- Article
Role of ACTN3 R577X Polymorphism in Mitochondrial Myokines After Endurance Exercise
- Leticia Aparecida da Silva Manoel,
- Antônio Alves de Fontes-Júnior and
- Maria Fernanda Cury-Boaventura
- + 7 authors
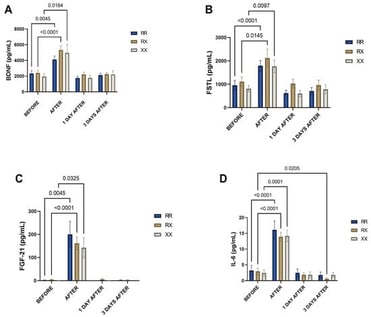
Objective: Resistance exercise can induce muscle damage that impairs sports performance and cellular repair. Myokines, particularly mitochondrial myokines, play an important role in regulating energy metabolism and muscle recovery. The ACTN3 R577X polymorphism, which alters the expression of α-actinin-3 in muscle fibers, may influence myokine responses by modulating exercise adaptation and recovery. Methods: Seventy-five amateur runners (30–55 years) from the São Paulo International Marathon were evaluated. Plasma levels of mitochondrial myokines (BDNF, FGF-21, FSTL, IL-6, apelin, IL-15, musclin, and myostatin) were measured before and after the race and correlated with ACTN3 R577X genotypes. Results: In this study, the genotypic frequencies of the ACTN3 R577X polymorphism were 36% (RR), 39% (RX), and 14% (XX). Plasma concentrations of BDNF, FSTL, FGF-21, and IL-6 increased immediately after running across all genotypes, with no significant differences observed between genotypes. In contrast, plasma levels of myostatin, musclin, IL-15, and apelin decreased during the recovery period only among runners carrying the R allele. Conclusions: Mitochondrial myokine responses to resistance exercise were not substantially different among genotypes of the ACTN3 R577X polymorphism. However, myokines associated with protein breakdown and bioenergetic adaptation were reduced during the recovery period in runners carrying the R allele, which may impact muscle repair and bioenergetic adaptation.
26 January 2026