- Article
Antibodies Against SARS-CoV-2 Nucleocapsid Protein Possess Autoimmune Properties
- Alexandra Rak,
- Yana Zabrodskaya and
- Pei-Fong Wong
- + 1 author
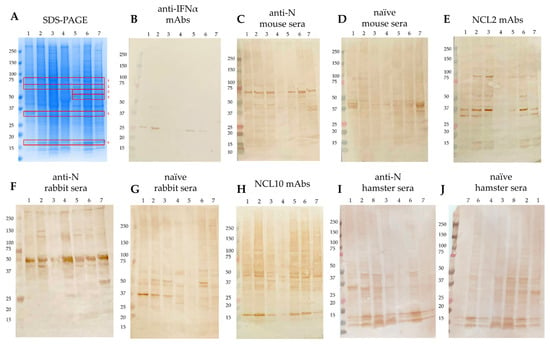
Background/Objectives: Notwithstanding the declaration by the World Health Organization in May 2023 regarding the conclusion of the COVID-19 pandemic, new cases of this potentially lethal infection continue to be documented globally, exerting a sustained influence on the worldwide economy and social structures. Contemporary SARS-CoV-2 variants, while associated with a reduced propensity for severe acute pathology, retain the capacity to induce long-term post-COVID syndrome, including in ambulatory patient populations. This clinical phenomenon may be attributable to potential autoimmune reactions hypothetically triggered by antiviral antibodies, thereby underscoring the need for developing novel, universal vaccines against COVID-19. The nucleocapsid protein (N), being one of its most conserved and highly immunogenic components of SARS-CoV-2, presents a promising target for such investigative efforts. However, the protective role of anti-N antibodies, generated during natural infection or through immunization with N-based vaccines, alongside the potential adverse effects associated with their production, remains to be fully elucidated. In the present study, we aim to identify potential sites of homology in structures or sequences between the SARS-CoV-2 N protein and human antigens detected using hyperimmune sera against N protein obtained from mice, rabbits, and hamsters. Methods: We employed Western blot analysis of lysates from human cell lines (MCF7, HEK293T, THP-1, CaCo2, Hep2, T98G, A549) coupled with mass spectrometric identification to assess the cross-reactivity of polyclonal and monoclonal antibodies generated against recombinant SARS-CoV-2 N protein with human self-antigens. Results: We showed that anti-N antibodies developed in mice and rabbits exhibit pronounced immunoreactivity towards specific components of the human proteome. In contrast, anti-N immunoglobulins from hamsters showed no non-specific cross-reactivity with either hamster or human proteomic extracts because of the lack of autoreactivity or immunogenicity differences. Subsequent mass spectrometric analysis of the immunoreactive bands identified principal autoantigenic targets, which were predominantly heat shock proteins (including HSP90-beta, HSP70, mitochondrial HSP60, and HSPA8), histones (H2B, H3.1–3), and key metabolic enzymes (G6PD, GP3, PKM, members of the 1st family of aldo-keto reductases). Conclusions: The results obtained herein highlight the differences in the development of anti-N humoral responses in humans and in the Syrian hamster model. These data provide a foundational basis for formulating clinical recommendations to predict possible autoimmune consequences in COVID-19 convalescents and are of critical importance for the rational design of future N protein-based, cross-protective vaccine candidates against novel coronavirus infections.
22 December 2025