Abstract
Biodegradable plastics are increasingly proposed as environmentally friendly alternatives for disposable dishes or glasses in addition to their more conventional uses as foils and in bags. If produced from certified degradable materials, such items are expected to degrade rapidly during state-of-the-art composting. However, conditions prescribed for the testing and certification of materials differ from those typically applied in industrial composting, and operators of the corresponding plants have found that degradation is incomplete. In this study the degradation of commercially available biodegradable bags as well as disposable sparkling wine glasses was studied in a series of pilot-scale composting campaigns closely mimicking state-of-the-art composting conditions. The materials were characterized regarding their chemical composition, structure, and crystallinity, as well as the changes thereof throughout the process. Evidence is given that parameters such as crystallinity change significantly during composting, which may inhibit breakdown during the process and thus have unknown consequences for the subsequent environmental impact.
1. Introduction
For several decades, plastic pollution has mainly been discussed in the context of the aqueous environment, such as oceans, lakes, and rivers, while the pollution of land has been less considered. However, any contamination, e.g., of arable soil is of concern, since microplastic found there may easily enter the food web [1]. In addition, microplastic has been shown to change the characteristics of the soil. Film fragments may influence the density of the soil, while fibers have been suspected of changing its storage capacity for water [2,3]. The chemical nature of the plastic has in addition been shown to influence the adsorption of heavy metals and other toxins [4,5,6].
The polymer types most commonly found in the environment are commodity plastics such as polyethylene (PE), polypropylene (PP), polystyrene (PS), polyethylene terephthalate (PET), polyurethane (PU), and polyvinylchloride (PVC) [7,8]. However, increasingly, studies also find microplastic with signatures of biodegradable materials, mostly poly(lactic acid) (PLA) and poly(butylene adipate terephthalate) (PBAT) [9,10]. PBAT has been shown to influence soil characteristics, inter alia the propensity for the release of CO2 into the atmosphere [11].
Biodegradable plastics are often proposed as an environmentally friendly alternative to conventional plastics for one-way-use items such as disposable dishes, but also for uses such as bags for biowaste collection. The assumption is that, when properly disposed of, e.g., in the solid household biowaste, such materials will degrade during state-of-the-art biowaste treatment, whereas the environmental burden is reduced even in case of improper disposal, as such a material should degrade in the environment within an acceptable time span. However, these assumptions have recently been challenged by studies demonstrating that quality composts produced in state-of-the-art technical composting plants may still contain significant numbers of microplastic fragments with signatures of biodegradable materials [10,12]. Microplastic particles from biodegradable materials may therefore enter the environment through the organic fertilizer (compost) produced in these plants. The fate of such microplastic in the various environmental compartments is still not completely understood [13].
One obvious reason for failing to fully degrade during industrial composting is the complexity of the composting process itself. Several requirements must be met in technical composing. The temperature must, e.g., be sufficient to assure sanitization, whereas the duration of the process is typically just long enough to obtain the desired degree of rotting. The 12 weeks prescribed in the DIN EN 13432 [14] to test and certify materials for compostability are generally not reached in industrial composting.
In order to gain a more systematic insight into the degradation of items made from biodegradable materials under the condition of technical composting, we mirrored such conditions in model pilot-scale composting experiments. The degradation of three types of compostable bags designated for biowaste collection and one type of disposable sparkling wine glass was studied. In addition, factors such as temperature and moisture were systematically investigated under abiotic conditions.
2. Materials and Methods
2.1. Materials
Suppliers of chemicals were Carl Roth GmbH and Sigma-Aldrich. Solvents and reagents were technical grade and used as received. Deuterated solvents were from Deutero and stored over molecular sieves. The biowaste bags, all certified as compostable according to DIN EN 13432 [14], were from EMIL DEISS KG (GmbH + Co., Hamburg, Germany) or Naturabiomat GmbH (Rheine, Germany). Hereafter they will be referred to as Bag 1, Bag 2, and Bag 3. The disposable glasses were purchased at www.avocadostore.de (accessed on 10 May 2025) (EAN: 8027499022124) and according to the supplier were made from a PLA also certified as compostable according to DIN EN 13432.
2.2. Composting
Composting was carried out with biowaste donated by the local municipal composting plant in steel barrels (height: 790 mm; diameter: 570 mm) equipped with drainage (Figure S1). Prior to the experiments, contaminants such as glass, metal, and plastics were removed from the biowaste.
For optimal composting, the C/N ratio should be between 20 and 30 according to DIN EN 14045 [15]. To determine the C/N ratio, the components of the biowaste were weighed and calculated using the values recommended by the German Compost Quality Association (Bundesgütegemeinschaft Kompost e.V.) [16]. The waste was divided into three fractions: kitchen waste (C/N: 12–20), grass clippings (C/N: 15–35), and structural material consisting of branches and shrub cuttings (C/N: 100–150). These values are comparable with the literature [17,18,19]. The resulting average C/N ratios were as follows: composter 1: 28.1; composter 2: 28.7; and composter 3: 27.2. The calculation was performed based on the wet weight.
At minimum 10% structural material was added to the biowaste to assure sufficient aeration during composting. For the determination of the dry weight (DW), five 30 mL aliquots were weighed into 250 mL Schott-Duran beakers and dried at 105 °C (Memmert UM 500, Memmert, Schwabach, Germany) for at least 24 h. Afterwards, the beakers were allowed to cool to room temperature in a desiccator and the DW was determined by weighing the beakers. The bags (16 per experiment) and disposable glass cups (12 per experiment) to be tested were filled with biowaste and placed together with additional bulk biowaste (total approximately 140 L) into the barrels (Table S1).
The barrel was placed on a rotation device (Figure S2) and rotated once per week at the same time the oxygen level was measured (oxygen sensor GOX 100, Greisinger electronic GmbH; Regenstauf, Germany). The temperature was measured with a rod thermometer (TFA Dostmann; Wertheim-Reicholzheim, Germany). A heating jacket (ISOHEAT PT100, SAF-Wärmetechnik; Ubstadt-Weiher, Germany) ensured that the rim of the compost had the same temperature as the bulk. The pH was measured by taking 50 g samples of a given compost, mixing them with 250 mL of deionized water followed by 20 min constant agitation and the pH measurement (pH meter VWR International; Darmstadt, Germany).
2.3. Abiotic Decomposition
A total of 12 pieces 5 × 5 cm each were cut from the bags and placed in 0.5 L of 0.01 M Tris buffer in 1 L Schott DURAN beakers, covered with aluminum foil (total amounts: Bag1, 0.76 g; Bag2, 0.6 g; and Bag3 0.94 g). In case of the disposable glasses, five stems were used (total amount: 37.13 g), since it was only possible to obtain test pieces of equal geometry from the stems. The temperature and pH were adjusted as indicated, the latter with 0.1 M HCL or 0.1 M NaOH. For temperature control, the beakers were placed in a Memmert UM 500 warming oven (Memmert GmbH & Co. KG, Schwabach, Germany).
2.4. Fragment Recovery
The compost from every composter was divided into 2.5 kg piles and each pile suspended in 10 L of demineralized water. The sludge was first sieved with a 10 mm sieve. All particles/fragments retained by the sieve were collected with tweezers. The material having passed the 10 mm sieve was then sieved with a 5 mm sieve, followed by sieving with 2 mm, 1 mm, and 0.5 mm sieves. All sieves were from Retsch GmbH (test sieves, ISO 3310-1 [20]; body/mesh, S-Steel; body, 200 mm × 50 mm, Haan, Germany). Recovery of particles from the abiotic decomposition experiments was analogous.
2.5. Analytics
IR spectra were taken with a Perkin Elmer Spectrum Two, 1H-NMR measurements using a Bruker Avance 300. Deuterated δ(CDCl3) = 7.26 ppm served as solvent. The software MestreNova (version 14.3.1) was used for the evaluation of the data.
Polymer molecular masses and their dispersity were determined by GPC (1200 series, Agilent Technologies) equipped with an SDV XL gel column (particle size 5 µm, separation range 100 to 3,000,000 Da) and a refractive index detector. The samples were dissolved in chloroform and filtered with a 0.22 µm PTFE filter prior to injection (injection volume 20 µL). Chloroform also served as eluent (flow rate 0.5 mL min−1). Toluene was used as internal standard. The system was calibrated using narrow-dispersion polystyrene standards.
WAXS measures were carried out on a Bruker D8 ADVANCE using the Cu-Kα radiation (λ = 0.154 nm). A transmittance program in the 2θ angle range between 5° and 45° with a scanning speed of 0.05° min−1 at 25 °C was selected for the XRD profiles. Fragments that were smaller than the templates from the device were fixated with polyimide tape. The software Origin (OriginPro 2025) was used to calculate crystallinities. The peaks were fitted with the tool pulse analyzer, and the values were calculated using the following equation with AC as crystallin areas and AA for the amorphous halo:
The instrument was calibrated using a suitable standard, and its resolution was validated using a crystalline standard.
Crystallinities were in addition determined by DSC using a NETZSCH DSC 204 F1 Phoenix® apparatus (Selb, Germany)—heating rate 10 K min−1, nitrogen flow. The aluminum crucibles were from THEPRO. The data were evaluated using the software Proteus 8.0. The crystallinity was calculated as follows:
with ΔHc crystallization enthalpy, ΔHm melting enthalpy, and ΔHf theoretical heat of fusion for a 100% crystalline PLA (93 J g−1 [21]).
The DSC instrument was calibrated using an indium standard, and its accuracy was validated using a certified reference material. Regular maintenance was performed for all analytical instruments to ensure optimal performance.
3. Results and Discussion
3.1. Physico-Chemical Characterization of Disposable Bags and Glasses
The chemical composition of the bags was analyzed by 1H-NMR spectroscopy after dissolution in chloroform using CDCl3 as the internal standard (Figure S3). All bags contained PLA and PBAT in a ratio of roughly 20:80. The 1H-NMR of Bag 2 showed besides the typical PLA quartet between 5.10 ppm and 5.25 ppm a multiplet shifted to higher values. This indicates that besides poly(L-lactic acid) (PLLA), poly(D,L-lactic acid) was also present. The 1H-NMR of the disposable sparkling wine glass corresponded to pure PLLA. In addition, the composition of the PBAT regarding the monomeric units butylene terephthalate (BT) and butylene adipate (BA) were calculated from the 1H-NMR peaks, see Figures S4–S6 for details. The ratios of the two monomeric units BT:BA within the PBAT were identical for all bags, namely 49 wt%: 51 wt%.
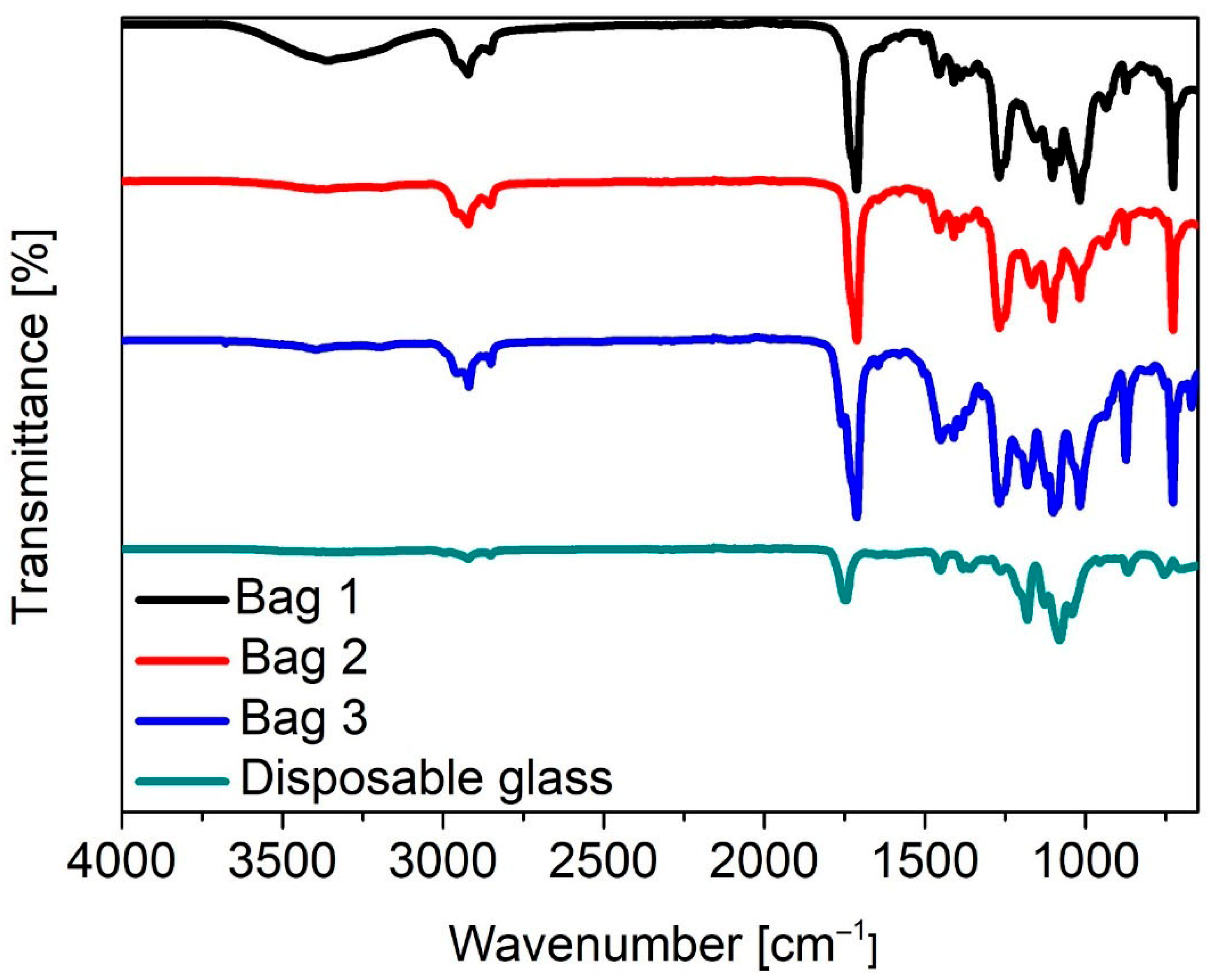
Subsequently the materials were analyzed by infrared (IR) spectroscopy (Figure 1). While the presence of PLA and PBAT was verified as expected, for the bags, additional signals were observed that could not be assigned to either PLA or PBAT, e.g., the hydroxy stretching vibrations at 3280 cm−1. These signals likely stem from additives that do not dissolve in chloroform and therefore were not detected in the 1H-NMR. The unresolved residue amounted to 15 wt% for Bag 1, 26 wt% for Bag 2, and 22 wt% for Bag 3.
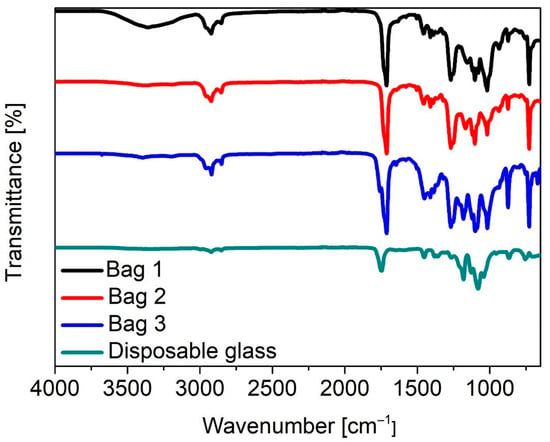
Figure 1.
IR spectra of the tested materials as indicated.
To elucidate the nature of these additives, the corresponding IR spectra were compared to those of the original bags and the dissolved polymer fraction recovered from the chloroform solution via the evaporation of the solvent (Figure S7). Metal oxides and thermoplastic starch (TPS) could be identified as additives. The presence of Mg, Ti, Ca, and Si in the residue could in addition be verified by EDX measurements (Figure S8).
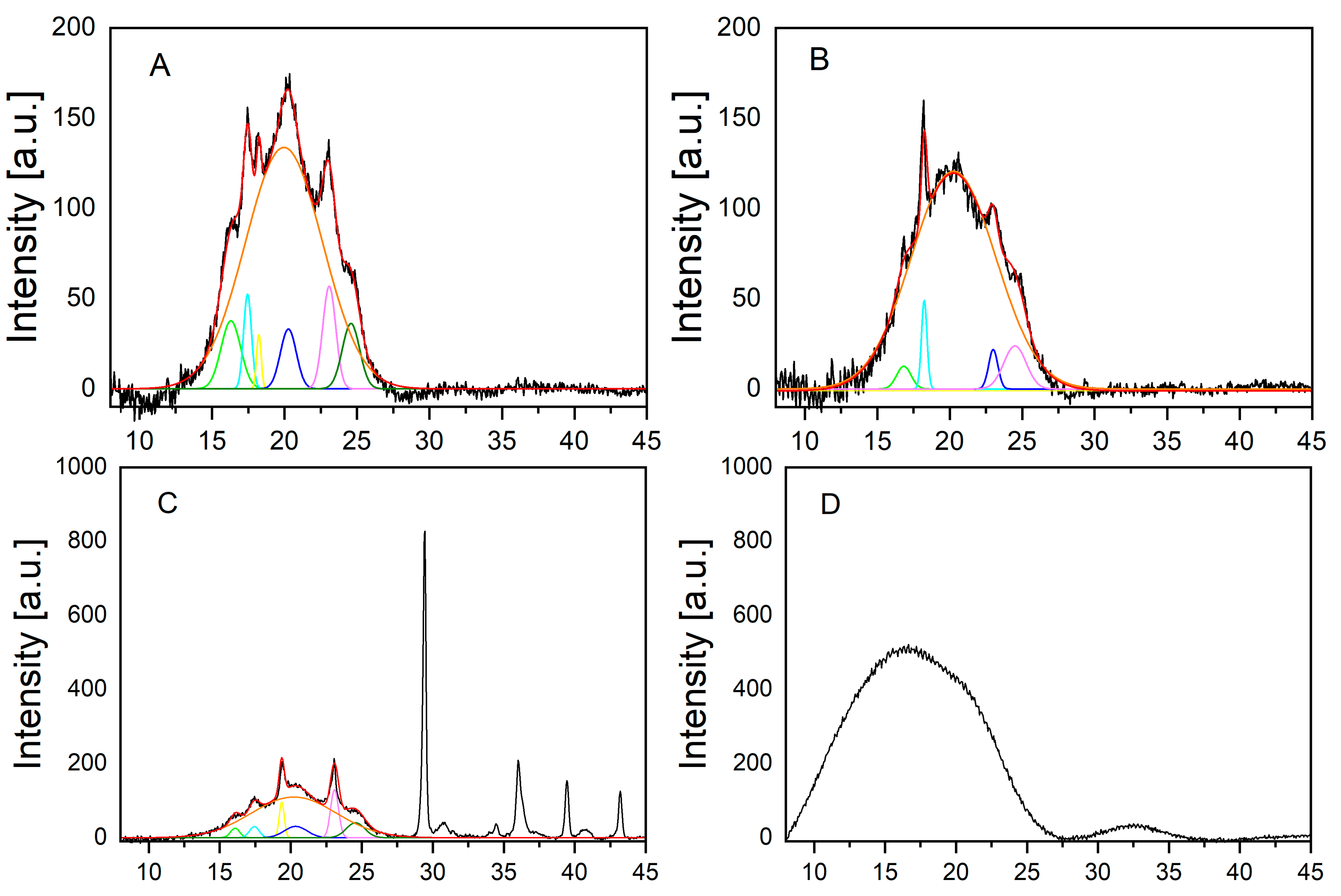
Finally, the crystallinity of the investigated materials was determined by wide-angle-X-ray scattering (WAXS) (Figure 2). Crystallinity improves the mechanical stability of a polymeric material. However, the degree of crystallinity is also a decisive factor for the biodegradability of polymer materials. It has already been shown that high crystallinity leads to slower biodegradation rates [22,23]. Whereas the bags contained between 14 and 27% of crystalline parts (for details see Table 1 below), neither the stem nor the cup of the pristine disposable glasses showed any crystallinity in the WAXS measurements.
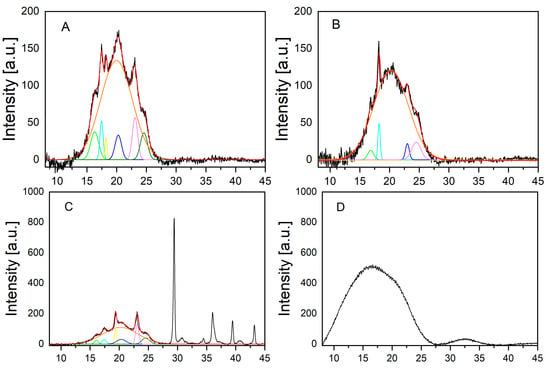
Figure 2.
WAXS measurement of Bags 1 to 3 and the disposable glass: (A) bag 1; (B) bag 2; (C) bag 3; (D) disposable glass. The jagged curve (black) is the signal of the measurement. The curve directly above the signal is the fitted curve (red). The smooth curve directly below represents the halo, which represents the amorphous part (orange). The smaller peaks in the lower part of the respective graph (in different colors) show the crystalline part, which are the peaks deviating from the halo.

Table 1.
Development of the crystallinity of bags 1 to 3 during composting and abiotic decomposition: analysis by WAXS.
3.2. Degradation of Disposable Bags and Glasses Under Composting Conditions
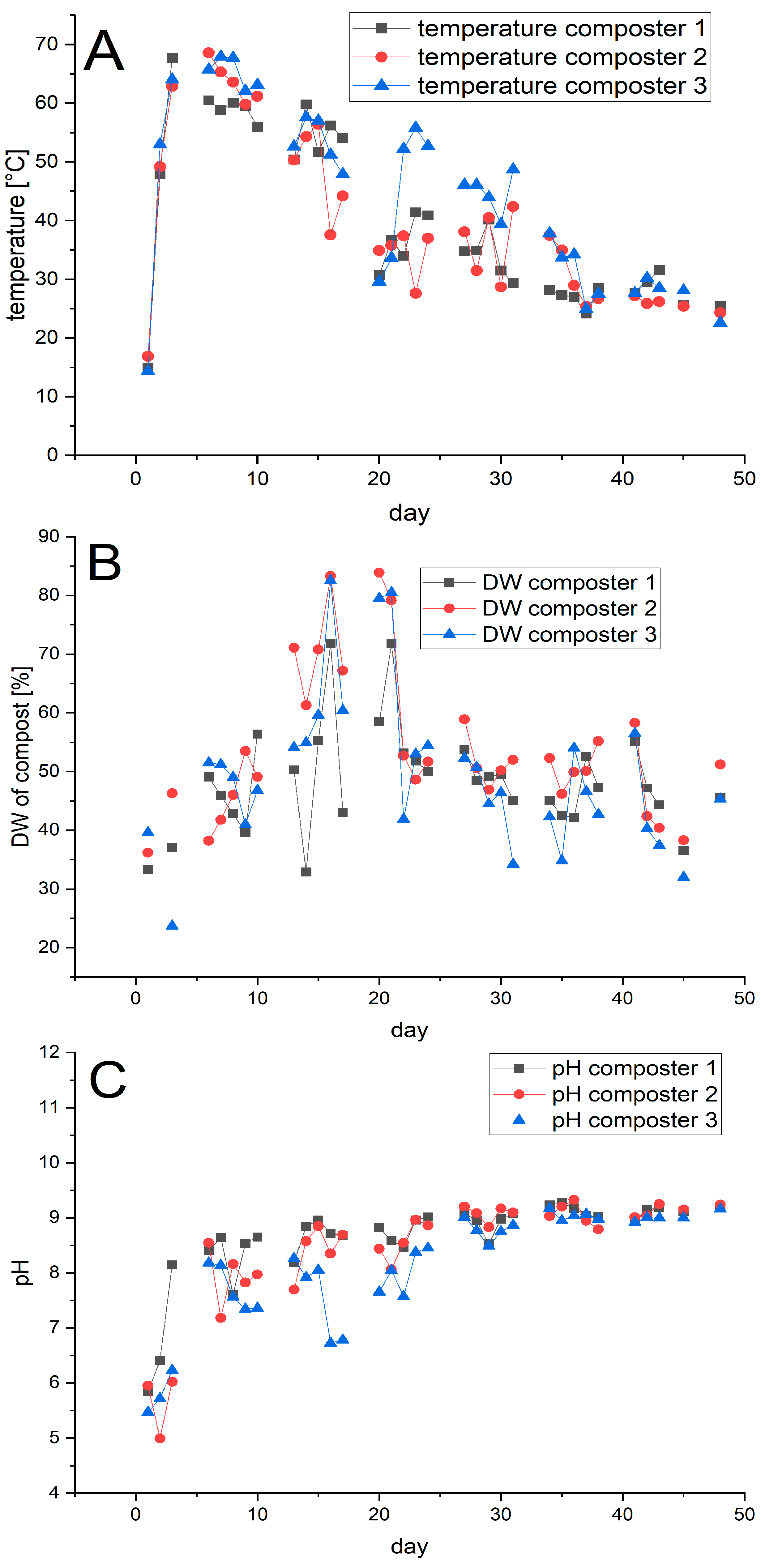
Composting was carried out for 48 days in three pilot composters (volume: 200 L; composting volume 140 L). Bags 2 and 3 were introduced into composter 1, Bag 1 into composter 2, while composter 3 was used to treat the disposable glasses. In each experiment, 12 bags or glasses were used, see Table S1 for further details. The composters were monitored daily for temperature, dry weight, and pH (Figure 3).
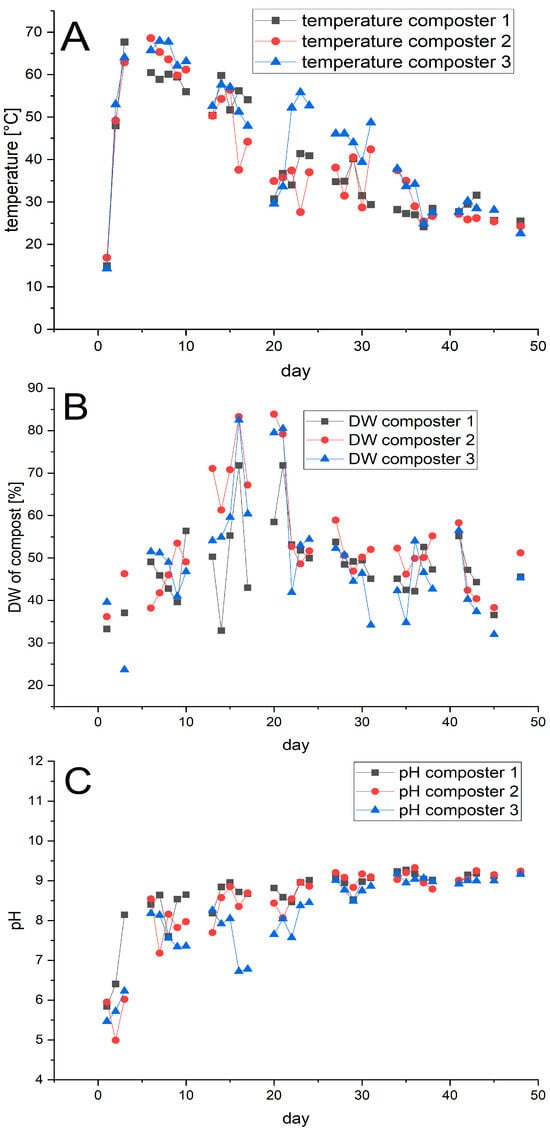
Figure 3.
Profile of the temperature (A), dry weight (DW) (B), and pH (C) in the three composters. Composting was performed in parallel. Bags 2 and 3 were composted in composter 1, bag 1 in composter 2, and the disposable glasses in composter 3.
The temperature showed the expected process phases [24,25]. After an initial mesophilic phase of 1–2 days, the temperature entered a thermophilic phase with temperatures over 60 °C, where it remained for approximately 1 week, followed by cooling and maturation. Only in composter 1 was the temperature slightly lower (59 °C) on day 7. The fluctuations in the temperature are due to interventions like the weekly turnover of the compost or the addition of water to counteract drying, as indicated by the changes in the dry weight (DW).
The pH of the composts increased rapidly over the first two days to values around 8. Subsequently the value continued to increase gradually, reaching a final value around 9, which mirrors the development found in technical composting. Only in composter 3, containing the disposable glasses, did we repeatedly observe drops in the pH, presumed to be due to the release of lactic acid from the PLA. The drop in pH was counteracted by us through the addition of a base when necessary. The oxygen level in the composters was also monitored and never dropped below 13%, indicating sufficient aeration, as specified in DIN EN 14045 [15].
All three composters lost approximately 56% of organic mass during composting, which is reasonable in view of the typical values found in technical composting. When the produced composts were sieved down to a mesh size of 0.5 mm, 39.5% of the initially added mass of bag 1 was recovered from composter 2 and 36.4% of the initially added combined masses of bags 2 and 3 from composter 1. Visibly, some degradation of the bag material took place during composting (Figure 4). In the case of the disposable glasses, 81 wt% of the initially added material was recovered. Interestingly, most of the recovered glasses were still intact, albeit no longer transparent. Only 1.2–2.4 wt% of the material recovered in this case by the sieving procedure were in the 0.5 to 5 mm range, i.e., represented microplastic.

Figure 4.
Disposable bags and disposable glasses before and after composting for 48 days.
3.3. Degradation of Disposable Bags and Glasses Under Abiotic Conditions
For further insights into the possible disintegration processes, experiments were performed under abiotic conditions while adjusting similar physico-chemical settings (temperature, pH) as during composting. A total of 12 pieces, 5 × 5 cm each, were cut from the bags and placed into individual 1 L Schott jars filled with 0.5 L of 0.01 M Tris buffer, pH 7.4. In case of the disposable glasses, five stems were used since it was only possible to obtain test pieces of equal geometry from the stem.
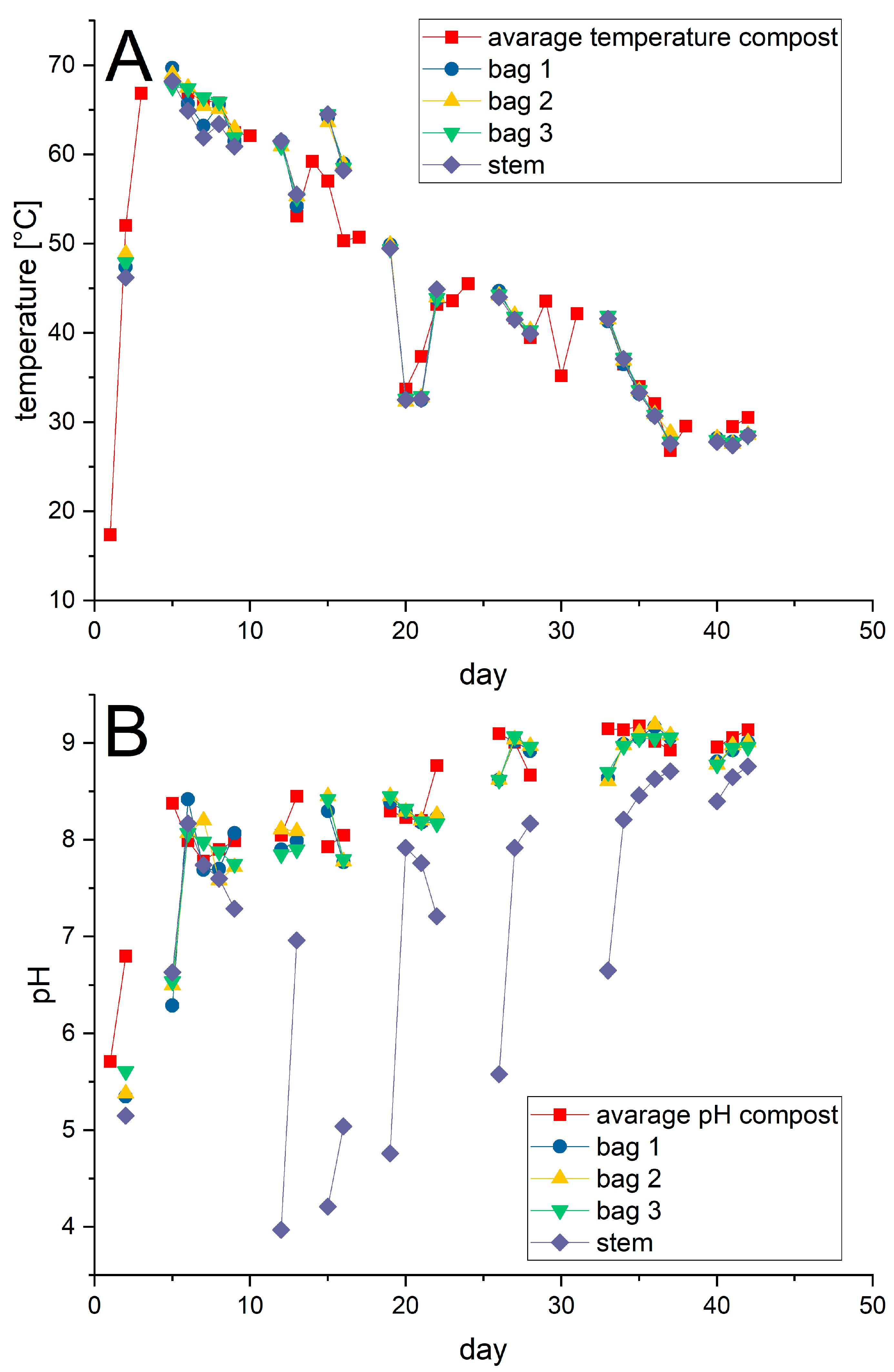
The average temperature and pH profiles recorded for the three composters were applied as closely as possible to the jars, Figure 5. Note that the values shown in Figure 5 were recorded prior to any subsequent adjustment in particular of the pH-value to the prescribed one. Whereas the temperature profile could be adjusted without difficulty in all cases, this was not the case for the pH in the jar containing the disposable glass stems. Starting on day 10 and lasting to day 35, the pH value in jar 3 repeatedly dropped dramatically to acidic levels requiring readjustment via the addition of a base. After day 35 the pH value in jar 3 became more stable and only small additional adjustments were necessary.
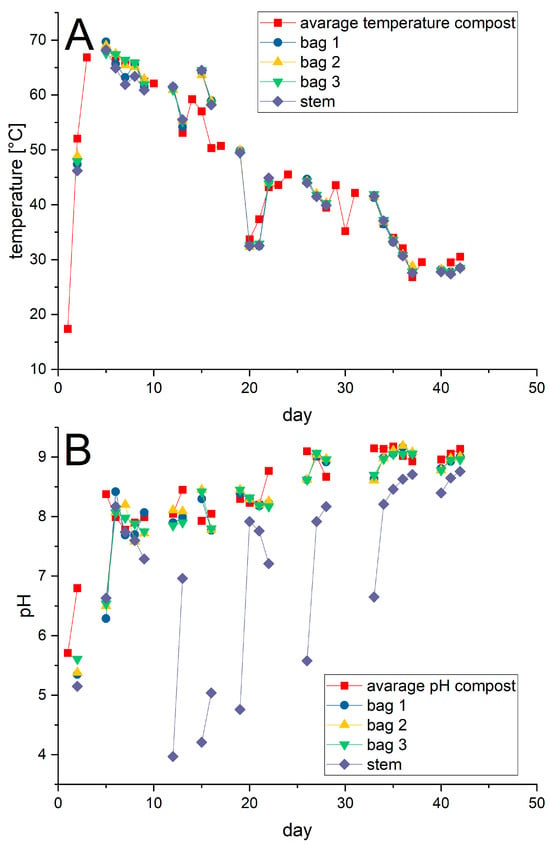
Figure 5.
Profiles of the temperatures (A) and pH values (B) recorded in the abiotic experiments. The average values calculated for temperature and pH from the composting experiments are given for comparison.
At the end of the experiments, 44.6 wt% of bag 1, 49.6 wt% of bag 2, 41.5 wt% of bag 3, and 80.8 wt% of the disposable glass stems could be recovered as distinct particles/fragments > 0.5 mm through the sieving procedure. However, in pronounced contrast to the composting experiments, >85 wt% of the particles generated by the disposable glass stems during abiotic decomposition were now found in the 0.5–5 mm fragment size range. Disintegration into microplastic therefore was much more pronounced during abiotic decomposition than during composting. In contrast, fragments of the bags found after abiotic decomposition tended to be >10 mm, i.e., slightly larger than those produced during composting. The differences in the breakdown behavior of the disposable glasses in the two sets of breakdown experiments may be linked to the repeated acidification of the environment in the case of the abiotic decomposition, which did not occur to a similar degree during composting.
3.4. Development of the Material Characteristics of the Disposable Bags During Degradation
For insight into the changes occurring in the materials during degradation, the material properties of the bags were monitored during composting and abiotic decomposition and compared to the data collected for the pristine materials. Table 2 compiles the development of the composition of the bags in terms of their relative PLA and PBAT content as determined by 1H-NMR.

Table 2.
Composition of the relative content of the respective dissolvable fractions regarding PLA and PBAT: calculation based on 1H-NMR measurements.
Under conditions of abiotic decomposition, the relative PLA content of the bags decreased, slightly for Bags 1 and 2, and completely in case of Bag 3. PLA is known to hydrolyze more quickly than PBAT. The reason that complete breakdown is observed only for Bag 3 may be due to differences in the production process. For instance, if the PLA is embedded in a PBAT matrix and thus has hardly any direct contact with the aqueous environment, it will be less susceptible to decomposition.
For composting, the relative losses in PLA were smaller. However, this may be due to a more equal loss of both PLA and PBAT during biotic degradation, since less than <40 wt% of the added material was recovered after composting, whereas up to 49 wt% of the originally added material was recovered after abiotic decomposition. None of the measurements indicated any change in the BT/BA ratio of the PBAT.
The only one parameter that changed significantly during both composting/abiotic decomposition was the crystallinity of the materials, which increased with time (Table 1).
A high degree of crystallinity had previously been observed for fragments of biodegradable plastic recovered from composts [10]. Tentatively, this has been explained by assuming that the more easily degraded amorphous parts, e.g., of a degradable bag, are preferably degraded, leaving the crystalline regions behind [25]. In addition, shorter chains produced during bulk breakdown would be better able to align, thereby forming crystalline regions [26]. Finally, an increase in crystallinity is expected to increase the brittleness of the material, which presumably also aids fragmentation. However, in these previous studies no direct link to the crystallinity of the corresponding pristine material could be established, as this was unknown. Our results now clearly show that the increased crystallinity observed in bag fragments recovered after composting/abiotic decomposition indeed correlates well with a preferential degradation of the amorphous parts of the bags.
If we assume an initial crystallinity of 20% and an average weight loss of 60% during 7 weeks of composting, with preferably the amorphous parts of the material degrading, an increase in crystallinity to roughly 40% must be expected. Incidentally, this supports previously voiced concerns that biodegradable polymers in the crystalline form may be enriched during biodegradation and enter the environment as more persistent microplastic [10,13].
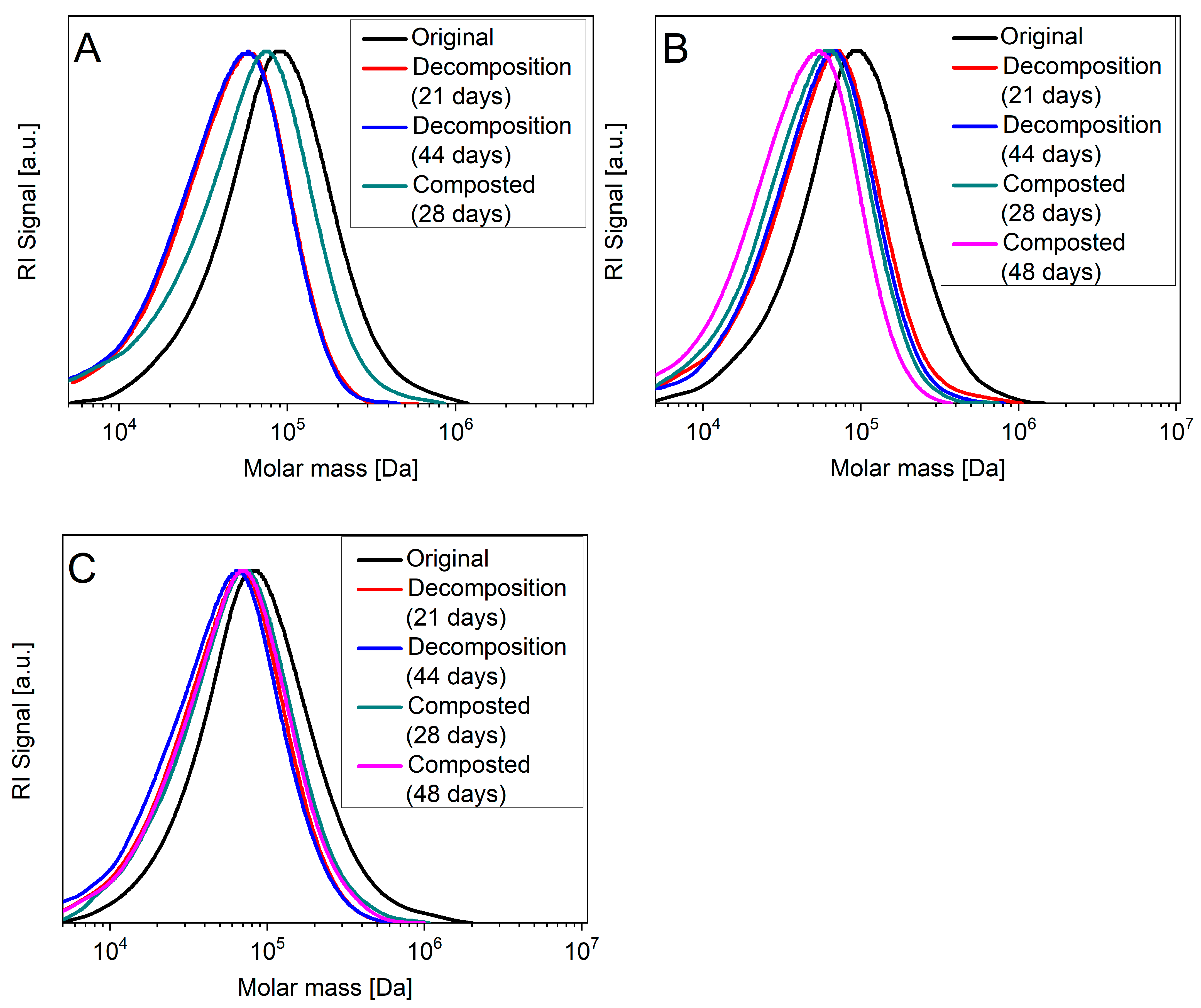
Additional insight into the degradation mechanism on a molecular basis can be obtained via GPC measurements of the molecular weight of the polymer chains after dissolution in chloroform. In case of bulk degradation, the GPC chromatograms should be shifted to lower molecular weights, whereas this will not be the case when decomposition is mainly due to surface degradation. The chromatograms for the bags are shown in Figure 6; data on the development of the average molecular weight can be found in Table S2.
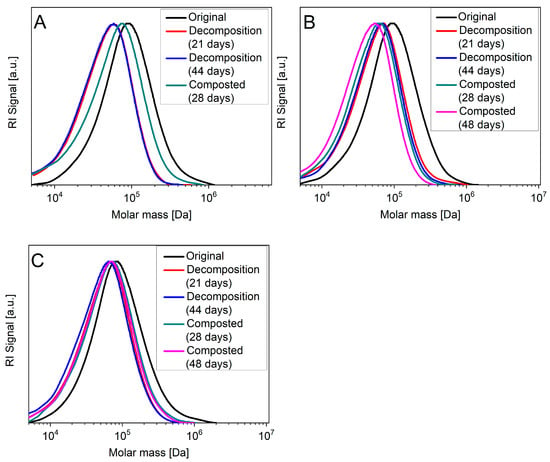
Figure 6.
GPC measurement of samples from bag 1–3: (A) bag 1, (B) bag 2 and (C) bag 3, which were composted or abiotically degraded for the indicated length of time. Samples were dissolved in chloroform prior to the measurement.
In all cases, the peaks in the GPC curves are shifted to lower molecular weights. However, the shift is not as pronounced as one would expect for a degradation process mainly driven by bulk degradation. In the case of bag 3, the shift is very small even in the case of abiotic decomposition, i.e., when the PLA was completely removed. It is likely that in this case the GPC curves correspond exclusively to the molecular weight of the less affected PBAT polymer chains.
Our study suggests that the breakdown of disposable bags made from PLA and PBAT mixtures under conditions closely mimicking industrial composting is less efficient than to be expected for a material certified as compostable according to DIN EN 13432. The relative increase in crystallinity over time may conceivably play a major role in hindering full degradation.
3.5. Degradation Behavior of the Disposable Glasses (PLA)
While the degradation behavior of the bags can thus be explained by a preferential degradation of the amorphous regions, the situation is more challenging in the case of the disposable glasses, which initially appeared to be highly amorphous and therefore had been expected to degrade well. Instead, the glasses were recovered in large amounts, mostly intact, albeit turbid, in the case of composting, and mostly as microplastic in the case of abiotic degradation.
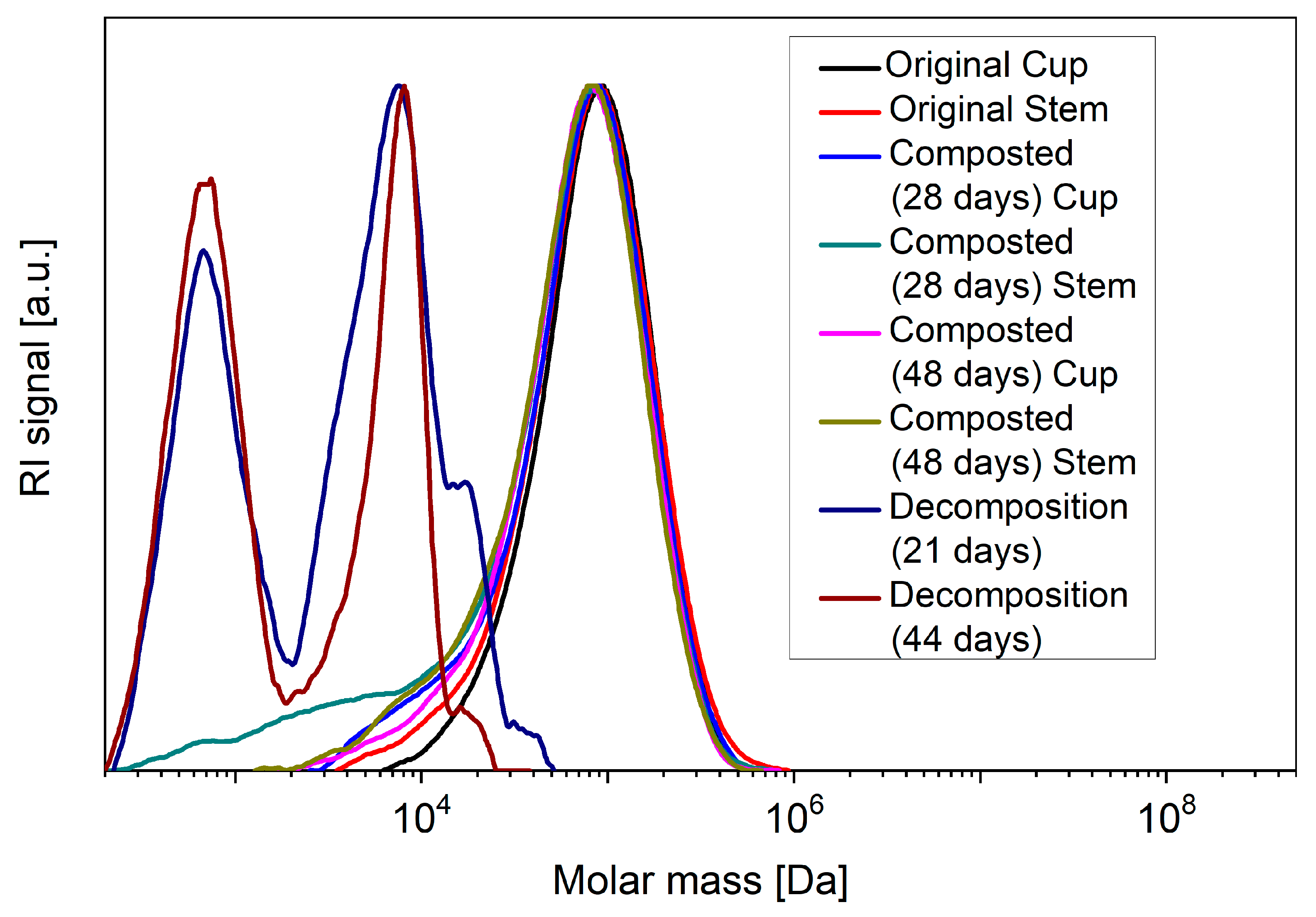
The GPC measurements (Figure 7) give clear evidence of bulk degradation during abiotic decomposition in the case of the glasses, as the molecular weight of the PLA polymer chains decreases massively with time. In addition, a bimodal distribution of the polymer chains argues for the presence of areas of different degradation kinetics. In the case of the composted cups and stems, on the other hand, the GPC measurements showed no reduction in the size of the PLA chains, while the subsequently performed WAXS measurements gave evidence that both during composting and abiotic decomposition the originally mostly amorphous PLA had become highly crystalline (Table 3), which incidentally would also explain the observed turbidity of the composted disposable glasses.
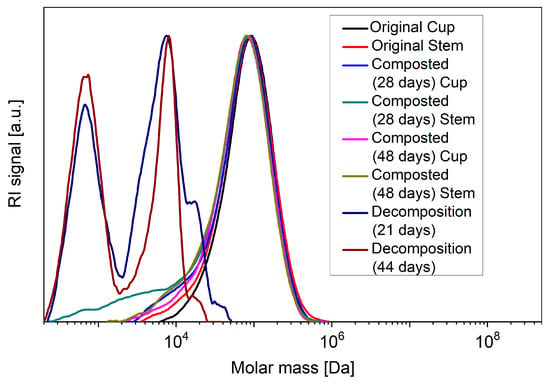
Figure 7.
GPC measurements according to the respective incubation time of the stem and cup parts of the disposable glasses dissolved in chloroform. In the case of the decomposition under abiotic conditions, only the stems were tested for reasons of reproducibility.

Table 3.
Crystallinities of material obtained from the disposable glasses originally and after the indicated number of days of composting or abiotic decomposition.
The formation of mainly microplastic during abiotic decomposition could hypothetically be explained by the assumption of two competing reactions. One is an initial rapid breakdown of the amorphous material into microplastic by acidic hydrolysis. The other reaction is crystallization, which renders the remaining fragments less susceptible to further breakdown. The presence of both crystalline and residual amorphous regions would in this case also provide an explanation for the bimodal distribution of the polymer chain length observed in the GPC measurements. Since the composts did not show similar drops in the pH, the glasses there underwent crystallization rather than hydrolysis and remained largely intact.
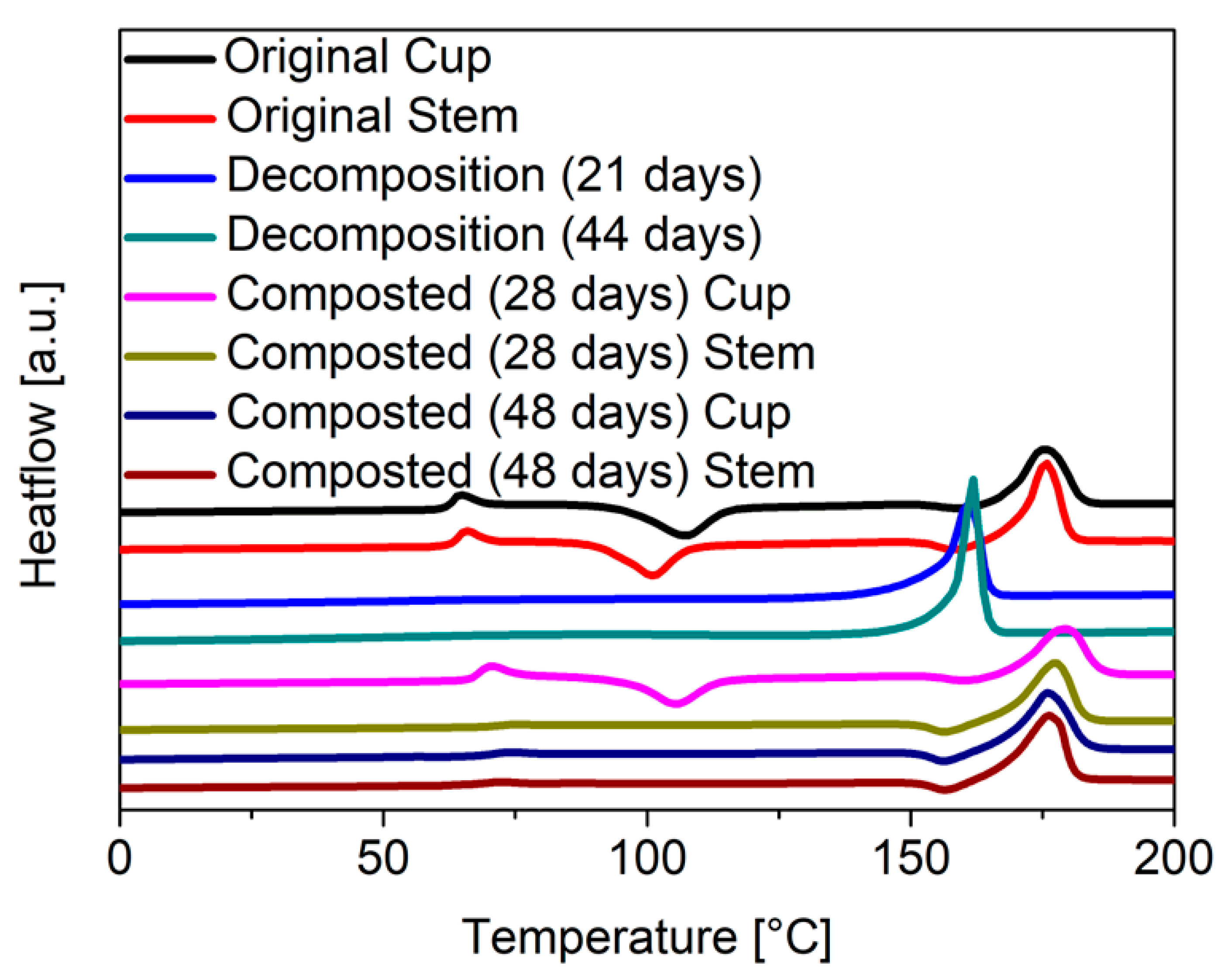
Phase transitions including melting and crystallization can be quantified by differential scanning calorimetry (DSC). The DSC scans of the original stem and cup (Figure 8) show the expected glass transition at a temperature (Tg) of 60 °C as well as a weak recrystallization transition at 110 °C and a melting peak at 170 °C. The degree of crystallinity can be calculated from the enthalpies of crystallization and melting. For a completely amorphous material, the enthalpies of melting and crystallization should be equal, and the crystallinity therefore calculate to zero. Here an initial crystallinity of 15% was calculated for the disposable glasses from the DCS data (Table 3). This stands in contrast with the WAXS measurements that had indicated a fully amorphous material. It is possible that the crystallinity detected by the DCS is due to the presence of small (<200 nm) nanocrystals, which are difficult to see in the WAXS. Such small nanocrystals would not influence the transparence of the disposable glass, while still improving the mechanical properties.
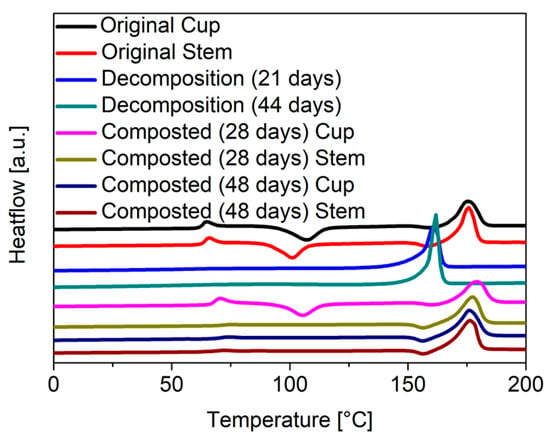
Figure 8.
DSC measurements of the stem and cup part from the disposable glasses according to the respective time period; heating rate: 10 K min−1 under nitrogen.
After abiotic decomposition, the DSC scans of the glass stems showed a pronounced melting peak, shifted to temperatures lower than for the fresh material. This shift indicates that the chain length has decreased and thus the energy required for melting was already sufficient at lower temperatures, which corroborates the GPC data discussed above. The peaks for the glass transition and crystallization enthalpies almost vanish compared to the original material, arguing for an increased crystallinity of the material recovered after abiotic decomposition.
In the case of the composted material, the peaks for glass transition and crystallization also vanish. The temperature of the melting peak remains in the same range as for the pristine material, which supports the GPC data indicating that the chain length of the PLA is hardly affected by composting.
The increase in crystallinity as well as the accompanying lack of degradation were subsequently hypothesized to be linked directly to the composting conditions, in particular the temperature. During the most intensive part of industrial composting, temperatures above 65 °C are reached, which is significantly higher than the glass transition temperature of PLA. Such temperatures are maintained for at least a week to assure sanitization of the biowaste. A week of tempering above Tg conceivably aids the recrystallization of an amorphous material.
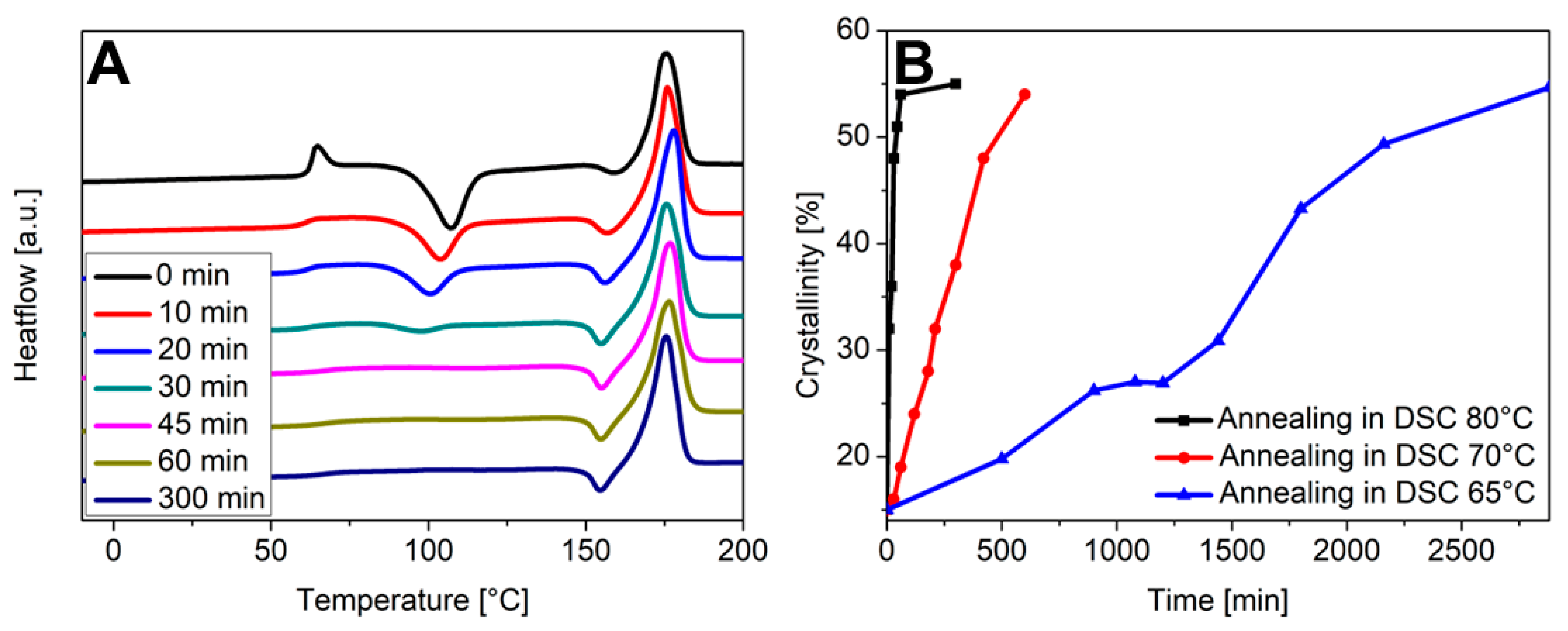
For a more quantitative look on the recrystallization kinetics, fragments of the disposable glass were tempered at 65 °C, 70 °C, and 80 °C for a defined time in the DCS and the crystallinity was determined. Figure 9A compiles the DSC measurements after tempering at 80 °C for the indicated time. The DSC data obtained for 70 °C and 65 °C are included in the Supplementary Material (Figure S9). In case of an incubation at 80 °C, the crystallinity increased from 15% to 54% within 60 min and remained constant thereafter. At 70 °C the trend was similar, but it took longer to reach the final crystallinity value. By comparison, it took 48 h of incubation at 65 °C to reach a crystallinity of 50% (Figure 9B).
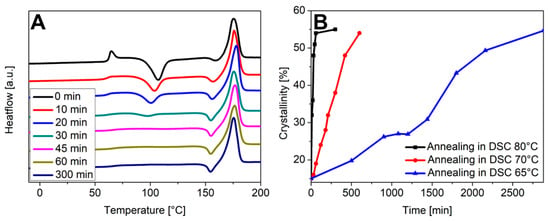
Figure 9.
(A) DSC measurements of fragments of the disposable glass after tempering at 80 °C for the indicated time. (B) Changes in crystallinity with time after tempering the fragments in the DSC at the indicated temperature.
State-of-the-art composting conditions therefore aid the conversion of the more easily degradable amorphous PLA into a much stabler crystalline form. Together with the presumed increased brittleness of the crystalline material, this increases the likelihood of contaminating the produced composts with large amounts of environmentally stable, crystalline PLA microplastic fragments.
The DSC measurements had been carried out under dry conditions. Composts are characterized by a high content of moisture, which may influence the crystallization speed. In order to investigate this aspect more closely, fragments of the disposable glass were also tempered in a water bath at 70 °C. After 90 min, the initially transparent fragments had become turbid (Figure S10). The increase in crystallinity was validated by DSC measurement (Figure S11). A first increase in crystallinity from the initial 15% to 21% was already obvious after 5 min in the water bath. After 40 min the transformation was complete, and the mean value of 53% crystallinity hardly changed anymore. Presumably the more efficient heat transfer in water had led to a much faster crystallization compared to the incubation of the material under dry conditions. In consequence, the high moisture content of a typical compost further promotes crystallization.
4. Conclusions
The fragmentation of certified biodegradable bags during composting into particles smaller than 0.5 mm was found to be more effective than that of PLA in the same composting conditions. A possible explanation for this observation is the higher crystallinity of PLA during composting treatment, compared to the three certified bags. The superior fragmentation behavior of the bags may also be attributed to their certification as finished products, in contrast to the disposable tableware, which is merely made from certified materials.
Moreover, the degradation and crystallization processes appear to occur in opposing temporal directions—i.e., the materials become crystalline faster than they degrade. The experiments showed that the composting process itself promotes the crystallization of bags and disposable glasses. This leads also to a slower biological degradation in the environment, when plastic particles are found in a huge amount in the fertilizers. This could result in the release of highly persistent fragments into the environment, potentially leading to an increased amount of microplastics with a reduced biodegradability.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/microplastics4030059/s1, Figure S1: Scheme of the composter. Figure S2: Turning device for the composter. Figure S3: 1H-NMR (300 MHz) of the materials. Figures S4–S6: Calculation of the PLA/PBAT from the 1H-NMR in Bags 1 to 3. Figure S7: IR measurement of the original bags, the chloroform dissolvable and insolvable fractions. Figure S8: EDX measurements of fragments from Bag 2. Figure S9: DSC measurements of disposable glass samples tempered at 65 °C and 70 °C, respectively. Figure S10: Photo of a fragment of the disposable glass before and after tempering. Figure S11: DSC measurements of a disposable glass sample tempered at 70 °C in water. Table S1: Experimental data of the composting runs. Table S2: Average molecular weights and changes thereof during degradation calculated from the GPC measurements.
Author Contributions
Conceptualization: R.F. and A.G.; Data curation: L.-C.L., T.S., R.F., and A.G.; Formal analysis: L.-C.L. and T.S.; Funding acquisition: R.F. and A.G.; Investigation: L.-C.L. and T.S.; Methodology: L.-C.L., T.S., R.F. and A.G.; Supervision: R.F. and A.G.; Validation: L.-C.L. and T.S.; Writing—original draft: L.-C.L., T.S., R.F. and A.G.; Writing—review and editing: R.F. and A.G. All authors have read and agreed to the published version of the manuscript.
Funding
This study was funded by the Deutsche Forschungsgemeinschaft (DFG), CRC 1357, Project 391977956. It was also funded by the Ministry of the Environment, Climate Protection and Energy, Baden-Wurttemberg, Germany (BWBAW20104). Additionally funded by the Open Access Publishing Fund of the University of Bayreuth.
Data Availability Statement
Data is contained within the article or Supplementary Material.
Acknowledgments
We thank A. Schott and L. Veh for their excellent assistance with the experimental work. The assistance offered by the Keylab “Small Scale Polymer Processing” of the Bavarian Polymer Institute at the University of Bayreuth is gratefully acknowledged.
Conflicts of Interest
The authors declare no conflict of interest.
Correction Statement
This article has been republished with a minor correction to the Data Availability Statement. This change does not affect the scientific content of the article.
References
- Sun, Q.; Li, J.; Wang, C.; Chen, A.; You, Y.; Yang, S.; Liu, H.; Jiang, G.; Wu, Y.; Li, Y. Research Progress on Distribution, Sources, Identification, Toxicity, and Biodegradation of Microplastics in the Ocean, Freshwater, and Soil Environment. Front. Environ. Sci. Eng. 2022, 16, 1. [Google Scholar] [CrossRef]
- Lozano, Y.M.; Lehnert, T.; Linck, L.T.; Lehmann, A.; Rillig, M.C. Microplastic Shape, Polymer Type, and Concentration Affect Soil Properties and Plant Biomass. Front. Plant Sci. 2021, 12, 616645. [Google Scholar] [CrossRef] [PubMed]
- Chia, R.W.; Lee, J.-Y.; Jang, J.; Kim, H.; Kwon, K.D. Soil Health and Microplastics: A Review of the Impacts of Microplastic Contamination on Soil Properties. J. Soils Sediments 2022, 22, 2690–2705. [Google Scholar] [CrossRef]
- Godoy, V.; Blázquez, G.; Calero, M.; Quesada, L.; Martín-Lara, M.A. The Potential of Microplastics as Carriers of Metals. Environ. Pollut. 2019, 255, 113363. [Google Scholar] [CrossRef] [PubMed]
- Vithanage, M.; Ramanayaka, S.; Hasinthara, S.; Navaratne, A. Compost as a Carrier for Microplastics and Plastic-Bound Toxic Metals into Agroecosystems. Curr. Opin. Environ. Sci. Health 2021, 24, 100297. [Google Scholar] [CrossRef]
- Sheng, Y.; Ye, X.; Zhou, Y.; Li, R. Microplastics (MPs) Act as Sources and Vector of Pollutants-Impact Hazards and Preventive Measures. Bull. Environ. Contam. Toxicol. 2021, 107, 722–729. [Google Scholar] [CrossRef] [PubMed]
- Cocca, M.; Pace, E.; Maria, E.; Gentile, G.; Montarsolo, A.; Mossotti, R. Proceedings of the International Conference on Microplastic Pollution in the Mediterranean Sea; Springer: Berlin/Heidelberg, Germany, 2017. [Google Scholar]
- Rochman, C.M.; Brookson, C.; Bikker, J.; Djuric, N.; Earn, A.; Bucci, K.; Athey, S.; Huntington, A.; McIlwraith, H.; Munno, K.; et al. Rethinking Microplastics as a Diverse Contaminant Suite. Environ. Toxicol. Chem. 2019, 38, 703–711. [Google Scholar] [CrossRef] [PubMed]
- Wei, X.-F.; Bohlén, M.; Lindblad, C.; Hedenqvist, M.; Hakonen, A. Microplastics Generated from a Biodegradable Plastic in Freshwater and Seawater. Water Res. 2021, 198, 117123. [Google Scholar] [CrossRef] [PubMed]
- Steiner, T.; Zhang, Y.; Möller, J.N.; Agarwal, S.; Löder, M.G.J.; Greiner, A.; Laforsch, C.; Freitag, R. Municipal Biowaste Treatment Plants Contribute to the Contamination of the Environment with Residues of Biodegradable Plastics with Putative Higher Persistence Potential. Sci. Rep. 2022, 12, 9021. [Google Scholar] [CrossRef] [PubMed]
- Rauscher, A.; Meyer, N.; Jakobs, A.; Bartnick, R.; Lueders, T.; Lehndorff, E. Biodegradable Microplastic Increases CO2 Emission and Alters Microbial Biomass and Bacterial Community Composition in Different Soil Types. Appl. Soil Ecol. 2023, 182, 104714. [Google Scholar] [CrossRef]
- Steiner, T.; Möller, J.N.; Löder, M.G.J.; Hilbrig, F.; Laforsch, C.; Freitag, R. Microplastic Contamination of Composts and Liquid Fertilizers from Municipal Biowaste Treatment Plants: Effects of the Operating Conditions. Waste Biomass Valor. 2023, 14, 873–887. [Google Scholar] [CrossRef]
- Bagheri, A.R.; Laforsch, C.; Greiner, A.; Agarwal, S. Fate of So-Called Biodegradable Polymers in Seawater and Freshwater. Glob. Chall. 2017, 1, 1700048. [Google Scholar] [CrossRef] [PubMed]
- DIN EN 13432:2000-12; Verpackung—Anforderungen an Die Verwertung Von Verpackungen Durch Kompostierung Und Biologischen Abbau—Prüfschema Und Bewertungskriterien Für Die Einstufung Von Verpackungen; Deutsche Fassung EN 13432:2000. Deutsches Institut für Normung: Berlin, Germany, 2000. [CrossRef]
- DIN EN 14045:2003-06; Verpackung—Bewertung Der Desintegration Von Verpackungsmaterialien in Praxisorientierten Prüfungen Unter Definierten Kompostierungsbedingungen; Deutsche Fassung EN 14045:2003. Deutsches Institut fur Normung: Berlin, Germany, 2003. [CrossRef]
- Kehres, B.; Mähl, B.; Clemens, J.; Cuhls, C.; Reinhold, J.; Müsken, J. Betrieb Von Kompostierungsanlagen—Mit Geringen Emissionen Klimarelevanter Gase; Bundesgütegemeinschaft Kompost e.V.: Köln, Germany, 2010. [Google Scholar]
- Rynk, R.; van de Kamp, M.; Willson, G.B.; Singley, M.E.; Richard, T.L.; Kolega, J.J.; Gouin, F.R.; Laliberty, L.; Kay, D.; Murphy, D.; et al. On-Farm Composting Handbook (NRAES 54); Northeast Regional Agricultural Engineering Service: Ithaca, NY, USA, 1992. [Google Scholar]
- Koch, K.; Plabst, M.; Schmidt, A.; Helmreich, B.; Drewes, J.E. Co-Digestion of Food Waste in a Municipal Wastewater Treatment Plant: Comparison of Batch Tests and Full-Scale Experiences. Waste Manag. 2016, 47, 28–33. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Hu, F.; Bowman, D.; Shi, W. Nitrous Oxide Production in Turfgrass Systems: Effects of Soil Properties and Grass Clipping Recycling. Appl. Soil Ecol. 2013, 67, 61–69. [Google Scholar] [CrossRef]
- DIN ISO 3310-1:2017-11; Test Sieves Technical Requirements and Testing Part 1: Test Sieves of Metal Wire Cloth (ISO_3310-1:2016). DIN Deutsches Institut für Normung: Berlin, Germany, 2017. [CrossRef]
- Mihai, M.; Huneault, M.A.; Favis, B.D.; Li, H. Extrusion Foaming of Semi-Crystalline PLA and PLA/Thermoplastic Starch Blends. Macromol. Biosci. 2007, 7, 907–920. [Google Scholar] [CrossRef] [PubMed]
- La Fuente, C.I.A.; Maniglia, B.C.; Tadini, C.C. Biodegradable Polymers: A Review about Biodegradation and Its Implications and Applications. Packag. Technol. Sci. 2023, 36, 81–95. [Google Scholar] [CrossRef]
- Kalita, N.K.; Nagar, M.K.; Mudenur, C.; Kalamdhad, A.; Katiyar, V. Biodegradation of Modified Poly (Lactic Acid) Based Biocomposite Films under Thermophilic Composting Conditions. Polym. Test. 2019, 76, 522–536. [Google Scholar] [CrossRef]
- Mouhoubi, R.; Lasschuijt, M.; Ramon Carrasco, S.; Gojzewski, H.; Wurm, F.R. End-of-Life Biodegradation? How to Assess the Composting of Polyesters in the Lab and the Field. Waste Manag. 2022, 154, 36–48. [Google Scholar] [CrossRef] [PubMed]
- Rudnik, E. Compostable Polymer Materials, 2nd ed.; Elsevier: Amsterdam, The Netherlands, 2019. [Google Scholar]
- Han, X.; Pan, J. A Model for Simultaneous Crystallisation and Biodegradation of Biodegradable Polymers. Biomaterials 2009, 30, 423–430. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).