Mesenchymal Stromal Cells: Heterogeneity and Therapeutical Applications
Abstract
1. Introduction
2. Donor-to-Donor Heterogeneity
3. Tissue Source-Dependent Heterogeneity
3.1. MSCs from Adult Sources
3.1.1. Bone Marrow-Derived MSCs (BM-MSCs)
3.1.2. Adipose Tissue-Derived MSCs (AT-MSCs)
3.1.3. Endometrium-Derived MSCs (E-MSCs)
3.1.4. Synovial Membrane-Derived MSCs (SD-MSCs)
3.1.5. Dental Tissue-Derived MSCs (D-MSCs)
3.2. MSCs from Fetal Sources
3.2.1. Cord Blood MSCs (CB-MSCs)
3.2.2. Umbilical Cord-Derived MSCs (UC-MSCs)
| Condition | MSC | Phase | Clinical Trial | Status | References |
|---|---|---|---|---|---|
| Intraventricular Hemorrhage | Allogeneic UC-MSC (intraventricular injection) | Phase I | NCT02274428 | Completed | [128] |
| Bronchopulmonary Dysplasia (BPD) | Allogeneic UC-MSC (intratracheal injection) | Phase I | NCT01632475 | Active, not re-cruiting | [129] |
| Cerebral Palsy | Allogeneic CB- and UC-MSC | Phase I/II | NCT03473301 | Completed | - |
| Hypoxic-Ischemic Encephalopathy | Allogeneic UC-MSC | Pilot phase I | NCT03635450 | Completed | [130] |
| Bronchopulmonary dysplasia (BPD) | UC-MSC | Phase II | NCT01828957 | Completed | [131] |
| Myocardial Infarction | Allogeneic UC-MSC | Phase I | NCT03798353 | Completed | - |
| Autism | UC-MSC | Phase II | NCT04089579 | Active, not recruiting | - |
| Allogeneic UC-MSC | Phase I | NCT03099239 | Completed | [132] |
3.2.3. Placenta-Derived MSCs (P-MSCs)
3.2.4. Amniotic Fluid-Derived MSCs (AF-MSCs)
4. Culture Conditions-Dependent Heterogeneity
4.1. Culture Medium
4.2. O2 Tension
5. Human Induced Pluripotent Stem Cell (iPSC)-Derived MSCs (iMSCs)
6. Conclusions and Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Friedenstein, A.J.; Chailakhjan, R.K.; Lalykina, K.S. The development of fibroblast colonies in monolayer cultures of guinea-pig bone marrow and spleen cells. Cell Prolif. 1970, 3, 393–403. [Google Scholar] [CrossRef]
- Caplan, A.I. Mesenchymal stem cells. J. Orthop. Res. 1991, 9, 641–650. [Google Scholar] [CrossRef] [PubMed]
- Owen, M. Marrow stromal stem cells. J. Cell Sci. Suppl. 1988, 10, 63–76. [Google Scholar] [CrossRef] [PubMed]
- Horwitz, E.M.; Le Blanc, K.; Dominici, M.; Mueller, I.; Slaper-Cortenbach, I.; Marini, F.C.; Deans, R.J.; Krause, D.S.; Keating, A.; International Society for Cellular Therapy. Clarification of the nomenclature for MSC: The International Society for Cellular Therapy position statement. Cytotherapy 2005, 7, 393–395. [Google Scholar] [CrossRef] [PubMed]
- Bianco, P.; Cao, X.; Frenette, P.S.; Mao, J.J.; Robey, P.G.; Simmons, P.J.; Wang, C.-Y. The meaning, the sense and the significance: Translating the science of mesenchymal stem cells into medicine. Nat. Med. 2013, 19, 35–42. [Google Scholar] [CrossRef]
- Pittenger, M.F.; Mackay, A.M.; Beck, S.C.; Jaiswal, R.K.; Douglas, R.; Mosca, J.D.; Moorman, M.A.; Simonetti, D.W.; Craig, S.; Marshak, D.R. Multilineage Potential of Adult Human Mesenchymal Stem Cells. Science 1999, 284, 143–147. [Google Scholar] [CrossRef]
- Bianco, P. Bone and the hematopoietic niche: A tale of two stem cells. Blood 2011, 117, 5281–5288. [Google Scholar] [CrossRef]
- da Silva Meirelles, L.; Chagastelles, P.C.; Nardi, N.B. Mesenchymal stem cells reside in virtually all post-natal organs and tissues. J. Cell Sci. 2006, 119 Pt 11, 2204–2213. [Google Scholar] [CrossRef]
- Dominici, M.; Le Blanc, K.; Mueller, I.; Slaper-Cortenbach, I.; Marini, F.; Krause, D.; Deans, R.; Keating, A.; Prockop, D.; Horwitz, E. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy 2006, 8, 315–317. [Google Scholar] [CrossRef]
- Phinney, D.G.; Prockop, D.J. Concise Review: Mesenchymal Stem/Multipotent Stromal Cells: The State of Transdifferentiation and Modes of Tissue Repair—Current Views. Stem Cells 2007, 25, 2896–2902. [Google Scholar] [CrossRef]
- Le Blanc, K.; Rasmusson, I.; Sundberg, B.; Götherström, C.; Hassan, M.; Uzunel, M.; Ringdén, O. Treatment of severe acute graft-versus-host disease with third party haploidentical mesenchymal stem cells. Lancet 2004, 363, 1439–1441. [Google Scholar] [CrossRef]
- Bartholomew, A.; Sturgeon, C.; Siatskas, M.; Ferrer, K.; McIntosh, K.; Patil, S.; Hardy, W.; Devine, S.; Ucker, D.; Deans, R.; et al. Mesenchymal stem cells suppress lymphocyte proliferation in vitro and prolong skin graft survival in vivo. Exp. Hematol. 2002, 30, 42–48. [Google Scholar] [CrossRef] [PubMed]
- Di Nicola, M.; Carlo-Stella, C.; Magni, M.; Milanesi, M.; Longoni, P.D.; Matteucci, P.; Grisanti, S.; Gianni, A.M. Human bone marrow stromal cells suppress T-lymphocyte proliferation induced by cellular or nonspecific mitogenic stimuli. Blood 2002, 99, 3838–3843. [Google Scholar] [CrossRef]
- Jiang, X.-X.; Zhang, Y.; Liu, B.; Zhang, S.-X.; Wu, Y.; Yu, X.-D.; Mao, N. Human mesenchymal stem cells inhibit differentiation and function of monocyte-derived dendritic cells. Blood 2005, 105, 4120–4126. [Google Scholar] [CrossRef] [PubMed]
- Nauta, A.J.; Kruisselbrink, A.B.; Lurvink, E.; Willemze, R.; Fibbe, W.E. Mesenchymal Stem Cells Inhibit Generation and Function of Both CD34+-Derived and Monocyte-Derived Dendritic Cells. J. Immunol. 2006, 177, 2080–2087. [Google Scholar] [CrossRef] [PubMed]
- Spaggiari, G.M.; Capobianco, A.; Becchetti, S.; Mingari, M.C.; Moretta, L. Mesenchymal stem cell-natural killer cell interactions: Evidence that activated NK cells are capable of killing MSCs, whereas MSCs can inhibit IL-2-induced NK-cell proliferation. Blood 2006, 107, 1484–1490. [Google Scholar] [CrossRef]
- Espagnolle, N.; Balguerie, A.; Arnaud, E.; Sensebé, L.; Varin, A. CD54-Mediated Interaction with Pro-inflammatory Macrophages Increases the Immunosuppressive Function of Human Mesenchymal Stromal Cells. Stem Cell Rep. 2017, 8, 961–976. [Google Scholar] [CrossRef]
- Franquesa, M.; Mensah, F.K.; Huizinga, R.; Strini, T.; Boon, L.; Lombardo, E.; DelaRosa, O.; Laman, J.D.; Grinyó, J.M.; Weimar, W.; et al. Human Adipose Tissue-Derived Mesenchymal Stem Cells Abrogate Plasmablast Formation and Induce Regulatory B Cells Independently of T Helper Cells. Stem Cells 2015, 33, 880–891. [Google Scholar] [CrossRef] [PubMed]
- Asari, S.; Itakura, S.; Ferreri, K.; Liu, C.-P.; Kuroda, Y.; Kandeel, F.; Mullen, Y. Mesenchymal stem cells suppress B-cell terminal differentiation. Exp. Hematol. 2009, 37, 604–615. [Google Scholar] [CrossRef] [PubMed]
- Healy, M.E.; Bergin, R.; Mahon, B.P.; English, K. Mesenchymal Stromal Cells Protect Against Caspase 3-Mediated Apoptosis of CD19+Peripheral B Cells Through Contact-Dependent Upregulation of VEGF. Stem Cells Dev. 2015, 24, 2391–2402. [Google Scholar] [CrossRef]
- Heldman, A.W.; DiFede, D.L.; Fishman, J.E.; Zambrano, J.P.; Trachtenberg, B.H.; Karantalis, V.; Mushtaq, M.; Williams, A.R.; Suncion, V.Y.; McNiece, I.K.; et al. Transendocardial Mesenchymal Stem Cells and Mononuclear Bone Marrow Cells for Ischemic Cardiomyopathy: The TAC-HFT Randomized Trial. JAMA 2014, 311, 62–73. [Google Scholar] [CrossRef] [PubMed]
- Fukumitsu, M.; Suzuki, K. Mesenchymal stem/stromal cell therapy for pulmonary arterial hypertension: Comprehensive review of preclinical studies. J. Cardiol. 2019, 74, 304–312. [Google Scholar] [CrossRef]
- Chen, S.-L.; Fang, W.-W.; Ye, F.; Liu, Y.-H.; Qian, J.; Shan, S.-J.; Zhang, J.-J.; Chunhua, R.Z.; Liao, L.-M.; Lin, S.; et al. Effect on left ventricular function of intracoronary transplantation of autologous bone marrow mesenchymal stem cell in patients with acute myocardial infarction. Am. J. Cardiol. 2004, 94, 92–95. [Google Scholar] [CrossRef] [PubMed]
- Garikipati, V.N.S.; Jadhav, S.; Pal, L.; Prakash, P.; Dikshit, M.; Nityanand, S. Mesenchymal Stem Cells from Fetal Heart Attenuate Myocardial Injury after Infarction: An In Vivo Serial Pinhole Gated SPECT-CT Study in Rats. PLoS ONE 2014, 9, e100982. [Google Scholar]
- Chiossone, L.; Conte, R.; Spaggiari, G.M.; Serra, M.; Romei, C.; Bellora, F.; Becchetti, F.; Andaloro, A.; Moretta, L.; Bottino, C. Mesenchymal Stromal Cells Induce Peculiar Alternatively Activated Macrophages Capable of Dampening Both Innate and Adaptive Immune Responses. Stem Cells 2016, 34, 1909–1921. [Google Scholar] [CrossRef]
- Dong, L.-H.; Jiang, Y.-Y.; Liu, Y.-J.; Cui, S.; Xia, C.-C.; Qu, C.; Jiang, X.; Qu, Y.-Q.; Chang, P.-Y.; Liu, F. The anti-fibrotic effects of mesenchymal stem cells on irradiated lungs via stimulating endogenous secretion of HGF and PGE2. Sci. Rep. 2015, 5, 8713. [Google Scholar] [CrossRef]
- Gomes, S.A.; Rangel, E.B.; Premer, C.; Dulce, R.A.; Cao, Y.; Florea, V.; Balkan, W.; Rodrigues, C.O.; Schally, A.V.; Hare, J.M. S-nitrosoglutathione reductase (GSNOR) enhances vasculogenesis by mesenchymal stem cells. Proc. Natl. Acad. Sci. USA 2013, 110, 2834–2839. [Google Scholar] [CrossRef]
- Phinney, D.G.; Kopen, G.; Righter, W.; Webster, S.; Tremain, N.; Prockop, D.J. Donor variation in the growth properties and osteogenic potential of human marrow stromal cells. J. Cell. Biochem. 1999, 75, 424–436. [Google Scholar] [CrossRef]
- Wagner, W.; Ho, A.D. Mesenchymal Stem Cell Preparations—Comparing Apples and Oranges. Stem Cell Rev. Rep. 2007, 3, 239–248. [Google Scholar] [CrossRef]
- Kanawa, M.; Igarashi, A.; Ronald, V.S.; Higashi, Y.; Kurihara, H.; Sugiyama, M.; Saskianti, T.; Pan, H.; Kato, Y. Age-dependent decrease in the chondrogenic potential of human bone marrow mesenchymal stromal cells expanded with fibroblast growth factor-2. Cytotherapy 2013, 15, 1062–1072. [Google Scholar] [CrossRef]
- Zaim, M.; Karaman, S.; Cetin, G.; Isik, S. Donor age and long-term culture affect differentiation and proliferation of human bone marrow mesenchymal stem cells. Ann. Hematol. 2012, 91, 1175–1186. [Google Scholar] [CrossRef] [PubMed]
- Siegel, G.; Kluba, T.; Hermanutz-Klein, U.; Bieback, K.; Northoff, H.; Schäfer, R. Phenotype, donor age and gender affect function of human bone marrow-derived mesenchymal stromal cells. BMC Med. 2013, 11, 146. [Google Scholar] [CrossRef] [PubMed]
- Alves, H.; van Ginkel, J.; Groen, N.; Hulsman, M.; Mentink, A.; Reinders, M.; van Blitterswijk, C.; de Boer, J. A Mesenchymal Stromal Cell Gene Signature for Donor Age. PLoS ONE 2012, 7, e42908. [Google Scholar] [CrossRef]
- Mareschi, K.; Ferrero, I.; Rustichelli, D.; Aschero, S.; Gammaitoni, L.; Aglietta, M.; Madon, E.; Fagioli, F. Expansion of mesenchymal stem cells isolated from pediatric and adult donor bone marrow. J. Cell. Biochem. 2006, 97, 744–754. [Google Scholar] [CrossRef] [PubMed]
- Psaroudis, R.T.; Singh, U.; Lora, M.; Jeon, P.; Boursiquot, A.; Stochaj, U.; Langlais, D.; Colmegna, I. CD26 is a senescence marker associated with reduced immunopotency of human adipose tissue-derived multipotent mesenchymal stromal cells. Stem Cell Res. Ther. 2022, 13, 358. [Google Scholar] [CrossRef]
- de Oliveira, G.L.V.; de Lima, K.W.A.; Colombini, A.M.; Pinheiro, D.G.; Panepucci, R.A.; Palma, P.V.B.; Brum, D.G.; Covas, D.T.; Simões, B.P.; de Oliveira, M.C.; et al. Bone marrow mesenchymal stromal cells isolated from multiple sclerosis patients have distinct gene expression profile and decreased suppressive function compared with healthy counterparts. Cell Transplant. 2015, 24, 151–165. [Google Scholar] [CrossRef]
- Ferrer, R.A.; Wobus, M.; List, C.; Wehner, R.; Schönefeldt, C.; Brocard, B.; Mohr, B.; Rauner, M.; Schmitz, M.; Stiehler, M.; et al. Mesenchymal stromal cells from patients with myelodyplastic syndrome display distinct functional alterations that are modulated by lenalidomide. Haematologica 2013, 98, 1677–1685. [Google Scholar] [CrossRef]
- Pachón-Peña, G.; Serena, C.; Ejarque, M.; Petriz, J.; Duran, X.; Oliva-Olivera, W.; Simó, R.; Tinahones, F.J.; Fernández-Veledo, S.; Vendrell, J. Obesity Determines the Immunophenotypic Profile and Functional Characteristics of Human Mesenchymal Stem Cells From Adipose Tissue. Stem Cells Transl. Med. 2016, 5, 464–475. [Google Scholar] [CrossRef]
- Wu, C.-L.; Diekman, B.O.; Jain, D.; Guilak, F. Diet-induced obesity alters the differentiation potential of stem cells isolated from bone marrow, adipose tissue and infrapatellar fat pad: The effects of free fatty acids. Int. J. Obes. 2012, 37, 1079–1087. [Google Scholar]
- Rodríguez, J.P.; Garat, S.; Gajardo, H.; Pino, A.M.; Seitz, G. Abnormal osteogenesis in osteoporotic patients is reflected by altered mesenchymal stem cells dynamics. J. Cell. Biochem. 1999, 75, 414–423. [Google Scholar] [CrossRef]
- Montecinos, L.; Reyes, P.; Rodríguez, J.P.; Ríos, S.; Martínez, J. Mesenchymal stem cells from osteoporotic patients produce a type I collagen-deficient extracellular matrix favoring adipogenic differentiation. J. Cell. Biochem. 2000, 79, 557–565. [Google Scholar]
- Selle, M.; Koch, J.D.; Ongsiek, A.; Ulbrich, L.; Ye, W.; Jiang, Z.; Krettek, C.; Neunaber, C.; Noack, S. Influence of age on stem cells depends on the sex of the bone marrow donor. J. Cell. Mol. Med. 2022, 26, 1594–1605. [Google Scholar] [CrossRef] [PubMed]
- Kannan, S.; Viswanathan, P.; Gupta, P.K.; Kolkundkar, U.K. Characteristics of Pooled Wharton’s Jelly Mesenchymal Stromal Cells (WJ-MSCs) and their Potential Role in Rheumatoid Arthritis Treatment. Stem Cell Rev. Rep. 2022, 18, 1851–1864. [Google Scholar] [CrossRef]
- Sammour, I.; Somashekar, S.; Huang, J.; Batlahally, S.; Breton, M.; Valasaki, K.; Khan, A.; Wu, S.; Young, K.C. The Effect of Gender on Mesenchymal Stem Cell (MSC) Efficacy in Neonatal Hyperoxia-Induced Lung Injury. PLoS ONE 2016, 11, e0164269. [Google Scholar] [CrossRef]
- Da Silva Meirelles, L.; Caplan, A.I.; Nardi, N.B. In Search of the In Vivo Identity of Mesenchymal Stem Cells. Stem Cells 2008, 26, 2287–2299. [Google Scholar] [CrossRef]
- Zhang, Z.-Y.; Teoh, S.-H.; Chong, M.S.; Schantz, J.T.; Fisk, N.M.; Choolani, M.A.; Chan, J. Superior Osteogenic Capacity for Bone Tissue Engineering of Fetal Compared with Perinatal and Adult Mesenchymal Stem Cells. Stem Cells 2009, 27, 126–137. [Google Scholar] [CrossRef]
- Anker, P.S.I.; Noort, W.A.; Scherjon, S.A.; Der Keur, C.K.-V.; Kruisselbrink, A.B.; Van Bezooijen, R.L.; Beekhuizen, W.; Willemze, R.; Kanhai, H.H.H.; E Fibbe, W. Mesenchymal stem cells in human second-trimester bone marrow, liver, lung, and spleen exhibit a similar immunophenotype but a heterogeneous multilineage differentiation potential. Haematologica 2003, 88, 845–852. [Google Scholar]
- Guillot, P.V.; De Bari, C.; Dell’Accio, F.; Kurata, H.; Polak, J.; Fisk, N.M. Comparative osteogenic transcription profiling of various fetal and adult mesenchymal stem cell sources. Differentiation 2008, 76, 946–957. [Google Scholar] [CrossRef] [PubMed]
- Heo, J.S.; Choi, Y.; Kim, H.-S.; Kim, H.O. Comparison of molecular profiles of human mesenchymal stem cells derived from bone marrow, umbilical cord blood, placenta and adipose tissue. Int. J. Mol. Med. 2015, 37, 115–125. [Google Scholar] [CrossRef]
- Noël, D.; Caton, D.; Roche, S.; Bony, C.; Lehmann, S.; Casteilla, L.; Jorgensen, C.; Cousin, B. Cell specific differences between human adipose-derived and mesenchymal–stromal cells despite similar differentiation potentials. Exp. Cell Res. 2008, 314, 1575–1584. [Google Scholar] [CrossRef]
- Sacchetti, B.; Funari, A.; Remoli, C.; Giannicola, G.; Kogler, G.; Liedtke, S.; Cossu, G.; Serafini, M.; Sampaolesi, M.; Tagliafico, E.; et al. No Identical “Mesenchymal Stem Cells” at Different Times and Sites: Human Committed Progenitors of Distinct Origin and Differentiation Potential Are Incorporated as Adventitial Cells in Microvessels. Stem Cell Rep. 2016, 6, 897–913. [Google Scholar] [CrossRef]
- Hochmann, S.; Ou, K.; Poupardin, R.; Mittermeir, M.; Textor, M.; Ali, S.; Wolf, M.; Ellinghaus, A.; Jacobi, D.; Elmiger, J.A.J.; et al. The enhancer landscape predetermines the skeletal regeneration capacity of stromal cells. Sci. Transl. Med. 2023, 15, eabm7477. [Google Scholar] [CrossRef] [PubMed]
- Bae, H.C.; Park, H.J.; Wang, S.Y.; Yang, H.R.; Lee, M.C.; Han, H.-S. Hypoxic condition enhances chondrogenesis in synovium-derived mesenchymal stem cells. Biomater. Res. 2018, 22, 28. [Google Scholar] [CrossRef]
- Zarychta-Wiśniewska, W.; Burdzińska, A.; Zielniok, K.; Koblowska, M.; Gala, K.; Pędzisz, P.; Nowicka, R.I.; Fogtman, A.; Aksamit, A.; Kulesza, A.; et al. The Influence of Cell Source and Donor Age on the Tenogenic Potential and Chemokine Secretion of Human Mesenchymal Stromal Cells. Stem Cells Int. 2019, 2019, 1613701. [Google Scholar] [CrossRef]
- Herrmann, M.; Hildebrand, M.; Menzel, U.; Fahy, N.; Alini, M.; Lang, S.; Benneker, L.; Verrier, S.; Stoddart, M.J.; Bara, J.J. Phenotypic Characterization of Bone Marrow Mononuclear Cells and Derived Stromal Cell Populations from Human Iliac Crest, Vertebral Body and Femoral Head. Int. J. Mol. Sci. 2019, 20, 3454. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Bai, J.; Ji, X.; Li, R.; Xuan, Y.; Wang, Y. Comprehensive characterization of four different populations of human mesenchymal stem cells as regards their immune properties, proliferation and differentiation. Int. J. Mol. Med. 2014, 34, 695–704. [Google Scholar] [CrossRef] [PubMed]
- Kern, S.; Eichler, H.; Stoeve, J.; Klüter, H.; Bieback, K. Comparative Analysis of Mesenchymal Stem Cells from Bone Marrow, Umbilical Cord Blood, or Adipose Tissue. Stem Cells 2006, 24, 1294–1301. [Google Scholar] [CrossRef]
- Almeida, A.; Lira, R.; Oliveira, M.; Martins, M.; Azevedo, Y.; Silva, K.R.; Carvalho, S.; Cortez, E.; Stumbo, A.C.; Carvalho, L.; et al. Bone marrow-derived mesenchymal stem cells transplantation ameliorates renal injury through anti-fibrotic and anti-inflammatory effects in chronic experimental renovascular disease. Biomed. J. 2021, 45, 629–641. [Google Scholar] [CrossRef]
- Li, C.Y.; Wu, X.-Y.; Tong, J.-B.; Yang, X.-X.; Zhao, J.-L.; Zheng, Q.-F.; Zhao, G.-B.; Ma, Z.-J. Comparative analysis of human mesenchymal stem cells from bone marrow and adipose tissue under xeno-free conditions for cell therapy. Stem Cell Res. Ther. 2015, 6, 55. [Google Scholar] [CrossRef]
- Bolli, R.; Hare, J.M.; Henry, T.D.; Lenneman, C.G.; March, K.L.; Miller, K.; Pepine, C.J.; Perin, E.C.; Traverse, J.H.; Willerson, J.T.; et al. Rationale and Design of the SENECA (StEm cell iNjECtion in cAncer survivors) Trial. Am. Heart J. 2018, 201, 54–62. [Google Scholar] [CrossRef]
- Gnecchi, M.; He, H.; Liang, O.D.; Melo, L.G.; Morello, F.; Mu, H.; Noiseux, N.; Zhang, L.; Pratt, R.E.; Ingwall, J.S.; et al. Paracrine action accounts for marked protection of ischemic heart by Akt-modified mesenchymal stem cells. Nat. Med. 2005, 11, 367–368. [Google Scholar] [CrossRef]
- Petrou, P.; Kassis, I.; Levin, N.; Paul, F.; Backner, Y.; Benoliel, T.; Oertel, F.C.; Scheel, M.; Hallimi, M.; Yaghmour, N.; et al. Beneficial effects of autologous mesenchymal stem cell transplantation in active progressive multiple sclerosis. Brain 2020, 143, 3574–3588. [Google Scholar] [CrossRef] [PubMed]
- Uccelli, A.; on behalf of the MESEMS study group; Laroni, A.; Brundin, L.; Clanet, M.; Fernandez, O.; Nabavi, S.M.; Muraro, P.A.; Oliveri, R.S.; Radue, E.W.; et al. MEsenchymal StEm cells for Multiple Sclerosis (MESEMS): A randomized, double blind, cross-over phase I/II clinical trial with autologous mesenchymal stem cells for the therapy of multiple sclerosis. Trials 2019, 20, 263. [Google Scholar] [CrossRef]
- Thebault, S.; Reaume, M.; Marrie, R.A.; Marriott, J.J.; Furlan, R.; Laroni, A.; A Booth, R.; Uccelli, A.; Freedman, M.S. High or increasing serum NfL is predictive of impending multiple sclerosis relapses. Mult. Scler. Relat. Disord. 2022, 59, 103535. [Google Scholar] [CrossRef] [PubMed]
- Kurtzberg, J.; Abdel-Azim, H.; Carpenter, P.; Chaudhury, S.; Horn, B.; Mahadeo, K.; Nemecek, E.; Neudorf, S.; Prasad, V.; Prockop, S.; et al. A Phase 3, Single-Arm, Prospective Study of Remestemcel-L, Ex Vivo Culture-Expanded Adult Human Mesenchymal Stromal Cells for the Treatment of Pediatric Patients Who Failed to Respond to Steroid Treatment for Acute Graft-versus-Host Disease. Biol. Blood Marrow Transplant. 2020, 26, 845–854. [Google Scholar] [CrossRef]
- Zuk, P.A.; Zhu, M.I.; Mizuno, H.; Huang, J.; Futrell, J.W.; Katz, A.J.; Benhaim, P.; Lorenz, H.P.; Hedrick, M.H. Multilineage Cells from Human Adipose Tissue: Implications for Cell-Based Therapies. Tissue Eng. 2001, 7, 211–228. [Google Scholar] [CrossRef]
- Kuhbier, J.W.; Weyand, B.; Radtke, C.; Vogt, P.M.; Kasper, C.; Reimers, K. Isolation, Characterization, Differentiation, and Application of Adipose-Derived Stem Cells. Adv. Biochem. Eng. Biotechnol. 2010, 123, 55–105. [Google Scholar]
- Bae, Y.K.; Kwon, J.H.; Kim, M.; Kim, G.-H.; Choi, S.J.; Oh, W.; Yang, Y.S.; Jin, H.J.; Jeon, H.B. Intracellular Calcium Determines the Adipogenic Differentiation Potential of Human Umbilical Cord Blood-Derived Mesenchymal Stem Cells via the Wnt5a/β-Catenin Signaling Pathway. Stem Cells Int. 2018, 2018, 6545071. [Google Scholar] [CrossRef]
- Fraser, J.K.; Wulur, I.; Alfonso, Z.; Hedrick, M.H. Adipose-Derived Stem Cells. In Mesenchymal Stem Cells: Methods and Protocols; Prockop, D.J., Bunnell, B.A., Phinney, D.G., Eds.; Humana Press: Totowa, NJ, USA, 2008; pp. 59–67. [Google Scholar]
- Choudhery, M.S.; Badowski, M.; Muise, A.; Pierce, J.; Harris, D.T. Donor age negatively impacts adipose tissue-derived mesenchymal stem cell expansion and differentiation. J. Transl. Med. 2014, 12, 8. [Google Scholar] [CrossRef] [PubMed]
- Rehman, J.; Traktuev, D.; Li, J.; Merfeld-Clauss, S.; Temm-Grove, C.J.; Bovenkerk, J.E.; Pell, C.L.; Johnstone, B.H.; Considine, R.V.; March, K.L. Secretion of Angiogenic and Antiapoptotic Factors by Human Adipose Stromal Cells. Circulation 2004, 109, 1292–1298. [Google Scholar] [CrossRef]
- Ivanova-Todorova, E.; Bochev, I.; Mourdjeva, M.; Dimitrov, R.; Bukarev, D.; Kyurkchiev, S.; Tivchev, P.; Altunkova, I.; Kyurkchiev, D.S. Adipose tissue-derived mesenchymal stem cells are more potent suppressors of dendritic cells differentiation compared to bone marrow-derived mesenchymal stem cells. Immunol. Lett. 2009, 126, 37–42. [Google Scholar] [CrossRef]
- Zhou, M.; Xi, J.; Cheng, Y.; Sun, D.; Shu, P.; Chi, S.; Tian, S.; Ye, S. Reprogrammed mesenchymal stem cells derived from iPSCs promote bone repair in steroid-associated osteonecrosis of the femoral head. Stem Cell Res. Ther. 2021, 12, 175. [Google Scholar] [CrossRef] [PubMed]
- Kotze, P.G.; Spinelli, A.; Warusavitarne, J.; Di Candido, F.; Sahnan, K.; Adegbola, S.O.; Danese, S. Darvadstrocel for the treatment of patients with perianal fistulas in Crohn’s disease. Drugs Today 2019, 55, 95–105. [Google Scholar] [CrossRef] [PubMed]
- García-Olmo, D.; García-Arranz, M.; García, L.G.; Cuellar, E.S.; Blanco, I.F.; Prianes, L.A.; Montes, J.A.R.; Pinto, F.L.; Marcos, D.H.; García-Sancho, L. Autologous stem cell transplantation for treatment of rectovaginal fistula in perianal Crohn’s disease: A new cell-based therapy. Int. J. Colorectal Dis. 2003, 18, 451–454. [Google Scholar] [CrossRef]
- Lee, W.Y.; Park, K.J.; Cho, Y.B.; Yoon, S.N.; Song, K.H.; Kim, D.S.; Jung, S.H.; Kim, M.; Yoo, H.-W.; Kim, I. Autologous adipose tissue-derived stem cells treatment demonstrated favorable and sustainable therapeutic effect for Crohn’s fistula. Stem Cells 2013, 31, 2575–2581. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Olmo, D.; Herreros, D.; Pascual, I.; Pascual, J.A.; Del-Valle, E.; Zorrilla, J.; De-La-Quintana, P.; Garcia-Arranz, M.; Pascual, M. Expanded adipose-derived stem cells for the treatment of complex perianal fistula: A phase II clinical trial. Dis. Colon. Rectum. 2009, 52, 79–86. [Google Scholar] [CrossRef]
- Panés, J.; García-Olmo, D.; Van Assche, G.; Colombel, J.F.; Reinisch, W.; Baumgart, D.C.; Dignass, A.; Nachury, M.; Ferrante, M.; Kazemi-Shirazi, L.; et al. Expanded allogeneic adipose-derived mesenchymal stem cells (Cx601) for complex perianal fistulas in Crohn’s disease: A phase 3 randomised, double-blind controlled trial. Lancet 2016, 388, 1281–1290. [Google Scholar] [CrossRef]
- Danisovic, L.; Oravcova, L.; Krajciova, L.; Novakova, Z.V.; Bohac, M.; Varga, I.; Vojtassak, J. Effect of long-term culture on the biological and morphological characteristics of human adipose tissue-derived stem Cells. J. Physiol. Pharmacol. 2017, 68, 149–158. [Google Scholar]
- Lotfy, A.; Salama, M.; Zahran, F.; Jones, E.; Badawy, A.; Sobh, M. Characterization of Mesenchymal Stem Cells Derived from Rat Bone Marrow and Adipose Tissue: A Comparative Study. Int. J. Stem Cells 2014, 7, 135–142. [Google Scholar] [CrossRef]
- Vidal, M.A.; Walker, N.J.; Napoli, E.; Borjesson, D.L.; Lange-Consiglio, A.; Romaldini, A.; Correani, A.; Corradetti, B.; Esposti, P.; Cannatà, M.F.; et al. Evaluation of Senescence in Mesenchymal Stem Cells Isolated from Equine Bone Marrow, Adipose Tissue, and Umbilical Cord Tissue. Stem Cells Dev. 2012, 21, 273–283. [Google Scholar] [CrossRef]
- Prianishnikov, V.A. On the concept of stem cell and a model of functional-morphological structure of the endometrium. Contraception 1978, 18, 213–223. [Google Scholar] [CrossRef] [PubMed]
- Wolff, E.F.; Wolff, A.B.; Du, H.; Taylor, H.S. Demonstration of Multipotent Stem Cells in the Adult Human Endometrium by In Vitro Chondrogenesis. Reprod. Sci. 2007, 14, 524–533. [Google Scholar] [CrossRef] [PubMed]
- Kazemnejad, S.; Zarnani, A.-H.; Khanmohammadi, M.; Mobini, S. Chondrogenic Differentiation of Menstrual Blood-Derived Stem Cells on Nanofibrous Scaffolds. Methods Mol. Biol. 2013, 1058, 149–169. [Google Scholar] [PubMed]
- Edwards, S.L.; Werkmeister, J.A.; Rosamilia, A.; Ramshaw, J.A.; White, J.F.; Gargett, C.E. Characterisation of clinical and newly fabricated meshes for pelvic organ prolapse repair. J. Mech. Behav. Biomed. Mater. 2013, 23, 53–61. [Google Scholar] [CrossRef]
- Zhou, C.; Yang, B.; Tian, Y.; Jiao, H.; Zheng, W.; Wang, J.; Guan, F. Immunomodulatory effect of human umbilical cord Wharton’s jelly-derived mesenchymal stem cells on lymphocytes. Cell. Immunol. 2011, 272, 33–38. [Google Scholar] [CrossRef]
- Xu, Y.; Hu, J.; Lv, Q.; Shi, C.; Qiu, M.; Xie, L.; Liu, W.; Yang, B.; Shan, W.; Cheng, Y.; et al. Endometrium-derived mesenchymal stem cells suppress progression of endometrial cancer via the DKK1-Wnt/β-catenin signaling pathway. Stem Cell Res. Ther. 2023, 14, 159. [Google Scholar] [CrossRef]
- Bozorgmehr, M.; Moazzeni, S.M.; Salehnia, M.; Sheikhian, A.; Nikoo, S.; Zarnani, A.-H. Menstrual blood-derived stromal stem cells inhibit optimal generation and maturation of human monocyte-derived dendritic cells. Immunol. Lett. 2014, 162 Pt B, 239–246. [Google Scholar] [CrossRef]
- De Bari, C.; Dell, F.; Tylzanowski, P.; Luyten, F.P. Multipotent mesenchymal stem cells from adult human synovial membrane. Arthritis. Rheum. 2001, 44, 1928–1942. [Google Scholar] [CrossRef]
- Fülber, J.; Maria, D.A.; da Silva, L.C.L.C.; Massoco, C.O.; Agreste, F.; Baccarin, R.Y.A. Comparative study of equine mesenchymal stem cells from healthy and injured synovial tissues: An in vitro assessment. Stem Cell Res. Ther. 2016, 7, 35. [Google Scholar] [CrossRef]
- Sekiya, I.; Katano, H.; Ozeki, N. Characteristics of MSCs in Synovial Fluid and Mode of Action of Intra-Articular Injections of Synovial MSCs in Knee Osteoarthritis. Int. J. Mol. Sci. 2021, 22, 2838. [Google Scholar] [CrossRef]
- Paradiso, F.; Lenna, S.; Isbell, R.; Garza, M.F.G.; Williams, M.; Varner, C.; Mcculloch, P.; Taraballi, F. Immunosuppressive potential evaluation of synovial fluid mesenchymal stem cells grown on 3D scaffolds as an alternative source of MSCs for osteoarthritis cartilage studies. Front. Biomater. Sci. 2022, 1, 989708. [Google Scholar] [CrossRef]
- Davatchi, F.; Abdollahi, B.S.; Mohyeddin, M.; Nikbin, B. Mesenchymal stem cell therapy for knee osteoarthritis: 5 years follow-up of three patients. Int. J. Rheum. Dis. 2015, 19, 219–225. [Google Scholar] [CrossRef] [PubMed]
- Orozco, L.; Munar, A.; Soler, R.; Alberca, M.; Soler, F.; Huguet, M.; Sentís, J.; Sánchez, A.; García-Sancho, J. Treatment of knee osteoarthritis with autologous mesenchymal stem cells: A pilot study. Transplantation 2013, 95, 1535–1541. [Google Scholar] [CrossRef] [PubMed]
- Davatchi, F.; Abdollahi, B.S.; Mohyeddin, M.; Shahram, F.; Nikbin, B. Mesenchymal stem cell therapy for knee osteoarthritis. Preliminary report of four patients. Int. J. Rheum. Dis. 2011, 14, 211–215. [Google Scholar] [CrossRef] [PubMed]
- Soler, R.; Orozco, L.; Munar, A.; Huguet, M.; López, R.; Vives, J.; Coll, R.; Codinach, M.; Garcia-Lopez, J. Final results of a phase I–II trial using ex vivo expanded autologous Mesenchymal Stromal Cells for the treatment of osteoarthritis of the knee confirming safety and suggesting cartilage regeneration. Knee 2016, 23, 647–654. [Google Scholar] [CrossRef]
- Arthur, A.; Rychkov, G.; Shi, S.; Koblar, S.A.; Gronthos, S. Adult Human Dental Pulp Stem Cells Differentiate Toward Functionally Active Neurons Under Appropriate Environmental Cues. Stem Cells 2008, 26, 1787–1795. [Google Scholar] [CrossRef]
- Pierdomenico, L.; Bonsi, L.; Calvitti, M.; Rondelli, D.; Arpinati, M.; Chirumbolo, G.; Becchetti, E.; Marchionni, C.; Alviano, F.; Fossati, V.; et al. Multipotent Mesenchymal Stem Cells with Immunosuppressive Activity Can Be Easily Isolated from Dental Pulp. Transplantation 2005, 80, 836–842. [Google Scholar] [CrossRef]
- Wada, N.; Menicanin, D.; Shi, S.; Bartold, P.M.; Gronthos, S. Immunomodulatory properties of human periodontal ligament stem cells. J. Cell. Physiol. 2009, 219, 667–676. [Google Scholar] [CrossRef]
- Huang, A.H.-C.; Snyder, B.R.; Cheng, P.-H.; Chan, A.W. Putative Dental Pulp-Derived Stem/Stromal Cells Promote Proliferation and Differentiation of Endogenous Neural Cells in the Hippocampus of Mice. Stem Cells 2008, 26, 2654–2663. [Google Scholar] [CrossRef]
- Kwack, K.H.; Lee, J.M.; Park, S.H.; Lee, H.W. Human Dental Pulp Stem Cells Suppress Alloantigen-induced Immunity by Stimulating T Cells to Release Transforming Growth Factor Beta. J. Endod. 2016, 43, 100–108. [Google Scholar] [CrossRef]
- Pisciotta, A.; Riccio, M.; Carnevale, G.; Lu, A.; De Biasi, S.; Gibellini, L.; La Sala, G.B.; Bruzzesi, G.; Ferrari, A.; Huard, J.; et al. Stem cells isolated from human dental pulp and amniotic fluid improve skeletal muscle histopathology in mdx/SCID mice. Stem Cell Res. Ther. 2015, 6, 156. [Google Scholar] [CrossRef] [PubMed]
- Rubinstein, P.; Rosenfield, R.E.; Adamson, J.W.; Stevens, C.E. Stored placental blood for unrelated bone marrow reconstitution. Blood 1993, 81, 1679–1690. [Google Scholar] [CrossRef] [PubMed]
- Mareschi, K.; Biasin, E.; Piacibello, W.; Aglietta, M.; Madon, E.; Fagioli, F. Isolation of human mesenchymal stem cells: Bone marrow versus umbilical cord blood. Haematologica 2001, 86, 1099–1100. [Google Scholar] [PubMed]
- Wexler, S.A.; Donaldson, C.; Denning-Kendall, P.; Rice, C.; Bradley, B.; Hows, J.M. Adult bone marrow is a rich source of human mesenchymal ‘stem’ cells but umbilical cord and mobilized adult blood are not. Br. J. Haematol. 2003, 121, 368–374. [Google Scholar] [CrossRef]
- Bieback, K.; Netsch, P. Isolation, Culture, and Characterization of Human Umbilical Cord Blood-Derived Mesenchymal Stromal Cells. Mesenchymal Stem Cells Methods Protoc. 2016, 1416, 245–258. [Google Scholar]
- Erices, A.; Conget, P.; Minguell, J.J. Mesenchymal progenitor cells in human umbilical cord blood. Br. J. Haematol. 2000, 109, 235–242. [Google Scholar] [CrossRef]
- Goodwin, H.; Bicknese, A.; Chien, S.-N.; Bogucki, B.; Oliver, D.; Quinn, C.; Wall, D. Multilineage differentiation activity by cells isolated from umbilical cord blood: Expression of bone, fat, and neural markers. Biol. Blood Marrow Transplant. 2001, 7, 581–588. [Google Scholar]
- Hass, R.; Kasper, C.; Böhm, S.; Jacobs, R. Different populations and sources of human mesenchymal stem cells (MSC): A comparison of adult and neonatal tissue-derived MSC. Cell Commun. Signal. 2011, 9, 12. [Google Scholar]
- Karagianni, M.; Brinkmann, I.; Kinzebach, S.; Grassl, M.; Weiss, C.; Bugert, P.; Bieback, K. A comparative analysis of the adipogenic potential in human mesenchymal stromal cells from cord blood and other sources. Cytotherapy 2013, 15, 76–88.e2. [Google Scholar] [CrossRef]
- da Silva, C.; Durandt, C.; Kallmeyer, K.; Ambele, M.A.; Pepper, M.S. The Role of Pref-1 during Adipogenic Differentiation: An Overview of Suggested Mechanisms. Int. J. Mol. Sci. 2020, 21, 4104. [Google Scholar]
- Liedtke, S.; Buchheiser, A.; Bosch, J.; Bosse, F.; Kruse, F.; Zhao, X.; Santourlidis, S.; Kögler, G. The HOX Code as a “biological fingerprint” to distinguish functionally distinct stem cell populations derived from cord blood. Stem Cell Res. 2010, 5, 40–50. [Google Scholar] [CrossRef]
- Liedtke, S.; Sacchetti, B.; Laitinen, A.; Donsante, S.; Klöckers, R.; Laitinen, S.; Riminucci, M.; Kogler, G. Low oxygen tension reveals distinct HOX codes in human cord blood-derived stromal cells associated with specific endochondral ossification capacities in vitro and in vivo. J. Tissue Eng. Regen. Med. 2017, 11, 2725–2736. [Google Scholar] [CrossRef] [PubMed]
- Krumlauf, R. Hox genes in vertebrate development. Cell 1994, 78, 191–201. [Google Scholar] [CrossRef] [PubMed]
- Duboule, D. The vertebrate limb: A model system to study theHox/hom gene network during development and evolution. Bioessays 1992, 14, 375–384. [Google Scholar]
- Ackema, K.B.; Charité, J.; Laitinen, A.; Lampinen, M.; Liedtke, S.; Kilpinen, L.; Kerkelä, E.; Sarkanen, J.-R.; Heinonen, T.; Kogler, G.; et al. Mesenchymal Stem Cells from Different Organs are Characterized by Distinct Topographic Hox Codes. Stem Cells Dev. 2008, 17, 979–992. [Google Scholar] [CrossRef] [PubMed]
- Kluth, S.M.; Buchheiser, A.; Houben, A.P.; Geyh, S.; Krenz, T.; Radke, T.F.; Wiek, C.; Hanenberg, H.; Reinecke, P.; Wernet, P.; et al. DLK-1 as a Marker to Distinguish Unrestricted Somatic Stem Cells and Mesenchymal Stromal Cells in Cord Blood. Stem Cells Dev. 2010, 19, 1471–1483. [Google Scholar] [CrossRef]
- Andreas Reinisch; Etchart, N.; Thomas, D.; Hofmann, N.A.; Fruehwirth, M.; Sinha, S.; Chan, C.K.; Senarath-Yapa, K.; Seo, E.-Y.; Wearda, T.; et al. Epigenetic and in vivo comparison of diverse MSC sources reveals an endochondral signature for human hematopoietic niche formation. Blood 2015, 125, 249–260. [Google Scholar]
- Amable, P.R.; Teixeira, M.V.T.; Carias, R.B.V.; Granjeiro, J.M.; Borojevic, R. Protein synthesis and secretion in human mesenchymal cells derived from bone marrow, adipose tissue and Wharton’s jelly. Stem Cell Res. Ther. 2014, 5, 53. [Google Scholar] [CrossRef]
- Majore, I.; Moretti, P.; Hass, R.; Kasper, C. Identification of subpopulations in mesenchymal stem cell-like cultures from human umbilical cord. Cell Commun. Signal. 2009, 7, 6. [Google Scholar] [CrossRef]
- Bosch, J.; Houben, A.P.; Radke, T.F.; Stapelkamp, D.; Bünemann, E.; Balan, P.; Buchheiser, A.; Liedtke, S.; Kögler, G. Distinct differentiation potential of “MSC” derived from cord blood and umbilical cord: Are cord-derived cells true mesenchymal stromal cells? Stem Cells Dev. 2012, 21, 1977–1988. [Google Scholar] [CrossRef]
- Li, F.; Zhang, K.; Liu, H.; Yang, T.; Xiao, D.-J.; Wang, Y.-S. The neuroprotective effect of mesenchymal stem cells is mediated through inhibition of apoptosis in hypoxic ischemic injury. World J. Pediatr. 2019, 16, 193–200. [Google Scholar] [CrossRef] [PubMed]
- Benny, M.; Courchia, B.; Shrager, S.; Sharma, M.; Chen, P.; Duara, J.; Valasaki, K.; Bellio, M.A.; Damianos, A.; Huang, J.; et al. Comparative Effects of Bone Marrow-derived Versus Umbilical Cord Tissue Mesenchymal Stem Cells in an Experimental Model of Bronchopulmonary Dysplasia. Stem Cells Transl. Med. 2022, 11, 189–199. [Google Scholar] [CrossRef] [PubMed]
- Min, H.; Xu, L.; Parrott, R.; Kurtzberg, J.; Filiano, A. Abstract 4 Umbilical Cord-Derived Mesenchymal Stromal Cells Suppress Neuroinflammation and Promote Remyelination in the Spinal Cord. Stem Cells Transl. Med. 2022, 11 (Suppl. S1), S6. [Google Scholar] [CrossRef]
- Petriv, T.; Tatarchuk, M.; Tsymbaliyk, Y.; Rybachuk, O.; Tsymbaliuk, Y.; Tsymbaliuk, V. Abstract 14 Umbilical Cord Mesenchymal Stromal/Stem Cells Application for Spasticity Treatment in Multiple Sclerosis. Stem Cells Transl. Med. 2022, 11 (Suppl. S1), S16. [Google Scholar] [CrossRef]
- Xu, L.; Saha, A.; Parrott, R.; O’neil, S.; Kurtzberg, J.; Filiano, A. Abstract 5 Human Umbilical Cord Blood-Derived Cell Therapy Product, DUOC-01, Promotes Remyelination by Driving the Differentiation of Oligodendrocyte Progenitor Cells. Stem Cells Transl. Med. 2022, 11 (Suppl. S1), S7. [Google Scholar] [CrossRef]
- Wehbe, T.; Saab, M.A.; Chahine, N.A.; Margossian, T. Mesenchymal stem cell therapy for refractory scleroderma: A report of 2 cases. Stem Cell Investig. 2016, 3, 48. [Google Scholar] [CrossRef]
- Ahn, S.Y.; Chang, Y.S.; Sung, S.I.; Park, W.S. Mesenchymal Stem Cells for Severe Intraventricular Hemorrhage in Preterm Infants: Phase I Dose-Escalation Clinical Trial. Stem Cells Transl. Med. 2018, 7, 847–856. [Google Scholar] [CrossRef]
- Ahn, S.Y.; Chang, Y.S.; Kim, J.H.; Sung, S.I.; Park, W.S. Two-Year Follow-Up Outcomes of Premature Infants Enrolled in the Phase I Trial of Mesenchymal Stem Cells Transplantation for Bronchopulmonary Dysplasia. J. Pediatr. 2017, 185, 49–54.e2. [Google Scholar] [CrossRef]
- Cotten, C.M.; Fisher, K.; Malcolm, W.; Gustafson, K.E.; Cheatham, L.; Marion, A.; Greenberg, R.; Kurtzberg, J. A Pilot Phase I Trial of Allogeneic Umbilical Cord Tissue-Derived Mesenchymal Stromal Cells in Neonates With Hypoxic-Ischemic Encephalopathy. Stem Cells Transl. Med. 2023, 12, 355–364. [Google Scholar] [CrossRef]
- Ahn, S.Y.; Chang, Y.S.; Lee, M.H.; Sung, S.I.; Lee, B.S.; Kim, K.S.; Kim, A.-R.; Park, W.S. Stem Cells for Bronchopulmonary Dysplasia in Preterm Infants: A Randomized Controlled Phase II Trial. Stem Cells Transl. Med. 2021, 10, 1129–1137. [Google Scholar] [CrossRef]
- Sun, J.M.; Dawson, G.; Franz, L.; Howard, J.; McLaughlin, C.; Kistler, B.; Waters-Pick, B.; Meadows, N.; Troy, J.; Kurtzberg, J. Infusion of human umbilical cord tissue mesenchymal stromal cells in children with autism spectrum disorder. Stem Cells Transl. Med. 2020, 9, 1137–1146. [Google Scholar] [CrossRef]
- Wu, M.; Zhang, R.; Zou, Q.; Chen, Y.; Zhou, M.; Li, X.; Ran, R.; Chen, Q. Comparison of the Biological Characteristics of Mesenchymal Stem Cells Derived from the Human Placenta and Umbilical Cord. Sci. Rep. 2018, 8, 5014. [Google Scholar] [CrossRef] [PubMed]
- Campagnoli, C.; Roberts, I.A.G.; Kumar, S.; Bennett, P.R.; Bellantuono, I.; Fisk, N.M. Identification of mesenchymal stem/progenitor cells in human first-trimester fetal blood, liver, and bone marrow. Blood 2001, 98, 2396–2402. [Google Scholar] [CrossRef] [PubMed]
- Spitzhorn, L.-S.; Rahman, S.; Schwindt, L.; Ho, H.-T.; Wruck, W.; Bohndorf, M.; Wehrmeyer, S.; Ncube, A.; Beyer, I.; Hagenbeck, C.; et al. Isolation and Molecular Characterization of Amniotic Fluid-Derived Mesenchymal Stem Cells Obtained from Caesarean Sections. Stem Cells Int. 2017, 2017, 5932706. [Google Scholar] [CrossRef]
- You, Q.; Tong, X.; Guan, Y.; Zhang, D.; Huang, M.; Zhang, Y.; Zheng, J. The Biological Characteristics of Human Third Trimester Amniotic Fluid Stem Cells. J. Int. Med. Res. 2009, 37, 105–112. [Google Scholar] [CrossRef]
- Tsai, M.; Lee, J.; Chang, Y.; Hwang, S. Isolation of human multipotent mesenchymal stem cells from second-trimester amniotic fluid using a novel two-stage culture protocol. Hum. Reprod. 2004, 19, 1450–1456. [Google Scholar] [CrossRef] [PubMed]
- Babaie, Y.; Herwig, R.; Greber, B.; Brink, T.C.; Wruck, W.; Groth, D.; Lehrach, H.; Burdon, T.; Adjaye, J. Analysis of Oct4-Dependent Transcriptional Networks Regulating Self-Renewal and Pluripotency in Human Embryonic Stem Cells. Stem Cells 2006, 25, 500–510. [Google Scholar] [CrossRef]
- Mirabella, T.; Gentili, C.; Daga, A.; Cancedda, R. Amniotic fluid stem cells in a bone microenvironment: Driving host angiogenic response. Stem Cell Res. 2013, 11, 540–551. [Google Scholar]
- Costa, L.A.; Eiro, N.; Fraile, M.; Gonzalez, L.O.; Saá, J.; Garcia-Portabella, P.; Vega, B.; Schneider, J.; Vizoso, F.J. Functional heterogeneity of mesenchymal stem cells from natural niches to culture conditions: Implications for further clinical uses. Cell. Mol. Life Sci. 2021, 78, 447–467. [Google Scholar]
- Yang, Y.-H.K.; Ogando, C.R.; See, C.W.; Chang, T.-Y.; Barabino, G.A. Changes in phenotype and differentiation potential of human mesenchymal stem cells aging in vitro. Stem Cell Res. Ther. 2018, 9, 131. [Google Scholar] [CrossRef]
- Hung, S.-P.; Ho, J.H.; Shih, Y.-R.V.; Lo, T.; Lee, O.K. Hypoxia promotes proliferation and osteogenic differentiation potentials of human mesenchymal stem cells. J. Orthop. Res. 2011, 30, 260–266. [Google Scholar] [CrossRef]
- Choi, J.R.; Pingguan-Murphy, B.; Abas, W.A.B.W.; Azmi, M.A.N.; Omar, S.Z.; Chua, K.H.; Safwani, W.K.Z.W. Impact of low oxygen tension on stemness, proliferation and differentiation potential of human adipose-derived stem cells. Biochem. Biophys. Res. Commun. 2014, 448, 218–224. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, Y.; Fujita, M.; Tanaka, Y.; Kojima, I.; Kanatani, Y.; Ishihara, M.; Tachibana, S. Low Oxygen Tension Enhances Proliferation and Maintains Stemness of Adipose Tissue–Derived Stromal Cells. BioRes. Open Access 2013, 2, 199–205. [Google Scholar] [CrossRef] [PubMed]
- Werle, S.B.; Chagastelles, P.; Pranke, P.; Casagrande, L. The effects of hypoxia on in vitro culture of dental-derived stem cells. Arch. Oral Biol. 2016, 68, 13–20. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.R.; Yong, K.W.; Safwani, W.K.Z.W. Effect of hypoxia on human adipose-derived mesenchymal stem cells and its potential clinical applications. Cell. Mol. Life Sci. 2017, 74, 2587–2600. [Google Scholar] [CrossRef]
- Gnecchi, M.; He, H.; Noiseux, N.; Liang, O.D.; Zhang, L.; Morello, F.; Mu, H.; Melo, L.G.; Pratt, R.E.; Ingwall, J.S.; et al. Evidence supporting paracrine hypothesis for Akt-modified mesenchymal stem cell-mediated cardiac protection and functional improvement. FASEB J. 2006, 20, 661–669. [Google Scholar] [CrossRef]
- Hu, X.; Yu, S.P.; Fraser, J.L.; Lu, Z.; Ogle, M.E.; Wang, J.-A.; Wei, L. Transplantation of hypoxia-preconditioned mesenchymal stem cells improves infarcted heart function via enhanced survival of implanted cells and angiogenesis. J. Thorac. Cardiovasc. Surg. 2008, 135, 799–808. [Google Scholar] [CrossRef]
- Frobel, J.; Hemeda, H.; Lenz, M.; Abagnale, G.; Joussen, S.; Denecke, B.; Šarić, T.; Zenke, M.; Wagner, W. Epigenetic Rejuvenation of Mesenchymal Stromal Cells Derived from Induced Pluripotent Stem Cells. Stem Cell Rep. 2014, 3, 414–422. [Google Scholar] [CrossRef]
- de Peppo, G.M.; IMarcos-Campos, Á.; Kahler, D.J.; Alsalman, D.; Shang, L.; Vunjak-Novakovic, G.; Marolt, D. Engineering bone tissue substitutes from human induced pluripotent stem cells. Proc. Natl. Acad. Sci. USA 2013, 110, 8680–8685. [Google Scholar] [CrossRef]
- Sheyn, D.; Ben-David, S.; Shapiro, G.; De Mel, S.; Bez, M.; Ornelas, L.; Sahabian, A.; Sareen, D.; Da, X.; Pelled, G.; et al. Human Induced Pluripotent Stem Cells Differentiate Into Functional Mesenchymal Stem Cells and Repair Bone Defects. Stem Cells Transl. Med. 2016, 5, 1447–1460. [Google Scholar] [CrossRef]
- Jungbluth, P.; Spitzhorn, L.-S.; Grassmann, J.; Tanner, S.; Latz, D.; Rahman, M.S.; Bohndorf, M.; Wruck, W.; Sager, M.; Grotheer, V.; et al. Human iPSC-derived iMSCs improve bone regeneration in mini-pigs. Bone Res. 2019, 7, 32. [Google Scholar] [CrossRef] [PubMed]
- Kamiya, D.; Takenaka-Ninagawa, N.; Motoike, S.; Kajiya, M.; Akaboshi, T.; Zhao, C.; Shibata, M.; Senda, S.; Toyooka, Y.; Sakurai, H.; et al. Induction of functional xeno-free MSCs from human iPSCs via a neural crest cell lineage. NPJ Regen. Med. 2022, 7, 47. [Google Scholar] [CrossRef]
- Yang, H.; Feng, R.; Fu, Q.; Xu, S.; Hao, X.; Qiu, Y.; Feng, T.; Zeng, Z.; Chen, M.; Zhang, S. Human induced pluripotent stem cell-derived mesenchymal stem cells promote healing via TNF-α-stimulated gene-6 in inflammatory bowel disease models. Cell Death Dis. 2019, 10, 718. [Google Scholar] [CrossRef] [PubMed]
- Eto, S.; Goto, M.; Soga, M.; Kaneko, Y.; Uehara, Y.; Mizuta, H.; Era, T. Mesenchymal stem cells derived from human iPS cells via mesoderm and neuroepithelium have different features and therapeutic potentials. PLoS ONE 2018, 13, e0200790. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Liao, J.; Fang, C.; Mo, C.; Zhou, G.; Luo, Y. One-step Derivation of Functional Mesenchymal Stem Cells from Human Pluripotent Stem Cells. Bio-Protoc. 2018, 8, e3080. [Google Scholar] [CrossRef]
- Ozay, E.I.; Vijayaraghavan, J.; Gonzalez-Perez, G.; Shanthalingam, S.; Sherman, H.L.; Garrigan, D.T., Jr.; Chandiran, K.; Torres, J.A.; Osborne, B.A.; Tew, G.N.; et al. Cymerus™ iPSC-MSCs significantly prolong survival in a pre-clinical, humanized mouse model of Graft-vs-host disease. Stem Cell Res. 2019, 35, 101401. [Google Scholar] [CrossRef]
- Bloor, A.J.C.; Patel, A.; Griffin, J.E.; Gilleece, M.H.; Radia, R.; Yeung, D.T.; Drier, D.; Larson, L.S.; Uenishi, G.I.; Hei, D.; et al. Production, safety and efficacy of iPSC-derived mesenchymal stromal cells in acute steroid-resistant graft versus host disease: A phase I, multicenter, open-label, dose-escalation study. Nat. Med. 2020, 26, 1720–1725. [Google Scholar]
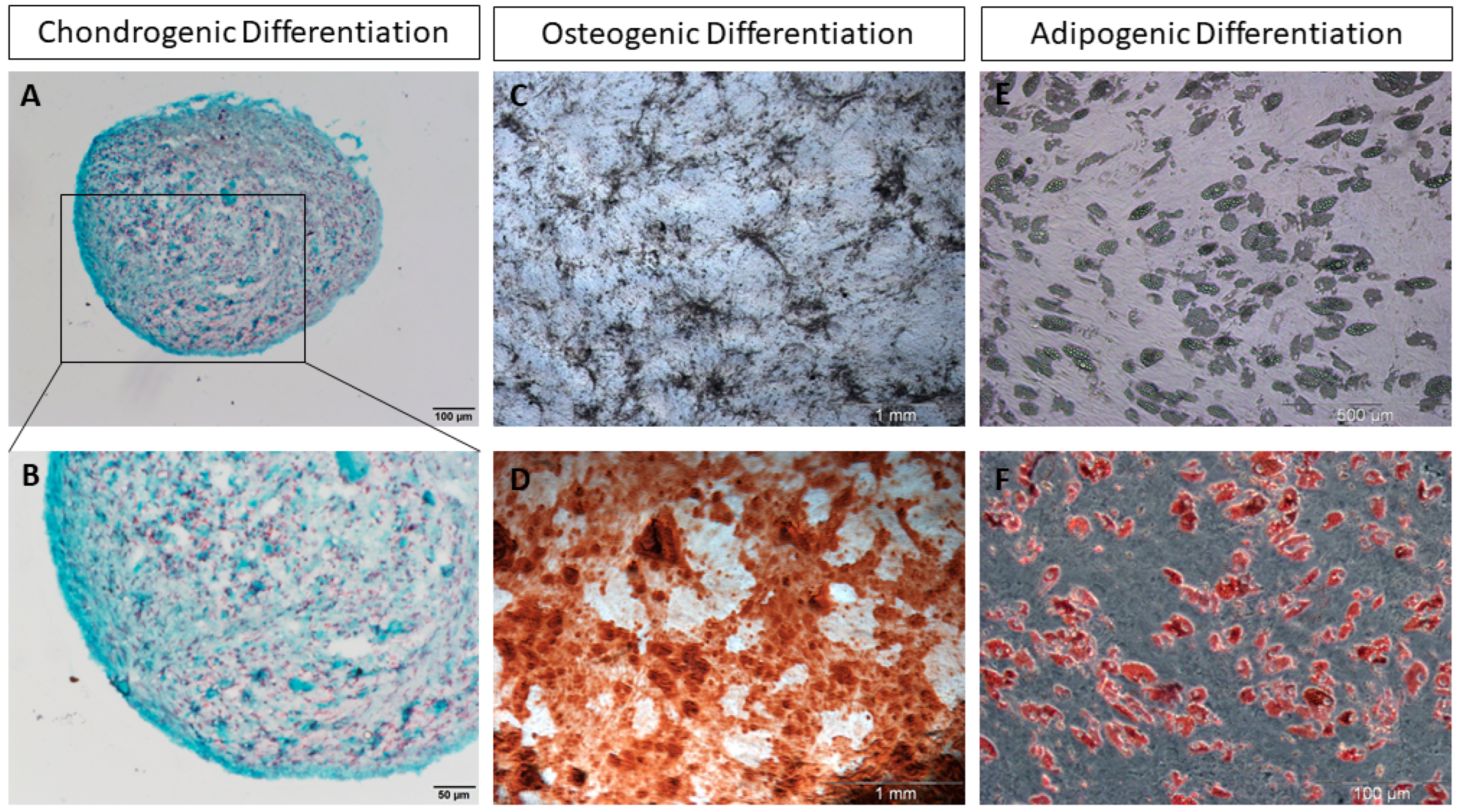
| Positive Markers | Physiological Function |
| CD44 | Hyaluronic receptor, surface adhesion, migration |
| CD73 | Lymphocyte-vascular adhesion protein 2 (Ecto-5’-nucleotidase) |
| CD90 | Cell adhesion, migration, apoptosis, fibrosis, T cell activation |
| CD105 | Activation and proliferation of endothelial cells |
| CD106 | Vascular cell adhesion molecule-1 (VCAM-1) |
| CD146 | Melanoma cell adhesion molecule (MCAM) |
| Negative Markers | Physiological Function |
| CD11b | Integrin αM subunit, NK Cells, neutrophils, monocytes, macrophages |
| CD14 | Lipopolysaccharide receptor, macrophages, monocytes |
| CD19 | B cell lymphocytes |
| CD34 | Adhesion molecule, hematopoietic stem cell |
| CD45 | B cell lymphocyte receptor complex |
| CD79 | B cell lymphocyte and B cell neoplasms |
| HLA-DR | MHC class II cell surface receptor |
| MSC Source | Isolation Technique |
|---|---|
| Bone marrow | Density gradient centrifugation, Ficoll gradient or red blood cell lysis of bone marrow aspirate |
| Adipose tissue | Enzymatic or non-enzymatic digestion after liposuction or lipectomy |
| Endometrium | Enzymatic digestion after scraping the myometrium of hysterectomy samples |
| Synovial membrane | Enzymatic digestion of synovium harvested from the inner joint side |
| Dental tissue | Extirpation of dental pulps after decoronation |
| Cord blood | Direct expansion |
| Umbilical cord | Enzymatic digestion or direct expansion of umbilical cord tissue |
| Wharton’s jelly | Vein removal, scraping and enzymatic digestion |
| Placenta | Enzymatic digestion |
| Amniotic fluid | Amniotic membrane perforation and tubing for fluid collection followed by density gradient centrifugation |
| Condition | MSC | Phase | Clinical Trial | Status | References |
|---|---|---|---|---|---|
| Multiple Sclerosis | Autologous BM-MSC | Phase II | NCT02166021 | Completed | [62] |
| Autologous BM-MSC | Phase II | NCT02239393 | Completed | [63,64] | |
| Post-traumatic Pulp Necrosis | Allogeneic BM-MSC | Phase II/III | NCT04545307 | Completed | - |
| Anthracycline-induced cardiomyopathy | Allogeneic BM-MSC | Phase I | NCT02509156 | Completed | [60] |
| SR-aGvHD | BM-MSC | Phase III | NCT02336230 | Completed | [65] |
| Liver Cirrhosis | Autologous BM-MSC | Phase III | NCT05080465 | Completed | - |
| Covid-19 Infection | Allogeneic BM-MSC | Phase I | NCT04397796 | Active, not recruiting | - |
| Chronic Myocardial Ischemia | Autologous BM-MSC | Phase II | NCT02462330 | Completed | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ouzin, M.; Kogler, G. Mesenchymal Stromal Cells: Heterogeneity and Therapeutical Applications. Cells 2023, 12, 2039. https://doi.org/10.3390/cells12162039
Ouzin M, Kogler G. Mesenchymal Stromal Cells: Heterogeneity and Therapeutical Applications. Cells. 2023; 12(16):2039. https://doi.org/10.3390/cells12162039
Chicago/Turabian StyleOuzin, Meryem, and Gesine Kogler. 2023. "Mesenchymal Stromal Cells: Heterogeneity and Therapeutical Applications" Cells 12, no. 16: 2039. https://doi.org/10.3390/cells12162039
APA StyleOuzin, M., & Kogler, G. (2023). Mesenchymal Stromal Cells: Heterogeneity and Therapeutical Applications. Cells, 12(16), 2039. https://doi.org/10.3390/cells12162039






