Abstract
The ADRENALINE pilot study explores the role of physical activity in health outcomes among patients with Chronic Lymphocytic Leukemia (CLL), focusing on disease markers, functional capacity, immune parameters, and quality of life. This baseline analysis includes treatment-naïve participants enrolled between September 2023 and August 2024, prior to randomization. Eleven patients (aged 47–78 years) underwent assessments of body composition, cardiovascular fitness, muscular strength, and immune profiling. Quality of life was evaluated using validated questionnaires (FACIT-F, EORTC QLQ-30/CLL17), and daily activity was objectively measured via accelerometry. Correlation analyses examined associations between physical activity, muscle strength, lean mass, and physical aptitude. Despite high self-reported physical function, participants demonstrated suboptimal body composition and cardiovascular fitness. Accelerometry revealed marked sedentary behavior, particularly among females, and overall activity levels were below current recommendations. Moderate-to-vigorous physical activity correlated positively with muscular strength and lean mass. Immune profiling identified a variability in key markers, warranting further investigation of their relationship with physical activity. These findings highlight the need for tailored interventions to increase activity and reduce sedentary time in CLL patients and support incorporating functional and immune monitoring into survivorship care.
1. Introduction
Chronic Lymphocytic Leukemia (CLL) is a hematological disease caused by the proliferation of monoclonal mature B lymphocytes [1], with a prevalence of 25% to 35% among all leukemias, and it is one of the most common forms in adults in Western countries [1]. Patients with CLL tend to adopt sedentary behaviors due to disease symptoms as it progresses, including fatigue, shortness of breath during regular physical activity, lymph node enlargement, low-grade fever, unexplained weight loss, night sweats, a feeling of fullness (related to an enlarged spleen or liver), and multiple infections [2]. As a result of these changes, there is often a notable decrease in physical fitness, with a consequent decrease in overall quality of life [3]. Additionally, the psychological impact of the disease, including anxiety and depression, further exacerbates this condition, underscoring the critical need for interventions that promote physical activity and help patients maintain independence in their daily activities.
Individuals with cancer are not any more likely to be physically inactive than those who have never had cancer; however, in a Canadian Community Health Survey of patients with current cancer or cancer history, it was found that physical activity in all studied groups were much lower than recommended [4]. While current treatment for patients with Chronic Lymphocytic Leukemia focuses on a multifaceted approach, aiming to address both physical and psychological challenges [5], many patients with CLL hematologic cancer still experience significant discomfort that hinders daily functioning and reduces their quality of life [6].
Recent studies have explored the impact of physical activity on CLL patients with research suggesting that many treatment-naïve CLL patients are physically inactive and have high levels on inflammation [7,8]. Regular physical activity has been shown to have significant positive effects in the general population, with moderate-to-vigorous exercise being associated with a reduced risk of infections [9], enhancing immune function, and counteracting chronic systemic inflammation [10], which are characteristic of CLL disease symptoms [11].
Physical activity may play a crucial role in maintaining an active lifestyle before, during, and after treatment [12,13], and regular physical activity may improve physical function enhancing mood, self-esteem, and overall well-being [14]. With the need for more robust research to determine the most effective exercise intervention for cancer populations, many studies have already provided strong evidence supporting the prescription of exercise therapy for managing metabolic syndrome-related disorders (insulin resistance, type 2 diabetes, dyslipidemia, hypertension, obesity), heart and pulmonary diseases (chronic obstructive pulmonary disease, coronary heart disease, chronic heart failure, intermittent claudication), and muscle, bone, and joint diseases (osteoarthritis, rheumatoid arthritis, osteoporosis, fibromyalgia, chronic fatigue syndrome), as well as cancer, depression, asthma, and type 1 diabetes [15,16]. Several controlled trials indicate significant improvements in cardiovascular fitness with moderate-intensity aerobic exercise performed approximately 2–3 times per week for 30–60 min [17,18,19] and in muscle fitness with structured resistance or high-intensity interval training conducted 2–3 times per week [20]. These exercise levels have also demonstrated beneficial effects on the immunologic response [20,21,22] and reductions in cancer-related fatigue [17,18]. Yet, in a study with a population of 606 survivors of hematologic cancer from Canada, only 22% met the combined exercise guidelines, and just 10% met the strength-only guidelines [23].
Objective assessments of physical activity with accelerometry provide accurate and objective data [24,25] regarding prognostic and quality of life measures such as objective quantification of time spent on sedentary behavior, light and moderate-to-vigorous physical activity, and body position [26,27]. This objective data on patients’ activity levels have been linked to better survival outcomes and lower disease symptoms [28,29], and by integrating this technology and data, clinicians can tailor interventions to enhance physical activity levels, potentially improving patients’ overall health and treatment response [30]. This approach offers a non-invasive, continuous monitoring tool that aligns with personalized medicine initiatives in oncology [31], facilitating the accomplishment of clinical interventions.
Regarding CLL patients, a common symptom reported by patients is fatigue. Based on current studies, the circadian clock has been shown to influence numerous immune cells at both the molecular and cellular levels [32]. The maturation of neutrophils, the most common leukocytes found in human blood, is rhythmic, and their antimicrobial activity behaves in a time-of-day-dependent manner [32]. Objectively assessed physical activity may shed light on the question of whether daily physical activity in CLL patients follows this circadian clock pattern.
The aim of the current study is to explore the relationship between daily physical activity levels and hematological, immunological, and physical and functional parameters; to evaluate the subjective perception of physical and functional capacity and quality of life; and to investigate whether the time of day influences physical activity patterns in patients diagnosed with CLL in the treatment-naïve phase.
2. Materials and Methods
2.1. Study Design
The present cross-sectional correlational study is part of the Randomized Controlled Clinical Trial of Exercise as Intervention in Chronic Lymphocytic Leukemia (ADRENALINE), a single-blind randomized controlled trial (RCT) study. The present manuscript reports baseline data from the undergoing research project, before the randomization and intervention.
Patients with confirmed CLL, selected using stringent inclusion and exclusion criteria, were consecutively recruited at the Hematology Department in the participating Hospital (São João University Hospital Center), which represents the largest hospital in the Porto region (Portugal). The data in the present study regarding initial evaluations (T0) of the ADRENALINE study and data collection covers the period from September 2023 to August 2024. During this period (T0 evaluation), no exposure to the ADRENALINE concept nor follow-up was made.
The study protocol has been approved by the Ethics Committees of São João University Hospital Center and the Faculty of Sport at the University of Porto. All patients provided written informed consent upon recruitment to participate in the study. The ADRENALINE trial is registered at ClinicalTrials.gov.
2.2. Participants
All patients received a screening assessment, including medical history, blood chemistry and hemogram, and a physical examination (for skin pallor, palpable cervical lymph nodes, and hepatomegaly) during routine hematologic workup before the assessment of health-related quality of life using questionnaires and fitness assessments. Objectively assessed levels of daily physical activity were acquired after baseline assessments using accelerometry technology to collect data on the physical activity (PA) patterns of the participants over a period of seven consecutive days, with a minimum of 10 h of daily assessment.
Inclusion criteria comprised a confirmed CLL diagnosis as per the International Workshop on CLL Guidelines [11], age between 45 and 80 years old, no history of previous treatment of CLL, ability to walk on a treadmill or cycle on an ergometer, ability to carry weights or use weight machines, having passed initial evaluations (Cardiopulmonary Exercise Test + electrocardiogram (CPET + ECG) for cardiac health and dynamometer for muscular health), and signed informed consent. Exclusion criteria included previous immunochemotherapy CLL treatments, ongoing engagement in a regular exercise program, an indication of disease progression or for starting treatment within 6 months (according to the recommendations of the International Workshop on CLL Guidelines), other primary tumors, inability to perform exercise (heart or advanced-stage respiratory, renal, hepatic, neurological, or osteoarticular disease), and being unable to travel to facilities or to comply with other study requirements. Figure 1 demonstrates the patient recruitment and data collection process of the present study within the development of the ADRENALINE study, with a convenience sample of eleven patients (64% male and 36% female).
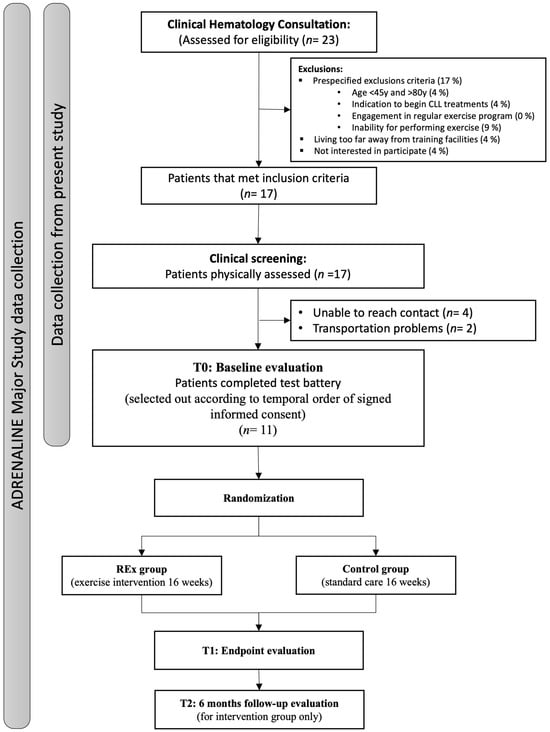
Figure 1.
Scheme of patient recruitment and data collection. REx = Resistance Exercise. CLL = Chronic Lymphocytic Leukemia.
2.3. Primary and Secondary Outcomes
Primary outcomes encompass the characterization of objective levels of physical activity using accelerometry and of quality of life and well-being using questionnaires, body composition measures, anthropometric, strength evaluation, and cardiac and respiratory capacity.
Secondary outcomes include medical assessments performed by clinicians during a routine hematology consultation before the baseline evaluations, including disease-related symptoms/complications, physical exam (for skin pallor, palpable cervical lymph nodes, and hepatomegaly), and clinical exams.
2.4. Evaluation Exercise Testing
At baseline, patients were evaluated in terms of (i) anthropometric and body composition measures and bone mineral density; (ii) strength; and (iii) cardiac and respiratory CPET on a cycle ergometer with a stress electrocardiogram with spirometry.
The CPET performed to assess cardiorespiratory fitness and potential cardiovascular and cardiorespiratory limitations followed international guidelines concerning standardization and interpretation strategies [33]. The CPET was performed on a Lode Corival CPET cycle ergometer (Lode, The Netherlands) under the supervision of a sports physician. Throughout the test, the electrocardiogram (ECG) and blood pressure were monitored, and heart rate (HR) and gas exchange variables were recorded continuously, averaged at 30 s intervals (COSMED Quark CPET, Rome, Italy). The protocol comprises 2 min rest without movement for respiratory adaptation followed by a 3 min unloaded warmup phase at 60 revolutions per minute (RPM) and zero watts. After warmup, the incremental exercise phase is initiated, with each step incrementing 15 watts per minute until 85% of the maximum heart rate is achieved or other standardized criteria for stopping are met [33]. After the incremental phase, the recovery phase takes place, with 2 min of active cool down with unloaded pedaling followed by 3 min of passive cool down without movement [34]. The highest recorded oxygen uptake (VO2peak) is used as an indicator of cardiorespiratory fitness. VO2peak represents the highest oxygen consumption achieved during the test rather than true maximal effort. This approach is commonly used when maximal exertion is not feasible, particularly in clinical populations.
Upper extremity muscle strength was assessed using a JAMAR PLUS handgrip dynamometer (Sammons Preston, Chicago, IL, USA), calibrated according to standard protocols, and using 3 trials for each hand, with a contraction of 2–4 s and with a 30 s interval between attempts [35,36]. For lower muscle strength, a BIODEX 850-000 System 4 Pro Isokinetic Dynamometer (Biodex Medical Systems, Shirley, NY, USA) was used following the standard procedures recommended by standard protocols, measuring extension/flexion of the dominant knee and using the Concentric/Concentric protocol with 60 deg/s speed to evaluate strength, with 5 repetitions for warmup and 3 repetitions for maximal effort after 1–2 min rest between the warmup and maximal effort phases [37].
Data collection of height and body mass was performed by standard anthropometric methods (SECA 217 stadiometer and SECA 899 digital floor scale). Body mass index was calculated from the ratio weight/height2 (kg/m2). Body composition and bone mineral density were collected using dual-energy X-ray absorptiometry [DEXA scans (Horizon Wi, Hologic, Marlborough, MA, USA)] to quantify percent body fat, fat mass, trunk fat mass, fat-free mass, and bone mineral density (from whole-body test) and hip bone mineral density (from non-dominant hip side).
Objectively assessed levels of physical activity (PA) were acquired using the GT9XLink accelerometer (Actigraph, Pensacola, FL, USA) to collect data on the PA patterns of participants over a period of seven consecutive days, with a minimum of four weekdays and one weekend day and of 10 h of daily assessment [38]. Accelerometers were worn on the dominant side over the hip and aligned with the kneecap, and patients were instructed to use them all day long except during baths, water sports, potential hazard activities, or during sleep. A report sheet for the description of daily accelerometer usage was also provided. Accelerometry analysis was conducted using the algorithm Troiano (2007) defining non-wear period with minimum length of 60 min, with a spike level to stop at 100 counts per minute, over seven consecutive days, with a minimum of four weekdays and one weekend day and of 10 h of daily assessment [38]. For scoring algorithms, Freedson VM3 2011 was used for energy expenditure, Freedson Adult 1998 for METs, and Troiano Adult 2008 for cutoff points and moderate-to-vigorous physical activity (MVPA). Outcomes are described as sum of minutes in each behavior.
All the referred tests were conducted at lab facilities, with medical surveillance during physical fitness protocols.
2.5. Clinical, Immunologic, and Inflammatory Testing
Clinicians performed clinical assessments during routine hematology consultations comprising standardized clinical assessments, including exercise- and disease-related symptoms/complications, hemogram and blood chemistry (hepatic, renal, mineral and metabolic markers), and physical exams (for skin pallor, palpable cervical lymph nodes, and hepatomegaly). Pre-intervention disease markers for characterization of CLL were also available (LDH, beta2-microglobulin, immunoglobulins, immunophenotyping and B cells characterization measurements). The CLL staging index (RAI and BINET) [39,40] and the Eastern Cooperative Oncology Group Performance Scale (ECOG) [41] were used due to being typical in the clinical practice of the participating hospital. Genetic characterization of the disease (needed for the CLL International Prognostic Index (CLL-IPI)) was not available since genetic markers are only required when the disease criteria evolve to the treatment phase.
2.6. Quality of Life
At baseline, two self-reported questionnaires were used to assess clinical status, well-being, and quality of life. The first is the Functional Assessment of Chronic Illness Therapy-Fatigue (FACIT-F) [42], a 27-item compilation of general questions divided into four Well-Being domains, where the FACT-General (FACT-G) score is determined by summing the totals from the four domains of physical, social/family, emotional, and functional well-being and the FACIT-Fatigue (FACIT-F) score is determined by adding the FACT-G total and the fatigue subscale score. The maximum score is 160, with higher scores indicating greater well-being for all domains. The second questionnaire is the European Organization for Research and Treatment of Cancer Quality of Life Questionnaires Core 30 (EORTC QLQ-C30) [43], consisting of 30 items evaluating health-related quality of life with five functional scales and with a Chronic Lymphocytic Leukemia Module (EORTC QLQ-CLL17), 17 items, incorporating three multi-item scales to assess symptom burden, physical condition/fatigue, and worries/fears about health and functioning, evaluating health-related quality of life (QoL). All the scales and single-item measures range in score from 0 to 100, where a high score for a functional scale represents a high/healthy level of functioning and a high score for the global health status/QoL represents a high QoL, but a high score for a symptom scale/item represents a high level of symptomatology/problems.
2.7. BIAS
Risk of bias was assessed with the JBI Critical Appraisal Checklist for Cross-Sectional Studies [44], part of the Joanna Briggs Institute’s critical appraisal tools. This checklist is specifically designed for cross-sectional studies, such as the present research, which assesses baseline differences between male and female participants. It evaluates potential biases in sample selection, measurement reliability, and statistical analysis. Additionally, it helps ensure the comparability of groups and the validity of data collection methods, thereby strengthening the study’s methodological rigor.
2.8. Statistical Methods
Sample size calculation for the greater ADRENALIN study (GPower software, mac version 3.1) was based on the means and standard deviations of prior studies [45]. An initial estimation indicated that a minimum of 16 subjects was required to detect differences with a power of 95% and a significance level of 5% (two-tailed). However, due to external constraints beyond our control—such as the distance between participants’ residences and training facilities, as well as the challenge of recruiting treatment-naïve patients without pharmacological intervention—only 11 participants were included in the study. For this convenience sample, a post hoc power analysis revealed a statistical power of 66.7% for detecting an effect size of 0.8. While this is below the conventional threshold of 80%, the significance of observed effects remains valid, as meaningful differences were detected despite the reduced sample size.
Descriptive statistics are presented as means and standard deviations (SDs) or medians and interquartile ranges. Normality analysis was checked and met, and it was conducted using the Shapiro–Wilk test. A T-Test was employed to analyze differences between genders. To analyze the relationship between variables, Pearson correlation coefficients were calculated, and all assumptions for Pearson correlation were checked and met. To evaluate the influence of MVPA, the participants were divided into tertiles based on their MVPA levels, creating three groups to compare the most active and the least active individuals. Parametric (ANOVA) and non-parametric (Kruskal–Wallis) tests were deployed to verify differences across these groups for continuous variables and goodness-of-fit chi-square for categorical variables. Effect size was assessed using Hedges’ g scores (due to the tendency to overestimate effect size with Cohen’s d on small samples), where values of 0.2, 0.5 and 0.8 were considered small, medium, and large effects, respectively [46]. Significance was maintained at p < 0.05.
For the questionnaire subdomains and composite scores, we followed recommendations from the authors (EORTC [43] and FACIT [42]) and from similar exercise studies in cancer [47,48,49]. Therefore, in line with recommendations and the independence of each subdomain, no adjustments for multiple comparisons between subdomains were performed.
Statistical analyses were conducted using IBM SPSS mac version 29 (Armonk, NY, USA), and accelerometry analyses were conducted using Actigraph Actilife version 6.13.6 (Pensacola, FL, USA).
3. Results
During the recruitment phase, from September 2023 to August 2024, twenty-three patients were assessed for eligibility in clinical hematology consultation, with six patients excluded due to not meeting the inclusion criteria. After clinical screening, we were unable to contact four patients, while another two had transportation issues that prevented them from complying with the physical fitness screening. Eleven participants were consented into the study. The complete flowchart can be consulted in Figure 1.
The total sample comprised eleven participants, seven men (64%) and four women (36%), with a mean (SD) age of 65.55 (9.73) (range: 47–78) years. Regarding baseline characteristics for body composition, this group of patients presented with overweight compared to the recommendations, with an overall average BMI = 26.72 (2.22), and with slight overfat, with body fat percentage values for male and female patients of 36.7 (5.7) and 24.3 (6.8) percent, respectively. Regarding bone health, measured by DEXA, the female group was in the osteopenia stage (T-score less than −1 SD from normal values), while in the male group, only three patients presented with this condition. None of the patients had osteoporosis; however, only four (36.4%) patients had healthy bone measures. There were relevant and statistically significant differences between genders in height, body fat percentage, lean mass, and femur neck bone mineral density, favoring male participants.
Overall mean time since diagnosis was 3.65 (range: 1.12–10.31) years. According to the standard criteria for clinical staging [39,40], participants were mostly in RAI stage I (54.5%), BINET stage A (72.7%), and ECOG status 0 (63.6%). Due to the inclusion and exclusion criteria, this was expected, since patients were in the surveillance phase, without treatment.
When analyzing the measured hematologic parameters, patients show percentages of lymphocyte counts above normal values (expected in CLL disease), while neutrophils appear particularly low [40.0% (11.7)]. Regarding immunologic parameters, only one patient had an unfavorable prognosis, with B2-microglobulin above 2.5 µg/L, an indicator of high tumoral charge and disease activity, while immunoglobulins appear controlled, with favorable values indicating low risk of infection. The complete description, at baseline, of patients’ demographic, body composition, hematologic, and immunologic data can be consulted in Table 1.

Table 1.
Baseline of demographic, body composition, hematologic, and immunologic data (n = 11). Values are means (standard deviations). BMI = body mass index. WBC = white blood cell. RBC = red blood cell. LDH = lactate dehydrogenase. † = Bold values are out of the normative reference intervals.
Regarding physical fitness parameters, they revealed a very low cardiovascular capacity, with mean values of 16.1 (5.1) mL/kg/min, ranging between 9.0 and 24.3 mL/kg/min VO2peak. None of the patients exceeded 135 watts of peak power in the cycle ergometer maximal test, reaching 85% maximal heart rate criteria, with a mean value of 84 (45.4) watts. Regarding strength characteristics, a peak torque knee extension of 113.1 (54.3) N.m and a flexion of 55.4 (21.3) N.m reveal a ratio of 52.6% between the agonist and the antagonist. There were relevant and statistically significant differences between genders in all physical fitness parameters, favoring male participants.
Concerning accelerometry, participants wore an accelerometry device for a minimum of 4 weekdays and 1 weekend day, as per the inclusion criteria for analysis. So, for our sample, the usability and feasibility of wearing the wearable device were accomplished and even exceeded. When analyzing sedentary behavior, the patients spent 58% of their time on this behavior, while light, moderate, and vigorous physical activity counts for 39%, 2.4%, and approximately 0%, respectively. When exploring daily physical activity and sedentary time, male patients showed less sedentary time and more physical activity when compared to female patients, with the male group spending 53.5% of their time on sedentary behavior versus 67.5% for the female group. When comparing light and moderate-to-vigorous PA, both genders performed more light PA between 12h00 and 18h00, but patterns of moderate-to-vigorous PA were different between genders, with the female group showing more MVPA between 7h00 and 12h00, while the male group did so around 12h00 until 18h00, as depicted in Figure 2.
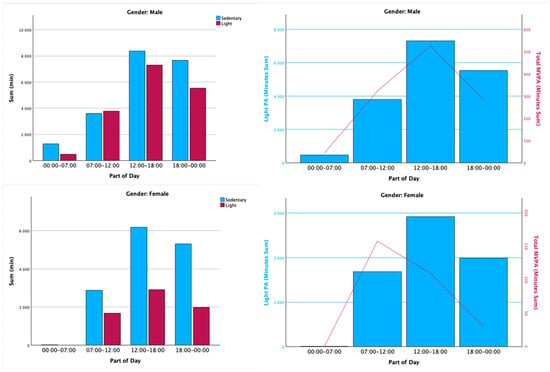
Figure 2.
Representative accelerometry analysis to illustrate the differences between parts of the day and physical activity patterns grouped by gender. MVPA = moderate-to-vigorous physical activity. PA = physical activity.
When analyzing quality of life questionnaires, our data reveal that in the EORTC, patients have a perception of high physical functioning, at 83.63 (13.79); this is also in line with FACIT-F, with the perception of high physical well-being, at 24.27 (2.49) of a maximum of 28 points. The complete description of participants’ baseline physical and functional capacity, accelerometry, and quality of life data can be consulted in Table 2.

Table 2.
Baseline of physical and functional capacity, accelerometry data, and quality of life questionnaires (n = 11). Values are means (standard deviations). FACIT-G = Functional Assessment of Cancer Therapy—General. FACIT-F = Functional Assessment of Cancer Therapy—Fatigue.
When analyzing the influence of MVPA on the outcomes of CLL patients, higher MVPA levels were associated, albeit without statistical significance, with a tendency toward lower B2-microglobulin levels and resting systolic blood pressure, as well as higher lean mass, BMI, and strength-related parameters, including left- and right-hand grip strength and isokinetic extension and flexion strength.
Highly positive and statistically significant correlations were found between total MVPA and peak torque knee extension (r = 0.78, p = 0.008), peak torque knee flexion (r = 0.77, p = 0.010), and grip strength of the right hand (r = 0.74, p = 0.015), as well as between lean mass and femur neck bone mass density (r = 0.66, p = 0.029), VO2peak (r = 0.86, p = 0.002), peak torque knee (extension (r = 0.82, p = 0.004) and flexion (r = 0.86, p = 0.001)), and peak grip force [left (r = 0.88, p = 0.001) and right (r = 0.82, p = 0.003)]. The association between lean mass and MVPA (r = −0.45 and r = −0.54, respectively) using the B2-microglobulin disease marker revealed a medium negative correlation, but without statistical significance. The complete description of correlations can be found in Table 3.

Table 3.
Correlations between total moderate-to-vigorous physical activity (MVPA) and lean mass against physical variables. (*) Correlation is significant at the 0.05 level (2-tailed). (**) Correlation is significant at the 0.01 level (2-tailed).
4. Discussion
The early dissemination of our study’s baseline data offers important contributions to the understanding of the characteristics of CLL in the treatment-naïve stage. The data collected to date offers valuable insights into the initial profile of patients, allowing sample characterization and identification of initial trends, which may diverge from common knowledge from the available literature and clinical practice. The present study addresses the characteristics of patients diagnosed with Chronic Lymphocytic Leukemia in the “watch and wait” phase and the relationship between PA levels and significant health outcomes, without immunochemotherapy treatment or indication for treatment initiation.
Participants obtained values above baseline normative data for healthy populations regarding body fat percentage. Although no universally accepted norms for body fat exist, the ACSM suggests optimal ranges of 10–22% for men and 20–32% for women as satisfactory for health [50]. In our sample, mean values were slightly higher, at 24.3% for men and 36.7% for women. It is important to note that an elevated body fat percentage may not solely reflect increased adiposity; it may also be influenced by the reduced muscle mass commonly observed in individuals with CLL, which increases the relative proportion of fat mass. This is consistent with prior reports of sarcopenia and impaired muscle strength in this population [51]. The higher body fat percentage in our cohort may therefore contribute to the symptom burden, as suggested by the statistically significant correlations between body fat and VO2peak, fatigue, physical functioning and average symptom burden (the latter showing a non-significant median correlation). These findings align with previous observations in CLL populations [17,18,20].
The unfavorable body composition profile may be partially explained by the physical condition parameter, where this group of patients scored low in cardiovascular fitness. The highest VO2peak value recorded was 24.3 mL/kg/min, observed in a male patient and below the minimum expected for a healthy adult female [50]. Current knowledge advises that an increment in VO2 is associated with lower levels of fatigue [17,18], higher levels of muscular resistance and bone mineral density [17,18,19], and improved physical functioning and well-being [14], which may directly impact daily tasks, help maintain daily physical activity, and reduce disease-related fatigue.
One factor that remains underexplored is the influence of strength and muscle mass on CLL patients. Several studies show a preference for low–moderate-intensity or endurance exercise over resistance training (65–70% of 1-RM), but as the literature suggests, exercise-induced myokine production may have a critical role in increasing cytotoxicity and the infiltration of immune cells into the tumor [52]. When comparing guidelines for aerobic-only, strength, and combined exercise in the hazard ratio for all-cause mortality, strength training was considered better than aerobic in the case of cardiovascular disease mortality and in cancer mortality, where isolated strength training demonstrated superior benefits compared to other modes of training [53]. The presence of low muscle mass when compared to healthy adults [54] is similar between our study and other interventions with exercise training in CLL patients; however, there are still few studies addressing the use of strength training in CLL patients. In our data, correlation analysis suggests that greater muscle mass is strongly associated with higher strength levels in patients with CLL, similar to what is observed in the general population. Given that one of the major symptoms of the disease is the loss of weight, this underscores the potential importance of incorporating strength training into the management of CLL to maintain muscle mass and physical function. Given that training intensity can influence long-term adherence, strength training—using weights at approximately 75–85% of one-repetition maximum—may be better tolerated than continuous aerobic endurance training. This is particularly relevant as fatigue and dyspnea during routine physical activity are among the most prominent symptoms of disease progression. The structure of strength training, which typically involves short sets with extended rest intervals (60–90 s), may facilitate greater respiratory comfort and improve exercise adherence.
One of the factors that may influence the self-awareness of this problem is that when analyzing the quality of life questionnaires, the individual participants did not report significant symptoms impairing their daily life quality. However, their perception of high physical functioning does not align with their fitness levels. Several longitudinal studies show that a higher perception of physical functioning by CLL patients is very close to the values of healthy controls [55,56]. When crossing this data with exercise interventions for CLL patients, we can confirm this tendency. In a study by Artese et al. [57], the data show that CLL patients with 31% body fat and a VO2 of 26.7 mL/kg/min, scored their physical well-being and functional well-being with a value of 27 and 25.7 (on a scale from 0 to 28), respectively; in another study by Furzer et al. [18], patients with 34.6% body fat and a 16.10 value of 75% VO2/kg reported a FACT-G score of 82 (on a scale from 0 to 108); another study with a wider sample of patients that also included CLL patients, by Courneya et al. [17], found a value of 147.1 on the FACT-An (on a scale from 0 to 188) in a group with 32.6% body fat and a VO2 of 25.4 mL/kg/min.
One piece of evidence that arises from our data is that MVPA is not significant for any parameter besides lymphocyte percentage. Even after exploring data with median and tertile ranking, MVPA appears to have no significant impact on the disease parameters in our sample. However, activity patterns suggest that the hours of peak MVPA and light PA lie between 7:00 and 18:00, in line with the latest studies, which speculate a lower risk of incidence of cardiovascular disease and cancer for those who are active during this timeframe [58,59]. The protective effect of chronoactivity may be one variable, but we cannot disregard that, besides the low number of patients in our sample, the number of minutes spent engaging in MVPA as compared to that recommended from major organizations [50,60], defined as at least 150 min of moderate-to-vigorous activity per week, was critically low in our study, barely reaching 19.61 min per day for this intensity. This is in contrast to the available literature that explores the association between CLL and physical activity patterns [20,61], which obtained higher values of objectively measured daily physical activity.
Regarding immunologic parameters, when comparing our sample values with normative values for the healthy population in Portugal [62], our CLL sample only presents above-normal percentages of lymphocytes and neutrophils, with patients showing percentages of lymphocytes that are high and above normal values (expected in CLL disease) and with neutrophils appearing particularly low, revealing an increased risk of infection. Studies concerning immunologic parameters of CLL patients in exercise interventions are scarce. To the best of our knowledge, when compared with the two available studies that implement immunologic analysis, those by Crane et al. [21] and Macdonald et al. [20], our sample presented similar values for main serologic data, differing only in terms of lower white blood cell counts, lower lymphocyte and monocyte percentages, higher neutrophil percentages, lower B2-microglobulin, and lower LDH and albumin. This may impact future study analyses, because our sample of patients has a more favorable diagnosis of disease.
4.1. Limitations
We acknowledge several limitations that may affect the interpretation and generalizability of our findings. The small sample size, ongoing recruitment, and wide age range reduce statistical power and may introduce variability in responses. Additionally, the use of self-reported questionnaires carries an inherent risk of bias and baseline differences between participants may have influenced the observed associations. As with any cross-sectional design, the associations identified do not imply causality. Despite these limitations, the data presented in this study provide a valuable baseline characterization of treatment-naïve patients with CLL.
4.2. Practical Implications
Given the low physical fitness observed in our sample, there are important practical implications. Exercise-based interventions should be integrated across diverse settings—from clinical facilities to community centers and home-based programs—to ensure both accessibility and long-term adherence. Tailored exercise protocols, guided by trained professionals, are essential to maximize potential benefits and minimize risks for this vulnerable population.
4.3. Future Research
Future research should include larger, multicenter cohorts to enhance statistical power and representativeness. Comparative studies examining different training modalities and intensities are needed to determine the most effective approaches for improving physical fitness in patients with CLL. Moreover, investigating immunological mechanisms—such as myokine and cytokine responses—may help clarify how exercise influences clinical outcomes, including frailty, infection risk, and time to disease progression. Such work will be critical for advancing evidence-based exercise guidelines for individuals living with CLL.
5. Conclusions
In conclusion, our findings show that treatment-naïve patients with CLL present body composition values that exceed baseline normative data for healthy populations. This may be partly explained by their low cardiovascular fitness, as indicated by the physical condition parameter. Despite this, patients did not report significant symptoms affecting their daily quality of life, suggesting a perception of high physical functioning that does not align with their objectively low fitness levels. Our results also indicate that greater muscle mass is strongly associated with higher strength levels in patients with CLL, a pattern consistent with what is observed in the general population. Although the existing literature increasingly links exercise training, muscle function, and immune function in older adults, the specific effects of strength training in CLL patients remain underexplored. Given that unintentional weight loss is one of the major symptoms of CLL, these findings highlight the potential importance of incorporating strength training to help preserve muscle mass and physical function in this population. Future research should investigate the impact of such interventions on physical fitness, quality of life, and well-being in patients with CLL.
Author Contributions
Conceptualization, P.C.; methodology, P.C.; validation, J.C.R.; investigation, P.C.; resources, J.M. and J.C.R.; data curation, P.C.; writing—original draft preparation, P.C.; writing—review and editing, R.R., A.P., J.M. and J.C.R.; supervision, R.R. and J.C.R.; project administration, P.C. and J.C.R.; funding acquisition, P.C., J.M. and J.C.R. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by a Research Fellowship for a Doctorate granted by the Foundation for Science and Technology (FCT—National Public Agency from Portugal) (FCT grant n.: 2021.07178.BD: DOI 10.54499/2021.07178.BD), and as sources of Outside Support for Research, we had support by way of equipment and facilities from the Research Center in Physical Activity, Health and Leisure (CIAFEL)—Faculty of Sports—University of Porto (FADEUP) and the Laboratory for Integrative and Translational Research in Population Health (ITR) (CIAFEL UID/00617/2025: doi:10.54499/UID/00617/2025 and ITR: LA/P/0064/2020).
Institutional Review Board Statement
This study was performed in line with the principles of the Declaration of Helsinki. Approval was granted by the Ethics Committee of Centro Hospitalar de São João (Approval Code: CE 431-19, Approval date: 23 May 2020), and by the Faculty of Sport—University of Porto (Approval Code: CEFADE 14_2024, Approval date: 17 April 2024), and informed consent was obtained from all individual participants included in the study.
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study. Written informed consent has been obtained from the patients to publish this paper.
Data Availability Statement
The datasets generated during the present study will not be publicly available but can be obtained from the corresponding author on reasonable request.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| CLL | Chronic Lymphocytic Leukemia |
| PA | Physical Activity |
| BMI | Body Mass Index |
References
- Ou, Y.; Long, Y.; Ji, L.; Zhan, Y.; Qiao, T.; Wang, X.; Chen, H.; Cheng, Y. Trends in Disease Burden of Chronic Lymphocytic Leukemia at the Global, Regional, and National Levels from 1990 to 2019, and Projections Until 2030: A Population-Based Epidemiologic Study. Front. Oncol. 2022, 12, 840616. [Google Scholar] [CrossRef]
- Yao, Y.; Lin, X.; Li, F.; Jin, J.; Wang, H. The global burden and attributable risk factors of chronic lymphocytic leukemia in 204 countries and territories from 1990 to 2019: Analysis based on the global burden of disease study 2019. Biomed. Eng. Online 2022, 21, 4. [Google Scholar] [CrossRef]
- Artese, A.L.; Sitlinger, A.; MacDonald, G.; Deal, M.A.; Hanson, E.D.; Pieper, C.F.; Weinberg, J.B.; Brander, D.M.; Bartlett, D.B. Quality of Life Changes Following High-intensity Interval Training in Older Adults with Chronic Lymphocytic Leukemia. Med. Sci. Sports Exerc. 2022, 54, 157–158. [Google Scholar] [CrossRef]
- Neil, S.E.; Gotay, C.C.; Campbell, K.L. Physical activity levels of cancer survivors in Canada: Findings from the Canadian Community Health Survey. J. Cancer Surviv. 2014, 8, 143–149. [Google Scholar] [CrossRef]
- Veliz, M.; Pinilla-Ibarz, J. Treatment of relapsed or refractory chronic lymphocytic leukemia. Cancer Control 2012, 19, 37–53. [Google Scholar] [CrossRef] [PubMed]
- Shanafelt, T.D.; Bowen, D.; Venkat, C.; Slager, S.L.; Zent, C.S.; Kay, N.E.; Reinalda, M.; Sloan, J.A.; Call, T.G. Quality of life in chronic lymphocytic leukemia: An international survey of 1482 patients. Br. J. Haematol. 2007, 139, 255–264. [Google Scholar] [CrossRef]
- Cunha, P.M.P.; Ribeiro, R.J.; Pizarro, A.; Mota, J.; Ribeiro, J.C.D. High-intensity interval training and strength conditioning in patients with chronic lymphocytic leukemia: A systematic review. Syst. Rev. 2025, 14, 116. [Google Scholar] [CrossRef]
- Sitlinger, A.; Thompson, D.P.; Deal, M.A.; Garcia, E.; Stewart, T.; Guadalupe, E.; Weinberg, J.B.; Bartlett, D.A.; Brander, D.M. Exercise and Chronic Lymphocytic Leukemia (CLL)–Relationships Among Physical Activity, Fitness, & Inflammation, and Their Impacts on CLL Patients. Blood 2018, 132, 3. [Google Scholar] [CrossRef]
- Chastin, S.F.M.; Abaraogu, U.; Bourgois, J.G.; Dall, P.M.; Darnborough, J.; Duncan, E.; Dumortier, J.; Pavón, D.J.; McParland, J.; Roberts, N.J.; et al. Effects of Regular Physical Activity on the Immune System, Vaccination and Risk of Community-Acquired Infectious Disease in the General Population: Systematic Review and Meta-Analysis. Sports Med. 2021, 51, 1673–1686. [Google Scholar] [CrossRef] [PubMed]
- Krüger, K.; Mooren, F.C.; Pilat, C. The Immunomodulatory Effects of Physical Activity. Curr. Pharm. Des. 2016, 22, 3730–3748. [Google Scholar] [CrossRef] [PubMed]
- Hallek, M.; Cheson, B.D.; Catovsky, D.; Caligaris-Cappio, F.; Dighiero, G.; Dohner, H.; Hillmen, P.; Keating, M.; Montserrat, E.; Chiorazzi, N.; et al. iwCLL guidelines for diagnosis, indications for treatment, response assessment, and supportive management of CLL. Blood 2018, 131, 2745–2760. [Google Scholar] [CrossRef]
- De Backer, I.C.; Vreugdenhil, G.; Nijziel, M.R.; Kester, A.D.; van Breda, E.; Schep, G. Long-term follow-up after cancer rehabilitation using high-intensity resistance training: Persistent improvement of physical performance and quality of life. Br. J. Cancer 2008, 99, 30–36. [Google Scholar] [CrossRef]
- Chen, L.J.; Peng, P.C.; Xu, Z.M.; Ding, X.F. The effects of exercise on the quality of life of patients with breast cancer: A systematic review and meta-analysis based on the QLQ-C30 quality of life scale. Gland Surg. 2023, 12, 633–650. [Google Scholar] [CrossRef]
- Campbell, K.L.; Winters-Stone, K.M.; Wiskemann, J.; May, A.M.; Schwartz, A.L.; Courneya, K.S.; Zucker, D.S.; Matthews, C.E.; Ligibel, J.A.; Gerber, L.H.; et al. Exercise Guidelines for Cancer Survivors: Consensus Statement from International Multidisciplinary Roundtable. Med. Sci. Sports Exerc. 2019, 51, 2375–2390. [Google Scholar] [CrossRef] [PubMed]
- Pedersen, B.K.; Saltin, B. Exercise as medicine—Evidence for prescribing exercise as therapy in 26 different chronic diseases. Scand. J. Med. Sci. Sport. 2015, 25, 1–72. [Google Scholar] [CrossRef] [PubMed]
- Luan, X.; Tian, X.Y.; Zhang, H.X.; Huang, R.; Li, N.; Chen, P.J.; Wang, R. Exercise as a prescription for patients with various diseases. J. Sport Health Sci. 2019, 8, 422–441. [Google Scholar] [CrossRef] [PubMed]
- Courneya, K.S.; Sellar, C.M.; Stevinson, C.; McNeely, M.L.; Peddle, C.J.; Friedenreich, C.M.; Tankel, K.; Basi, S.; Chua, N.; Mazurek, A.; et al. Randomized Controlled Trial of the Effects of Aerobic Exercise on Physical Functioning and Quality of Life in Lymphoma Patients. J. Clin. Oncol. 2009, 27, 4605–4612. [Google Scholar] [CrossRef]
- Furzer, B.J.; Ackland, T.R.; Wallman, K.E.; Petterson, A.S.; Gordon, S.M.; Wright, K.E.; Joske, D.J.L. A randomised controlled trial comparing the effects of a 12-week supervised exercise versus usual care on outcomes in haematological cancer patients. Support. Care Cancer 2016, 24, 1697–1707. [Google Scholar] [CrossRef]
- Nadler, M.B.; Desnoyers, A.; Langelier, D.M.; Amir, E. The Effect of Exercise on Quality of Life, Fatigue, Physical Function, and Safety in Advanced Solid Tumor Cancers: A Meta-analysis of Randomized Control Trials. J. Pain Symptom Manag. 2019, 58, 899. [Google Scholar] [CrossRef]
- MacDonald, G.; Sitlinger, A.; Deal, M.A.; Hanson, E.D.; Ferraro, S.; Pieper, C.F.; Weinberg, J.B.; Brander, D.M.; Bartlett, D.B. A pilot study of high-intensity interval training in older adults with treatment naive chronic lymphocytic leukemia. Sci. Rep. 2021, 11, 23137. [Google Scholar] [CrossRef]
- Crane, J.C.; Gordon, M.J.; Basen-Engquist, K.; Ferrajoli, A.; Markofski, M.M.; Lee, C.Y.; Fares, S.; Simpson, R.J.; LaVoy, E.C. Relationships between T-lymphocytes and physical function in adults with chronic lymphocytic leukemia: Results from the HEALTH4CLL pilot study. Eur. J. Haematol. 2023, 110, 732–742. [Google Scholar] [CrossRef]
- Sitlinger, A.; Brander, D.M.; Bartlett, D.B. Impact of exercise on the immune system and outcomes in hematologic malignancies. Blood Adv. 2020, 4, 1801–1811. [Google Scholar] [CrossRef]
- Vallerand, J.R.; Rhodes, R.E.; Walker, G.J.; Courneya, K.S. Correlates of meeting the combined and independent aerobic and strength exercise guidelines in hematologic cancer survivors. Int. J. Behav. Nutr. Phys. Act. 2017, 14, 44. [Google Scholar] [CrossRef]
- Troiano, R.P.; Berrigan, D.; Dodd, K.W.; Masse, L.C.; Tilert, T.; Mcdowell, M. Physical activity in the United States measured by accelerometer. Med. Sci. Sports Exerc. 2008, 40, 181–188. [Google Scholar] [CrossRef] [PubMed]
- Tudor-Locke, C.; Brashear, M.M.; Johnson, W.D.; Katzmarzyk, P.T. Accelerometer profiles of physical activity and inactivity in normal weight, overweight, and obese US men and women. Int. J. Behav. Nutr. Phys. Act. 2010, 7, 60. [Google Scholar] [CrossRef] [PubMed]
- Stevens, W.R.; Anderson, A.M.; Tulchin-Francis, K. Validation of Accelerometry Data to Identify Movement Patterns During Agility Testing. Front. Sports Act. Living 2020, 2, 563809. [Google Scholar] [CrossRef]
- Khakurel, J.; Porras, J.; Melkas, H.; Fu, B. A Comprehensive Framework of Usability Issues Related to the Wearable Devices. In Convergence of ICT and Smart Devices for Emerging Applications; Paiva, S., Paul, S., Eds.; Springer International Publishing: Cham, Switzerland, 2020; pp. 21–66. [Google Scholar]
- Bade, B.C.; Brooks, M.C.; Nietert, S.B.; Ulmer, A.; Thomas, D.D.; Nietert, P.J.; Scott, J.B.; Silvestri, G.A. Assessing the Correlation Between Physical Activity and Quality of Life in Advanced Lung Cancer. Integr. Cancer Ther. 2018, 17, 73–79. [Google Scholar] [CrossRef]
- Chan, C.; Sounderajah, V.; Normahani, P.; Acharya, A.; Markar, S.R.; Darzi, A.; Bicknell, C.; Riga, C. Wearable Activity Monitors in Home Based Exercise Therapy for Patients with Intermittent Claudication: A Systematic Review. Eur. J. Vasc. Endovasc. 2021, 61, 676–687. [Google Scholar] [CrossRef]
- Ormel, H.L.; van der Schoot, G.G.F.; Westerink, N.D.L.; Sluiter, W.J.; Gietema, J.A.; Walenkamp, A.M.E. Self-monitoring physical activity with a smartphone application in cancer patients: A randomized feasibility study (SMART-trial). Support. Care Cancer 2018, 26, 3915–3923. [Google Scholar] [CrossRef]
- Ferriolli, E.; Skipworth, R.J.E.; Hendry, P.; Scott, A.; Stensteth, J.; Dahele, M.; Wall, L.; Greig, C.; Fallon, M.; Strasser, F.; et al. Physical Activity Monitoring: A Responsive and Meaningful Patient-Centered Outcome for Surgery, Chemotherapy, or Radiotherapy? J. Pain Symptom Manag. 2012, 43, 1025–1035. [Google Scholar] [CrossRef] [PubMed]
- Ella, K.; Csepányi-Kömi, R.; Káldi, K. Circadian regulation of human peripheral neutrophils. Brain Behav. Immun. 2016, 57, 209–221. [Google Scholar] [CrossRef]
- Pritchard, A.; Burns, P.; Correia, J.; Jamieson, P.; Moxon, P.; Purvis, J.; Thomas, M.; Tighe, H.; Sylvester, K.P. ARTP statement on cardiopulmonary exercise testing 2021. BMJ Open Respir. Res. 2021, 8, e001121. [Google Scholar] [CrossRef]
- Radtke, T.; Vogiatzis, I.; Urquhart, D.S.; Laveneziana, P.; Casaburi, R.; Hebestreit, H.; Crook, S.; Kaltsakas, G.; Louvaris, Z.; Berton, D.C.; et al. Standardisation of cardiopulmonary exercise testing in chronic lung diseases: Summary of key findings from the ERS task force. Eur. Respir. J. 2019, 54, 1901441. [Google Scholar] [CrossRef]
- Harkonen, R.; Harju, R.; Alaranta, H. Accuracy of the Jamar dynamometer. J. Hand Ther. 1993, 6, 259–262. [Google Scholar] [CrossRef] [PubMed]
- National Institute for Health and Care Research. Procedure for Measuring Hand Grip Strength Using The JAMAR Dynamometer; NIHR Southampton Biomedical Research Centre: Southampton, UK, 2014; p. 6. [Google Scholar]
- Kannus, P. Isokinetic evaluation of muscular performance: Implications for muscle testing and rehabilitation. Int. J. Sports Med. 1994, 15 (Suppl. 1), S11–S18. [Google Scholar] [CrossRef]
- Broderick, J.M.; Ryan, J.; O’Donnell, D.M.; Hussey, J. A guide to assessing physical activity using accelerometry in cancer patients. Support. Care Cancer 2014, 22, 1121–1130. [Google Scholar] [CrossRef]
- Binet, J.L.; Auquier, A.; Dighiero, G.; Chastang, C.; Piguet, H.; Goasguen, J.; Vaugier, G.; Potron, G.; Colona, P.; Oberling, F.; et al. A New Prognostic Classification of Chronic Lymphocytic-Leukemia Derived from a Multivariate Survival Analysis. Cancer 1981, 48, 198–206. [Google Scholar] [CrossRef]
- Rai, K.R.; Sawitsky, A.; Cronkite, E.P.; Chanana, A.D.; Levy, R.N.; Pasternack, B.S. Clinical Staging of Chronic Lymphocytic Leukemia. Blood 1975, 46, 219–234. [Google Scholar] [CrossRef]
- Sorensen, J.B.; Klee, M.; Palshof, T.; Hansen, H.H. Performance status assessment in cancer patients. An inter-observer variability study. Br. J. Cancer 1993, 67, 773–775. [Google Scholar] [CrossRef] [PubMed]
- Webster, K.; Cella, D.; Yost, K. The Functional Assessment of Chronic Illness Therapy (FACIT) Measurement System: Properties, applications, and interpretation. Health Qual. Life Outcomes 2003, 1, 79. [Google Scholar] [CrossRef] [PubMed]
- Oerlemans, S.; Efficace, F.; Kieffer, J.M.; Kyriakou, C.; Xochelli, A.; Levedahl, K.; Petranovic, D.; Borges, F.C.; Bredart, A.; Shamieh, O.; et al. International validation of the EORTC QLQ-CLL17 questionnaire for assessment of health-related quality of life for patients with chronic lymphocytic leukaemia. Br. J. Haematol. 2022, 197, 431–441. [Google Scholar] [CrossRef]
- Joanna Briggs Institute. Checklist for Analytical Cross-Sectional Studies; Joanna Briggs Institute: Adelaide, Australia, 2020. [Google Scholar]
- Kang, H. Sample size determination and power analysis using the G*Power software. J. Educ. Eval. Health Prof. 2021, 18, 17. [Google Scholar] [CrossRef]
- Cohen, J. Statistical Power Analysis for the Behavioral Sciences, 2nd ed.; Routledge: New York, NY, USA, 1988. [Google Scholar]
- Yamada, P.M.; Teranishi-Hashimoto, C.; Bantum, E.O. Paired exercise has superior effects on psychosocial health compared to individual exercise in female cancer patients. Support. Care Cancer 2021, 29, 6305–6314. [Google Scholar] [CrossRef] [PubMed]
- Segal, R.J.; Reid, R.D.; Courneya, K.S.; Sigal, R.J.; Kenny, G.P.; Prud’Homme, D.G.; Malone, S.C.; Wells, G.A.; Scott, C.G.; D’Angelo, M.E.S. Randomized Controlled Trial of Resistance or Aerobic Exercise in Men Receiving Radiation Therapy for Prostate Cancer. J. Clin. Oncol. 2009, 27, 344–351. [Google Scholar] [CrossRef]
- Milne, H.M.; Wallman, K.E.; Gordon, S.; Courneya, K.S. Effects of a combined aerobic and resistance exercise program in breast cancer survivors: A randomized controlled trial. Breast Cancer Res. Treat. 2008, 108, 279–288. [Google Scholar] [CrossRef] [PubMed]
- ACSM. ACSM’s Guidelines for Exercise Testing and Prescription, 10th ed.; Wolters Kluwer Health: Philadelphia, PA, USA, 2018. [Google Scholar]
- Zeng, X.; Zhang, L.; Zhang, Y.; Jia, S.; Lin, T.; Zhao, X.; Huang, X. Prevalence and prognostic value of baseline sarcopenia in hematologic malignancies: A systematic review. Front. Oncol. 2023, 13, 1308544. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.S.; Galvao, D.A.; Newton, R.U.; Gray, E.; Taaffe, D.R. Exercise-induced myokines and their effect on prostate cancer. Nat. Rev. Urol. 2021, 18, 519–542. [Google Scholar] [CrossRef]
- Stamatakis, E.; Lee, I.M.; Bennie, J.; Freeston, J.; Hamer, M.; O’Donovan, G.; Ding, D.; Bauman, A.; Mavros, Y. Does Strength-Promoting Exercise Confer Unique Health Benefits? A Pooled Analysis of Data on 11 Population Cohorts with All-Cause, Cancer, and Cardiovascular Mortality Endpoints. Am. J. Epidemiol. 2018, 187, 1102–1112. [Google Scholar] [CrossRef]
- Imboden, M.T.; Swartz, A.M.; Finch, H.W.; Herber, M.P.; Kaminsky, L.A. Reference standards for lean mass measures using GE dual energy X-ray absorptiometry in Caucasian adults. PLoS ONE 2017, 12, e0176161. [Google Scholar] [CrossRef]
- Holzner, B.; Kemmler, G.; Kopp, M.; Nguyen-Van-Tam, D.; Sperner-Unterweger, B.; Greil, R. Quality of life of patients with chronic lymphocytic leukemia: Results of a longitudinal investigation over 1 yr. Eur. J. Haematol. 2004, 72, 381–389. [Google Scholar] [CrossRef]
- Youron, P.; Singh, C.; Jindal, N.; Malhotra, P.; Khadwal, A.; Jain, A.; Prakash, G.; Varma, N.; Varma, S.; Lad, D.P. Quality of life in patients of chronic lymphocytic leukemia using the EORTC QLQ-C30 and QLQ-CLL17 questionnaire. Eur. J. Haematol. 2020, 105, 755–762. [Google Scholar] [CrossRef]
- Artese, A.L.; Sitlinger, A.; MacDonald, G.; Deal, M.A.; Hanson, E.D.; Pieper, C.F.; Weinberg, J.B.; Brander, D.M.; Bartlett, D.B. Effects of high-intensity interval training on health-related quality of life in chronic lymphocytic leukemia: A pilot study. J. Geriatr. Oncol. 2022, 14, 101373. [Google Scholar] [CrossRef] [PubMed]
- Stein, M.J.; Baurecht, H.; Bohmann, P.; Fervers, B.; Fontvieille, E.; Freisling, H.; Friedenreich, C.M.; Konzok, J.; Peruchet-Noray, L.; Sedlmeier, A.M.; et al. Diurnal timing of physical activity and risk of colorectal cancer in the UK Biobank. BMC Med. 2024, 22, 399. [Google Scholar] [CrossRef] [PubMed]
- Albalak, G.; Stijntjes, M.; van Bodegom, D.; Jukema, J.W.; Atsma, D.E.; van Heemst, D.; Noordam, R. Setting your clock: Associations between timing of objective physical activity and cardiovascular disease risk in the general population. Eur. J. Prev. Cardiol. 2023, 30, 232–240. [Google Scholar] [CrossRef] [PubMed]
- Okely, A.D.; Kontsevaya, A.; Ng, J.; Abdeta, C. 2020 WHO guidelines on physical activity and sedentary behavior. Sports Med. Health Sci. 2021, 3, 115–118. [Google Scholar] [CrossRef]
- Persoon, S.; Chinapaw, M.J.M.; Buffart, L.M.; Liu, R.D.K.; Wijermans, P.; Koene, H.R.; Minnema, M.C.; Lugtenburg, P.J.; Marijt, E.W.A.; Brug, J.; et al. Randomized controlled trial on the effects of a supervised high intensity exercise program in patients with a hematologic malignancy treated with autologous stem cell transplantation: Results from the EXIST study. PLoS ONE 2017, 12, e0181313. [Google Scholar] [CrossRef]
- Central Administration of the Health System, IP, Portugal. VALORES LABORATORIAIS DE REFERÊNCIA (ADULTOS). Available online: https://www.acss.min-saude.pt/wp-content/uploads/2018/09/Tabela_Final.pdf (accessed on 5 March 2025).
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).