Abstract
Traditionally, referred to as the “Powerhouse of the Eukaryotic Cell”, mitochondria are essential for host defense in addition to producing ATP. Through processes like mitochondrial antiviral signaling (MAVS), the generation of reactive oxygen species (ROS), and the modification of inflammatory pathways, they respond to bacterial, fungal, viral, and parasitic infections while coordinating immune signaling, controlling cell death, and detecting pathogens. Pathogens, on the other hand, have developed ways to interfere with or harm mitochondrial function, which results in oxidative stress, cell death, altered metabolism, and compromised immune signaling. This type of mitochondrial dysfunction impairs the removal of infections and is linked to tissue damage, chronic inflammation, and long-term health issues. The dual roles of mitochondria in infection are highlighted in this review, which looks at both their defense mechanisms and the ways in which pathogens use them to increase their chances of survival.
1. Introduction
Mitochondria are key organelles taking part in one of the most important roles in energy production [1]. These organelles are found in all eukaryotic cells and are responsible for generating energy through oxidative phosphorylation, regulation of calcium levels, as well as activation of apoptosis and cell death. They also contribute to the synthesis of specific lipids such as cardiolipin and phosphatidylethanolamine, as well as heme; however the bulk of phospholipid biosynthesis takes place in other organelles like the endoplasmic reticulum [2]. For instance, recent research has shown that mitochondria and peroxisomes interact functionally through vesicular exchange, metabolite shuttling, membrane contact sites and shared redox management. In fact, peroxisomes may be able to affect immune signaling thresholds, membrane lipid environments, and mitochondrial redox status as a result of this crosstalk [2]. Moreover, mitochondria act as signaling platforms during stress and infections where they help coordinate the cell’s response to stress whether internal or external (including infections). This role activates the immune defenses and hence determines the cell’s fate [3]. During infections, mitochondria shape the immune response through generating Reactive Oxygen Species (ROS) that aids in eliminating pathogens, activating Pattern Recognition Receptors (PRRs) that are essential for detecting microbial components, serving as platforms for Mitochondrial Antiviral Signaling (MAVS) that are responsible for triggering interferon responses to RNA viruses, and regulating cell death pathways (including apoptosis-programmed cell death) which assists in limiting pathogen spread [4]. Peroxisomes frequently support these immune responses by contributing to ROS production and antiviral defense, which reflects coordinated organelle crosstalk during infection. It has been demonstrated that peroxisomal MAVS, in particular, triggers a quick, interferon-independent antiviral response that enhances the slower, interferon-dependent signaling from mitochondrial MAVS, thereby guaranteeing a strong and prompt innate immune defense [5].
As for their genome, mitochondria possess their own genome which reflects bacterial evolutionary origin. The endosymbiotic theory, which postulates that mitochondria evolved from a symbiotic relationship between an ancestral alpha-proteobacterium and a primitive eukaryotic cell, is strongly supported by mitochondrial DNA (mtDNA). Because one cell lived inside another and eventually developed into a permanent organelle, this is known as endosymbiosis [6]. Furthermore, the fact that mitochondria have their own circular DNA and can proliferate despite the aid of the host cell, resembling bacterial genomes in both structure and sequence, lends credence to this theory [7]. Although most of the mitochondrial proteins are encoded by nuclear genes, a small number of respiratory proteins and mitochondrial tRNAs are still encoded by the mitochondrial genome [8]. Mitochondrial biogenesis necessitates the coordination of gene expression from both nuclear and mitochondrial sources and that’s in order to guarantee the proper assembly and functionality of the wide set of proteins that make up the mitochondrial respiratory chain [9]. Although small amounts of proteins are encoded by the mtDNA, deficiencies or mutations in this type of DNA lead to a variety of diseases. Such diseases do not adhere to Mendelian inheritance patterns; rather, they are passed down through maternal lineage only and exhibit varying degrees of severity in their expression [10]. This reflects the heteroplasmy present within the mtDNA population, indicating that both wild-type and mutant mtDNA coexist. These diseases frequently result in pathological alterations within tissues that are significantly reliant on optimal mitochondrial performance, especially in oxidative phosphorylation, and have a restricted capacity to boost glycolysis as a compensatory response, which suggests a direct correlation between efficient energy generation and mitochondrial functionality [11].
Other than mtDNA mutations or deficiencies, mitochondrial dysfunction can be induced by several mechanisms. In fact, a variety of pathogens have the capacity to disrupt mitochondrial function, thereby compromising host defenses, promoting their own replication, or triggering the death of host cells [12]. As previously mentioned, this dysfunction may present itself in several ways, including damage or degradation of mtDNA, inhibition of enzymes within the Electron Transport Chain (ETC), which results in reduced ATP production and an increase in ROS, direct targeting of mitochondrial membranes or signaling proteins, and alterations in mitochondrial dynamics, such as fission and fusion [13].
Importantly, different tissues have different mitochondrial responses. The dynamics, immune signaling ability, metabolic activity, and gene expression of mitochondria vary depending on the tissue. For example, immune cell mitochondria are specialized for rapid ROS production and cytokine signaling, lung mitochondria are especially sensitive to oxidative stress, and liver mitochondria have high metabolic flexibility. As a result, depending on the tissue, pathogen-induced mitochondrial dysfunction may present differently, resulting in a range of pathological outcomes and immune responses [14]. The significance of taking tissue-specific mitochondrial features into account when examining infection biology and host defense mechanisms has been emphasized more and more in recent studies [15].
This review explores how mitochondria can be disrupted during bacterial, viral, fungal, and parasitic infections, and how this disruption affects individuals, noting that responses can vary across tissues.
2. Mitochondria Structure and Dynamics
Mitochondria, aerobic and anaerobic, are regarded as one of the major characteristics of eukaryotic cells [16]. Mitochondria are composed of two separate membranes: the outer membrane (OM) and the inner membrane (IM). Additionally, they contain two functionally different compartments: the intermembrane space (IMS) and the matrix [17]. The OM is characterized by a smooth, lipid-rich surface that incorporates ancestral beta-barrel proteins, such as voltage-dependent anion-selective channels (VDACs) and porins, which allow the passage of molecules with a size of up to 10 kDa through the membrane [18]. The latter also has alpha-helical membrane proteins, mainly featuring a single transmembrane domain, including MFF and MIRO, which serve as receptors for the mitochondrial division dynamin and motility machinery, respectively. This highlights the significance of the OM in regulating mitochondrial activity and facilitating cellular communication [18]. As for the IMS, it is the most diminutive compartment, containing about 5% of the mitochondrial proteome. Nevertheless, the IMS plays a vital role as it enables various pathways for protein and lipid biogenesis, metal homeostasis, redox regulation, oxidative folding, apoptosis, and the dynamics and structure of mitochondria [19]. The IM is the most protein-rich membrane found in eukaryotic cells, exhibiting high impermeability and forming structures known as cristae. It is acknowledged as the functional nucleus of the organelle, containing the ETC, also referred to as the respiratory chain (RC), which includes respiratory enzyme complexes (RCs; in humans, complexes I–IV) and a turbine-like ATP synthase apparatus (RC V) that dimerizes to mold and facilitate the development of cristae [17]. The mitochondrial matrix contains a multitude of biosynthetic reactions along with the mitochondrial genetic system. It serves as the site for the tricarboxylic acid cycle (TCA), which oxidizes the carbon backbones of nutrients into NADH- and FADH2- reducing equivalents. These equivalents are utilized by the RCs to produce chemiosmotic energy for ATP synthesis, in addition to being involved in various other biosynthetic pathways. This compartmentalization is crucial for the proper functioning of both the organelle and the cell [19].
Regarding mitochondrial dynamics, there are two opposing processes (fission and fusion) that play a very important role in the maintenance of both mitochondrial and cellular homeostasis. Mitochondrial fission helps in the separation of damaged mitochondria, which are then eliminated through mitophagy—a selective type of autophagy that is crucial for maintaining quality control. However, excessive or uncontrolled fission can result in mitochondrial fragmentation, which may initiate apoptosis and play a role in several diseases including neurodegeneration and cancer [20]. In contrast, mitochondrial fusion facilitates the blending of mitochondrial contents, which aids in preserving the integrity of mtDNA, compensating for localized damage, and optimizing ATP production, thus improving cellular resilience in the face of metabolic and oxidative stress [21]. Hence, a fine balance between fission and fusion is extremely essential for mitochondrial function. Any disruption in these two processes can increase the risk of developing diseases such as Parkinson’s disease, Alzheimer’s disease, and cardiomyopathies [22,23,24].
These dynamic processes are controlled by certain proteins. Fusion is facilitated by mitofusin 1 (MFN1) and mitofusin 2 (MFN2) located on the outer mitochondrial membrane, as well as optic atrophy 1 (OPA1) found on the inner membrane. These GTPase proteins allow for membrane tethering and fusion, thereby enhancing mitochondrial networking, bioenergetic efficiency, and resistance to apoptosis [25,26]. On the contrary, fission is mainly driven by dynamin-related protein 1 (DRP1) which is a cytosolic GTPase that is recruited to the mitochondria with the help of adaptor proteins including FIS1, MFF, and MiD49/51. Once there, they all together form ring-like structures that constrict and separate the mitochondria [27,28]. The insurance of these proteins’ proper regulation indicates good mitochondrial quality control, distribution during cell division, as well as adaptation to metabolic demands. Unfortunately, any dysfunction in these proteins can be associated with a lot of disorders which may include metabolic diseases, neurodegeneration, and cancer [29].
Mitochondrial dynamics possess a significant responsiveness to pathogenic stress and are essential in host-pathogen interactions throughout infections. Many bacterial and viral pathogens intentionally alter mitochondrial fission and fusion processes to escape immune responses, influence host cell death, or establish advantageous intracellular conditions [30]. Upon infection, an increase in mitochondrial fission is usually noted. The latter is driven by the activation and recruitment of DRP1 which results in mitochondrial fragmentation. This process enhances mitophagy, reduces MAVS, and may hinder immune responses [31]. For example, viruses like hepatitis C virus (HCV) and influenza A trigger DRP1-mediated fission to suppress interferon production and enhance viral replication [32,33]. Conversely, certain pathogens such as Mycobacterium tuberculosis and Listeria monocytogenes can interfere with fusion machinery (MFN2 and OPA1), leading to mitochondrial stress and influencing apoptosis [34,35]. Such changes can be a cause of metabolic reprogramming of the immune cells, more importantly macrophages, which shifts them to altering cytokine production [36]. Therefore, the dynamic restructuring of mitochondria in response to infection is not only a result of stress; it is also a calculated approach employed by both the host and the pathogen to affect immune responses and determine cell fate.
3. Signaling in Homeostasis and Stress
Apart from generating energy, mitochondria act as key cellular stress sensors and responders, integrating into the cellular signaling networks that decide whether a cell will survive, repair, or undergo apoptosis. They are particularly well suited to respond to perturbations in a cell’s balance, including oxidative stress, DNA injuries, calcium overload, or even lack of nutrients, and respond accordingly [37]. One of the primary methods by which this occurs is through the generation of ROS during the process of oxidative phosphorylation. Moderate levels of ROS can trigger adaptive responses, while excess ROS can result in mitochondrial damage, depolarized membranes, and activation of stress pathways such as the mitochondrial UPR (mTOR) [38]. Additionally, mitochondria control the buffering of calcium within cells, and calcium dysregulation caused by stress may trigger the opening of the mitochondrial permeability transition pore (mPTP), which can result in necrosis or apoptosis [39]. Moreover, mitochondria host key components of innate immune signaling, including MAVS, which, upon viral detection, triggers type I interferon responses [40]. When mitochondria are subjected to prolonged or extreme stress, they release cytochrome c and other pro-apoptotic factors into the cytosol, which triggers caspase cascades and initiates apoptosis [41]. Hence, mitochondria can detect both internal and external stressors, while also coordinating downstream signaling pathways to either restore equilibrium or remove damaged cells.
The production of ROS, which are natural byproducts of the ETC during oxidative phosphorylation, is a key aspect of mitochondrial function under both normal and stressful conditions. Although electrons are transferred along ETC complexes I–IV to produce ATP, only a small amount of electrons leak, especially from complexes I and III, where they react with molecular oxygen to form superoxide anion (O2−), the main ROS in the mitochondria [42]. Mitochondrial superoxide dismutase (SOD2) quickly converts superoxide into hydrogen peroxide (H2O2), a comparatively stable molecule that can diffuse into the cytosol and function as a second messenger in redox signaling. ROS have significant signaling functions at the physiological level, influencing pathways related to inflammation, metabolism, and hypoxia adaptation by modulating transcription factors like NRF2, NF-κB, and HIF-1α [43]. However, oxidative stress results when ROS production surpasses the capacity of antioxidant systems (such as glutathione and peroxiredoxins), harming proteins, lipids, and DNA. DNA includes mtDNA, which is particularly susceptible because of its proximity to ETC. Additionally, oxidized mtDNA fragments can enter the cytosol and trigger innate immune sensors such as cGAS-STING, which connect inflammation and autoimmune reactions to mitochondrial dysfunction [44]. As a result, mitochondrial ROS and ETC byproducts possess a dual role necessary for redox signaling and stress adaptation, but when they are dysregulated, they may be cytotoxic.
By activating transcription factors involved in immune regulation and antioxidant defense, mitochondrial ROS play a key role in modulating cellular responses to stress at moderate levels. A major regulator of inflammation and innate immunity, NF-κB (nuclear factor kappa-light-chain-enhancer of activated B cells) is involved in one important pathway. By altering redox-sensitive cysteine residues on elements of the IκB kinase (IKK) complex, ROS can activate NF-κB. This causes IκBα, the NF-κB inhibitor, to become phosphorylated and degraded. As a result, NF-κB is able to move into the nucleus and stimulate the production of immune modulators, adhesion molecules, and pro-inflammatory cytokines like IL-6 and TNF-α [45]. At the same time, a unique redox-sensitive mechanism activates NRF2 (nuclear factor erythroid 2-related factor 2). KEAP1, a sensor protein that targets NRF2 for ubiquitination and degradation, sequesters NRF2 in the cytoplasm under homeostatic conditions. Critical cysteine residues on KEAP1 undergo oxidation when ROS levels rise, resulting in conformational changes that stop NRF2 degradation [46]. Stabilized NRF2 builds up and moves into the nucleus, where it attaches itself to antioxidant response elements (ARE) in DNA and triggers genes related to the production of glutathione, detoxification enzymes (like NQO1), and ROS scavenging systems [47]. ROS function as molecular switches that trigger both pro-inflammatory and cytoprotective responses via these parallel pathways, giving cells the ability to precisely control how they respond to stress and infection. Upon cellular stress, mitochondria participate actively in immune and death pathways in addition to producing reactive signals. The release of mtDNA into the cytosol or extracellular space is one important stress response. Because of its bacterial ancestry and unmethylated CpG motifs, oxidized mtDNA is known to be a DAMP. It triggers type I interferon responses, or TLR9, which promote inflammation, by activating innate immune pathways like the cGAS–STING axis [44,48].
The dual protective and pathological roles of ROS in infection are illustrated by recent studies showing that dysregulated mitochondrial ROS during viral infections like SARS-CoV-2 not only increase inflammatory signaling and tissue damage but, when appropriately controlled, can also improve antiviral defenses [49]. Additionally, it has been demonstrated that improving pathogen clearance through pharmacological or genetic modulation of mitochondrial ROS highlights the potential for therapeutic intervention [50].
At the same time, mitochondria are key players in controlling intrinsic apoptosis. Cytochrome c is released into the cytosol when pro-apoptotic BCL-2 family proteins (such as BAX and BAK) permeabilize the outer mitochondrial membrane in response to extreme or irreversible stress. The apoptosome is created when cytochrome c binds procaspase-9 and apoptotic protease-activating factor 1 (Apaf-1). This triggers caspase-9 and downstream executioner caspases, such as caspase-3, resulting in programmed cell death [41]. Calcium (Ca2+) and mitochondrial membrane potential (ΔΨm) homeostasis tightly control these death signals. Stress can cause the mPTP to open and depolarize, which indicates a loss of ΔΨm. Moreover, excessive Ca2+ influx into mitochondria, which is frequently brought on by oxidative damage or ER stress, can increase ROS production and encourage mPTP opening, which can result in cell death, rupture, and swelling [51]. Collectively, these incidents demonstrate the crucial function of mitochondria as integrators of stress signals, able to trigger apoptosis, metabolic adaptation, or immune activation based on the intensity of the insult.
4. MAVS and Anti-Viral Signaling
Through the action of MAVS protein, mitochondria serve as key sites for innate immune responses to viral infections. When viral RNA is detected, MAVS, a crucial adaptor protein found on the outer mitochondrial membrane, coordinates downstream signaling. Cytosolic PRRs like retinoic acid-inducible gene I (RIG-I) and melanoma differentiation-associated protein 5 (MDA5) pick up viral double-stranded or triphosphorylated RNA when an RNA virus infects a host. These sensors change their conformation and communicate with MAVS through CARD–CARD (caspase activation and recruitment domain) interactions [52]. On the mitochondrial surface, activated MAVS forms sizable prion-like aggregates that attract and activate downstream kinases such as IKKε and TBK1. Type I interferons (IFN-α/β) and pro-inflammatory cytokines are transcriptionally activated when these kinases phosphorylate the NF-κB pathway and interferon regulatory factors IRF3 and IRF7, causing their nuclear translocation [53]. By increasing interferon-stimulated genes (ISGs), improving viral clearance, and triggering the immune system, this starts an antiviral state in infected and nearby cells. Yet a lot of viruses have developed defenses against MAVS signaling (Figure 1). The NS3/4A protease, for instance, is expressed by the HCV and cleaves MAVS from the mitochondrial membrane, thereby silencing the pathway [54]. The Influenza A virus uses the NS1 protein to inhibit RIG-I activation and MAVS downstream signaling [55], whereas SARS-CoV-2 encodes multiple proteins (like ORF9b) that target MAVS or upstream sensors to reduce interferon production [56]. Recent research additionally demonstrates that SARS-CoV-2 and other emerging RNA viruses suppress MAVS, which leads to excessive ROS production and tissue damage in addition to viral persistence. This shows how mitochondrial antiviral signaling can have both protective and pathological effects [57,58]. Furthermore, it has been demonstrated that pharmacologically activating MAVS or stabilizing its mitochondrial aggregates improves viral clearance and interferon responses, highlighting its potential as a therapeutic target [59,60].
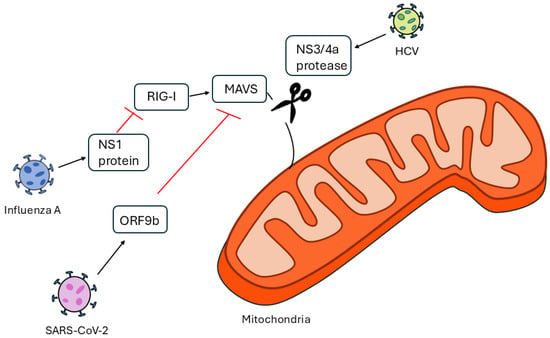
Figure 1.
Mechanisms of MAVS inhibition by HCV, Influenza A, and SARS-CoV-2.
Depending on the pathogen and tissue context, these mitochondrial changes have different functional effects. Changes in the mitochondria brought on by viruses can have both beneficial and harmful effects: Initially supporting antiviral defenses, MAVS and ROS signaling are frequently manipulated to support viral replication [61]. Although mild mitochondrial stress responses may momentarily activate protective signaling, the majority of bacterial effects are pathological, compromising host bioenergetics and impairing immune defenses [62]. Moreover, even if certain ROS-mediated reactions may momentarily strengthen host defense, parasitic mitochondrial effects are typically pathological, promoting tissue damage and aiding parasite survival [63]. Though restricted stress responses in some cells can support protective signaling, fungal-induced mitochondrial dysfunction is primarily pathological, impairing host energy metabolism and immune responses. Together, these distinctions highlight the importance of tissue-specific mitochondrial responses and their functional outcomes in shaping infection biology [64].
The evolutionary arms race at the mitochondrial interface, where MAVS is essential for identifying infection and starting host defense, is exemplified by these viral tactics.
5. Infection-Induced Mitochondrial Dysfunction
A common tactic used by a variety of pathogens to evade host defenses and increase their persistence is infection-induced mitochondrial dysfunction (Figure 2). By triggering fission, inhibiting oxidative phosphorylation, and encouraging mitophagy, viral pathogens like influenza A, hepatitis C virus, and SARS-CoV-2 alter mitochondrial dynamics [35]. This results in a reduction in MAVS-mediated antiviral signaling, an excess of ROS, and mtDNA leakage, which feeds inflammatory cascades [61]. Similarly, bacterial pathogens such as Helicobacter pylori, Salmonella enterica, and Listeria monocytogenes release effectors that interfere with host apoptosis pathways, fragment mitochondria, and alter membrane potential in order to improve intracellular survival [65]. Importantly, new research on viral infections has shown that the degree and type of these mitochondrial disruptions can vary among tissues and cell types [58], highlighting the importance of tissue-specific mitochondrial responses in determining infection outcomes. In addition to targeting mitochondria to alter host metabolism and immune signaling, parasitic infections like Toxoplasma gondii and Plasmodium falciparum also cause mitochondrial stress and ROS production, which in turn triggers the activation of the inflammasome [66]. As for fungal infections, to promote fungal invasion and elude immune responses, fungi like Candida albicans also cause oxidative stress and mitochondrial dysfunction in host cells. They also interfere with ATP synthesis and alter apoptosis [67]. Together, these changes impair host bioenergetics, cause DAMPs to be released, and influence immune responses, emphasizing mitochondria as a key player in infection-driven pathology [68]. An overview of the pathogen-specific mitochondrial alterations, along with their associated host outcomes, tissue/cell context, and functional outcomes, is presented in Table 1.
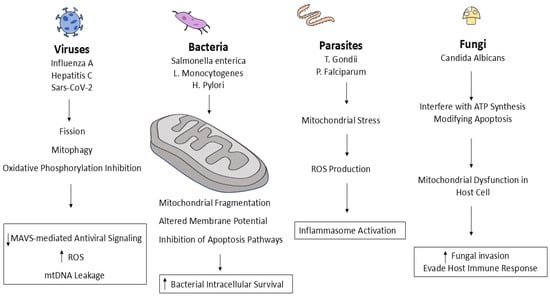
Figure 2.
Pathogen-induced mitochondrial dysfunction.

Table 1.
Infection induced mitochondrial dysfunction.
6. Consequences on Host Physiology
Mitochondrial disruption can harm the host physiology in many ways. One of those ways might be through reduced ATP synthesis. When the electron transport chain and the mitochondrial membrane are damaged, oxidative phosphorylation is weakened, which results in a lack of energy. Host defense and tissue repair are hampered by this lack of energy, which impacts cellular functions, especially in tissues with high energy demands like muscles, neurons, and immune cells [21].
Also, host physiology can be harmed through increased ROS production. Uncontrolled damage to mitochondria results in increased ROS production, even though moderate levels of ROS function as signaling molecules. Overproduction of ROS causes oxidative stress, which damages proteins, lipids, and DNA, exacerbating cellular damage and inflammation [40]. mtDNA leakage: Mitochondrial membrane permeabilization allows mtDNA to escape into the cytosol or extracellular space, where it acts as a DAMP. These triggers activate innate immune sensors such as cGAS–STING and TLR9, provoking type I interferon responses and inflammatory cascades that can amplify tissue damage [41]. Another method is known as Cytokine storms; Excessive DAMP release and uncontrolled mitochondrial signals can cause a “cytokine storm,” which is a severe form of systemic inflammation. Significant tissue damage, organ failure, and increased mortality rates are the outcomes of this hyperinflammatory state, which is seen in severe viral infections like COVID-19 and influenza [61]. Lastly, damage to the host can be achieved through chronic infection and mitochondrial fatigue. Immune cell metabolism and function are hampered by persistent mitochondrial stress and dysfunction, which is known as “mitochondrial exhaustion”. This disorder impairs the removal of pathogens, which leads to persistent infections and associated pathological conditions like cancer, neurodegeneration, or fibrosis [33].
7. Conclusions
As a conclusion, in addition to being essential cellular powerhouses, mitochondria also play a crucial role in immune signaling, stress adaptation, and programmed cell death regulation. They serve as vital sentinels during infections, identifying pathogens and coordinating inflammatory and antiviral reactions, but various pathogens also take advantage of or damage them to increase microbial survival. Significant effects on host physiology result from this reciprocal interaction, such as immune dysregulation, excessive oxidative stress, metabolic disruption, and the advancement of chronic diseases. Gaining a better understanding of these intricate host-pathogen-mitochondria interactions will be essential for creating innovative treatment plans that protect mitochondrial integrity, boost host defense, and lessen infection-related disease. In the end, focusing on mitochondrial pathways presents a promising avenue for upcoming immunomodulatory and anti-infective treatments.
Author Contributions
G.G. conceived this work, managed the manuscript writing and corrected it; R.A. wrote the review. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
No new data were created or analyzed in this study. Data sharing is not applicable to this article.
Conflicts of Interest
The authors have no competing interests to declare that are relevant to the content of this article.
Abbreviations
Apaf-1 (Apoptotic Protease Activating Factor 1), ARE (Antioxidant Response Element), ATP (Adenosine Triphosphate), Ca2+ (Calcium Ion), CARD (Caspase Activation and Recruitment Domain), cGAS (Cyclic GMP-AMP Synthase), DAMP (Damage-Associated Molecular Pattern), DRP1 (Dynamin-Related Protein 1), ETC (Electron Transport Chain), FIS1 (Fission 1 Protein), H2O2 (Hydrogen Peroxide), IKK (IκB Kinase), IKKε (IκB Kinase Epsilon), IL-6 (Interleukin 6), IM (Inner Membrane), IMS (Intermembrane Space), IRF3/IRF7 (Interferon Regulatory Factor 3/7), ISGs (Interferon-Stimulated Genes), KEAP1 (Kelch-Like ECH-Associated Protein 1), MAVS (Mitochondrial Antiviral Signaling Protein), MDA5 (Melanoma Differentiation-Associated Protein 5), MFN1 (Mitofusin 1), MFN2 (Mitofusin 2), MFF (Mitochondrial Fission Factor), MIRO (Mitochondrial Rho GTPase), MiD49/51 (Mitochondrial Dynamics Proteins 49 and 51), mPTP (Mitochondrial Permeability Transition Pore), mtDNA (Mitochondrial DNA), NF-κB (Nuclear Factor Kappa-Light-Chain-Enhancer of Activated B Cells), NRF2 (Nuclear Factor Erythroid 2–Related Factor 2), NS1 (Non-Structural Protein 1), NS3/4A (Non-Structural Protein 3/4A), OM (Outer Membrane), OPA1 (Optic Atrophy 1), ORF9b (Open Reading Frame 9b), PRRs (Pattern Recognition Receptors), RCs (Respiratory Complexes), RIG-I (Retinoic Acid-Inducible Gene I), ROS (Reactive Oxygen Species), SOD2 (Superoxide Dismutase 2), STING (Stimulator of Interferon Genes), TBK1 (TANK-Binding Kinase 1), TCA (Tricarboxylic Acid Cycle), TLR9 (Toll-Like Receptor 9), TNF-α (Tumor Necrosis Factor Alpha), VDACs (Voltage-Dependent Anion Channels), ΔΨm (Mitochondrial Membrane Potential).
References
- Cooper, G.M. Glossary. In The Cell: A Molecular Approach, 2nd ed.; Sinauer Associates: Sunderland, MA, USA, 2000. [Google Scholar]
- Osellame, L.D.; Blacker, T.S.; Duchen, M.R. Cellular and molecular mechanisms of mitochondrial function. Best Pract. Res. Clin. Endocrinol. Metab. 2012, 26, 711–723. [Google Scholar] [CrossRef]
- Tan, J.X.; Finkel, T. Mitochondria as intracellular signaling platforms in health and disease. J. Cell Biol. 2020, 219, e202002179. [Google Scholar] [CrossRef]
- West, A.P.; Shadel, G.S. Mitochondrial DNA in innate immune responses and inflammatory pathology. Nat. Rev. Immunol. 2017, 17, 363. [Google Scholar] [CrossRef] [PubMed]
- Kumar, R.; Islinger, M.; Worthy, H.; Carmichael, R.; Schrader, M. The peroxisome: An update on mysteries 3.0. Histochem. Cell Biol. 2024, 161, 99–132. [Google Scholar] [CrossRef]
- Gray, M.W. Mitochondrial evolution. Cold Spring Harb. Perspect. Biol. 2012, 4, a011403. [Google Scholar] [CrossRef] [PubMed]
- Lane, N.; Martin, W. The energetics of genome complexity. Nature 2010, 467, 929–934. [Google Scholar] [CrossRef]
- Chial, H. mtDNA and Mitochondrial Diseases. Learn Science at Scitable. Available online: http://www.nature.com/scitable/topicpage/mtdna-and-mitochondrial-diseases-903 (accessed on 22 July 2025).
- Zhang, F.; Lee, A.; Freitas, A.V.; Herb, J.T.; Wang, Z.-H.; Gupta, S.; Chen, Z.; Xu, H. A transcription network underlies the dual genomic coordination of mitochondrial biogenesis. eLife 2024, 13, RP96536. [Google Scholar] [CrossRef] [PubMed]
- National Academies of Sciences, Engineering and Medicine. Science and Policy Context. In Mitochondrial Replacement Techniques: Ethical, Social, and Policy Considerations; Claiborne, A., English, R., Kahn, J., Eds.; National Academies Press: Washington, DC, USA, 2016. [Google Scholar]
- Wen, H.; Deng, H.; Li, B.; Chen, J.; Zhu, J.; Zhang, X.; Yoshida, S.; Zhou, Y. Mitochondrial diseases: From molecular mechanisms to therapeutic advances. Signal Transduct. Target. Ther. 2025, 10, 9. [Google Scholar] [CrossRef]
- Rossmann, M.P.; Dubois, S.M.; Agarwal, S.; Zon, L.I. Mitochondrial function in development and disease. Dis. Model. Mech. 2021, 14, dmm048912. [Google Scholar] [CrossRef]
- Bartman, S.; Coppotelli, G.; Ross, J.M. Mitochondrial Dysfunction: A Key Player in Brain Aging and Diseases. Curr. Issues Mol. Biol. 2024, 46, 1987–2026. [Google Scholar] [CrossRef]
- Weinberg, S.E.; Chandel, N.S. Mitochondria reactive oxygen species signaling in immune responses. Immunity 2025, 58, 1904–1921. [Google Scholar] [CrossRef]
- Sadeesh, E.M.; Lahamge, M.S.; Kumari, S.; Singh, P. Tissue-Specific Diversity of Nuclear-Encoded Mitochondrial Genes Related to Lipid and Carbohydrate Metabolism in Buffalo. Mol. Biotechnol. 2025, 57, 17. [Google Scholar] [CrossRef]
- Picard, M.; Taivassalo, T.; Gouspillou, G.; Hepple, R.T. Mitochondria: Isolation, structure and function. J. Physiol. 2011, 589, 4413–4421. [Google Scholar] [CrossRef]
- Iovine, J.C.; Claypool, S.M.; Alder, N.N. Mitochondrial Compartmentalization: Emerging Themes in Structure and Function. Trends Biochem. Sci. 2021, 46, 902. [Google Scholar] [CrossRef]
- Varughese, J.T.; Buchanan, S.K.; Pitt, A.S. The Role of Voltage-Dependent Anion Channel in Mitochondrial Dysfunction and Human Disease. Cells 2021, 10, 1737. [Google Scholar] [CrossRef]
- Suomalainen, A.; Nunnari, J. Mitochondria at the crossroads of health and disease. Cell 2024, 187, 2601–2627. [Google Scholar] [CrossRef]
- Youle, R.J.; van der Bliek, A.M. Mitochondrial Fission, Fusion, and Stress. Science 2012, 337, 1062–1065. [Google Scholar] [CrossRef]
- Pazour, G.J.; Bloodgood, R.A. Chapter 5 Targeting Proteins to the Ciliary Membrane. In Current Topics in Developmental Biology; Ciliary Function in Mammalian Development; Academic Press: Oxford, UK, 2008; Volume 85, pp. 115–149. [Google Scholar]
- van der Bliek, A.M.; Shen, Q.; Kawajiri, S. Mechanisms of mitochondrial fission and fusion. Cold Spring Harb. Perspect. Biol. 2013, 5, a011072. [Google Scholar] [CrossRef]
- Archer, S.L. Mitochondrial Dynamics—Mitochondrial Fission and Fusion in Human Diseases. N. Engl. J. Med. 2013, 369, 2236–2251. [Google Scholar] [CrossRef]
- Westermann, B. Mitochondrial fusion and fission in cell life and death. Nat. Rev. Mol. Cell Biol. 2010, 11, 872–884. [Google Scholar] [CrossRef]
- Chen, H.; Detmer, S.A.; Ewald, A.J.; Griffin, E.E.; Fraser, S.E.; Chan, D.C. Mitofusins Mfn1 and Mfn2 coordinately regulate mitochondrial fusion and are essential for embryonic development. J. Cell Biol. 2003, 160, 189–200. [Google Scholar] [CrossRef]
- Song, Z.; Chen, H.; Fiket, M.; Alexander, C.; Chan, D.C. OPA1 processing controls mitochondrial fusion and is regulated by mRNA splicing, membrane potential, and Yme1L. J. Cell Biol. 2007, 178, 749–755. [Google Scholar] [CrossRef]
- Losón, O.C.; Song, Z.; Chen, H.; Chan, D.C. Fis1, Mff, MiD49, and MiD51 mediate Drp1 recruitment in mitochondrial fission. Mol. Biol. Cell 2013, 24, 659–667. [Google Scholar] [CrossRef]
- Smirnova, E.; Griparic, L.; Shurland, D.-L.; van der Bliek, A.M. Dynamin-related Protein Drp1 Is Required for Mitochondrial Division in Mammalian Cells. Mol. Biol. Cell 2001, 12, 2245–2256. [Google Scholar] [CrossRef]
- Chan, D.C. Fusion and Fission: Interlinked Processes Critical for Mitochondrial Health. Annu. Rev. Genet. 2012, 46, 265–287. [Google Scholar] [CrossRef]
- Castanier, C.; Garcin, D.; Vazquez, A.; Arnoult, D. Mitochondrial dynamics regulate the RIG-I-like receptor antiviral pathway. EMBO Rep. 2010, 11, 133–138. [Google Scholar] [CrossRef]
- Koshiba, T. Mitochondrial-mediated antiviral immunity. Biochim. Biophys. Acta BBA Mol. Cell Res. 2013, 1833, 225–232. [Google Scholar] [CrossRef]
- Kim, S.-J.; Syed, G.H.; Khan, M.; Chiu, W.-W.; Sohail, M.A.; Gish, R.G.; Siddiqui, A. Hepatitis C virus triggers mitochondrial fission and attenuates apoptosis to promote viral persistence. Proc. Natl. Acad. Sci. USA 2014, 111, 6413–6418. [Google Scholar] [CrossRef]
- Kordyukova, L.V.; Serebryakova, M.V.; Baratova, L.A.; Veit, M. Site-specific attachment of palmitate or stearate to cytoplasmic versus transmembrane cysteines is a common feature of viral spike proteins. Virology 2010, 398, 49–56. [Google Scholar] [CrossRef]
- Khan, S.; Raj, D.; Jaiswal, K.; Lahiri, A. Modulation of host mitochondrial dynamics during bacterial infection. Mitochondrion 2020, 53, 140–149. [Google Scholar] [CrossRef]
- Tiku, V.; Tan, M.-W.; Dikic, I. Mitochondrial Functions in Infection and Immunity. Trends Cell Biol. 2020, 30, 263–275. [Google Scholar] [CrossRef]
- Mills, E.L.; Kelly, B.; O’Neill, L.A.J. Mitochondria are the powerhouses of immunity. Nat. Immunol. 2017, 18, 488–498. [Google Scholar] [CrossRef]
- Casanova, A.; Wevers, A.; Navarro-Ledesma, S.; Pruimboom, L. Mitochondria: It is all about energy. Front. Physiol. 2023, 14, 1114231. [Google Scholar] [CrossRef]
- Shadel, G.S.; Horvath, T.L. Mitochondrial ROS Signaling in Organismal Homeostasis. Cell 2015, 163, 560–569. [Google Scholar] [CrossRef]
- Duchen, M.R. Mitochondria and calcium: From cell signalling to cell death. J. Physiol. 2000, 529, 57–68. [Google Scholar] [CrossRef]
- West, A.P.; Shadel, G.S.; Ghosh, S. Mitochondria in innate immune responses. Nat. Rev. Immunol. 2011, 11, 389–402. [Google Scholar] [CrossRef]
- Wang, C.; Youle, R.J. The Role of Mitochondria in Apoptosis. Annu. Rev. Genet. 2009, 43, 95–118. [Google Scholar] [CrossRef]
- Murphy, M.P. How mitochondria produce reactive oxygen species. Biochem. J. 2009, 417, 1–13. [Google Scholar] [CrossRef]
- Schieber, M.; Chandel, N.S. ROS Function in Redox Signaling and Oxidative Stress. Curr. Biol. 2014, 24, R453–R462. [Google Scholar] [CrossRef]
- Zhong, Z.; Sanchez-Lopez, E.; Karin, M. Autophagy, NLRP3 inflammasome and auto-inflammatory/immune diseases. Clin. Exp. Rheumatol. 2016, 34, 12–16. [Google Scholar]
- Morgan, M.J.; Liu, Z. Crosstalk of reactive oxygen species and NF-κB signaling. Cell Res. 2011, 21, 103–115. [Google Scholar] [CrossRef]
- Ngo, V.; Duennwald, M.L. Nrf2 and Oxidative Stress: A General Overview of Mechanisms and Implications in Human Disease. Antioxidants 2022, 11, 2345. [Google Scholar] [CrossRef]
- Sies, H.; Jones, D.P. Reactive oxygen species (ROS) as pleiotropic physiological signalling agents. Nat. Rev. Mol. Cell Biol. 2020, 21, 363–383. [Google Scholar] [CrossRef]
- Riley, J.S.; Tait, S.W. Mitochondrial DNA in inflammation and immunity. EMBO Rep. 2020, 21, e49799. [Google Scholar] [CrossRef]
- Xie, J.; Yuan, C.; Yang, S.; Ma, Z.; Li, W.; Mao, L.; Jiao, P.; Liu, W. The role of reactive oxygen species in severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection-induced cell death. Cell. Mol. Biol. Lett. 2024, 29, 138. [Google Scholar] [CrossRef]
- Chen, S.; Li, Q.; Shi, H.; Li, F.; Duan, Y.; Guo, Q. New insights into the role of mitochondrial dynamics in oxidative stress-induced diseases. Biomed. Pharmacother. 2024, 178, 117084. [Google Scholar] [CrossRef]
- Marchi, S.; Patergnani, S.; Missiroli, S.; Morciano, G.; Rimessi, A.; Wieckowski, M.R.; Giorgi, C.; Pinton, P. Mitochondrial and endoplasmic reticulum calcium homeostasis and cell death. Cell Calcium 2018, 69, 62–72. [Google Scholar] [CrossRef]
- Seth, R.B.; Sun, L.; Ea, C.-K.; Chen, Z.J. Identification and Characterization of MAVS, a Mitochondrial Antiviral Signaling Protein that Activates NF-κB and IRF3. Cell 2005, 122, 669–682. [Google Scholar] [CrossRef]
- Kawai, T.; Takahashi, K.; Sato, S.; Coban, C.; Kumar, H.; Kato, H.; Ishii, K.J.; Takeuchi, O.; Akira, S. IPS-1, an adaptor triggering RIG-I- and Mda5-mediated type I interferon induction. Nat. Immunol. 2005, 6, 981–988. [Google Scholar] [CrossRef]
- Li, X.-D.; Sun, L.; Seth, R.B.; Pineda, G.; Chen, Z.J. Hepatitis C virus protease NS3/4A cleaves mitochondrial antiviral signaling protein off the mitochondria to evade innate immunity. Proc. Natl. Acad. Sci. USA 2005, 102, 17717–17722. [Google Scholar] [CrossRef]
- Gack, M.U.; Albrecht, R.A.; Urano, T.; Inn, K.S.; Huang, I.C.; Carnero, E.; Farzan, M.; Inoue, S.; Jung, J.U.; García-Sastre, A. Influenza A virus NS1 targets the ubiquitin ligase TRIM25 to evade recognition by the host viral RNA sensor RIG-I. Cell Host Microbe 2009, 5, 439–449. [Google Scholar] [CrossRef]
- Jiang, H.; Zhang, H.; Meng, Q.; Xie, J.; Li, Y.; Chen, H.; Zheng, Y.; Wang, X.; Qi, H.; Zhang, J.; et al. SARS-CoV-2 Orf9b suppresses type I interferon responses by targeting TOM70. Cell. Mol. Immunol. 2020, 17, 998–1000. [Google Scholar] [CrossRef]
- Shoraka, S.; Samarasinghe, A.E.; Ghaemi, A.; Mohebbi, S.R. Host mitochondria: More than an organelle in SARS-CoV-2 infection. Front. Cell. Infect. Microbiol. 2023, 13, 1228275. [Google Scholar] [CrossRef]
- Chen, T.-H.; Jeng, T.-H.; Lee, M.-Y.; Wang, H.-C.; Tsai, K.-F.; Chou, C.-K. Viral mitochondriopathy in COVID-19. Redox Biol. 2025, 85, 103766. [Google Scholar] [CrossRef]
- Qi, Y.; Yin, J.; Xia, W.; Yang, S. Exploring the role of mitochondrial antiviral signaling protein in cardiac diseases. Front. Immunol. 2025, 16, 1540774. [Google Scholar] [CrossRef]
- Gao, J.; Ding, M.; Xiyang, Y.; Qin, S.; Shukla, D.; Xu, J.; Miyagi, M.; Fujioka, H.; Liang, J.; Wang, X. Aggregatin is a mitochondrial regulator of MAVS activation to drive innate immunity. J. Immunol. Baltim. 2025, 214, 238–252. [Google Scholar] [CrossRef]
- Trishna, S.; Lavon, A.; Shteinfer-Kuzmine, A.; Dafa-Berger, A.; Shoshan-Barmatz, V. Overexpression of the mitochondrial anti-viral signaling protein, MAVS, in cancers is associated with cell survival and inflammation. Mol. Ther. Nucleic Acids 2023, 33, 713–732. [Google Scholar] [CrossRef]
- Kumar, M.; Sharma, S.; Mazumder, S. Role of UPRmt and mitochondrial dynamics in host immunity: It takes two to tango. Front. Cell. Infect. Microbiol. 2023, 13, 1135203. [Google Scholar] [CrossRef]
- Pawłowska, M.; Mila-Kierzenkowska, C.; Szczegielniak, J.; Woźniak, A. Oxidative Stress in Parasitic Diseases—Reactive Oxygen Species as Mediators of Interactions between the Host and the Parasites. Antioxidants 2023, 13, 38. [Google Scholar] [CrossRef]
- Zong, Y.; Li, H.; Liao, P.; Chen, L.; Pan, Y.; Zheng, Y.; Zhang, C.; Liu, D.; Zheng, M.; Gao, J. Mitochondrial dysfunction: Mechanisms and advances in therapy. Signal Transduct. Target. Ther. 2024, 9, 124. [Google Scholar] [CrossRef]
- Spier, A.; Stavru, F.; Cossart, P. Interaction between Intracellular Bacterial Pathogens and Host Cell Mitochondria. Microbiol. Spectr. 2019, 7, 10–1128. [Google Scholar] [CrossRef]
- Andrieux, P.; Chevillard, C.; Cunha-Neto, E.; Nunes, J.P.S. Mitochondria as a Cellular Hub in Infection and Inflammation. Int. J. Mol. Sci. 2021, 22, 11338. [Google Scholar] [CrossRef] [PubMed]
- Shingu-Vazquez, M.; Traven, A. Mitochondria and fungal pathogenesis: Drug tolerance, virulence, and potential for antifungal therapy. Eukaryot. Cell 2011, 10, 1376–1383. [Google Scholar] [CrossRef]
- Nakahira, K.; Hisata, S.; Choi, A.M.K. The Roles of Mitochondrial Damage-Associated Molecular Patterns in Diseases. Antioxid. Redox Signal. 2015, 23, 1329–1350. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).