts should be submitted online at www.mdpi.com by registering and logging in to this website. Once you are registered, click here to go to the submission form. Manuscripts can be submitted until the deadline. All submissions that pass pre-check are peer-reviewed. Accepted papers will be published continuously in the journal (as soon as accepted) and will be listed together on the collection website. Research articles, review articles as well as short communications are invited. For planned papers, a title and short abstract (about 250 words) can be sent to the Editorial Office for assessment.
Submitted manuscripts should not have been published previously, nor be under consideration for publication elsewhere (except conference proceedings papers). All manuscripts are thoroughly refereed through a single-blind peer-review process. A guide for authors and other relevant information for submission of manuscripts is available on the Instructions for Authors page. Cells is an international peer-reviewed open access semimonthly journal published by MDPI.
Please visit the Instructions for Authors page before submitting a manuscript. The Article Processing Charge (APC) for publication in this open access journal is 2700 CHF (Swiss Francs). Submitted papers should be well formatted and use good English. Authors may use MDPI's English editing service prior to publication or during author revisions.
Keywords
- stem cells
- stem cell niche
- pericytes
- tissue engineering
- regenerative medicine
- reproduction
- iPSC
- stem cell differentiation
- organoids
- disease modelling
- stem cell aging
- research ethics
- cancer stem cells