The Cytotoxicity of Biodegradable Microplastics and Nanoplastics: Current Status and Research Prospects
Abstract
1. Introduction
2. Methods: Literature Search Strategy
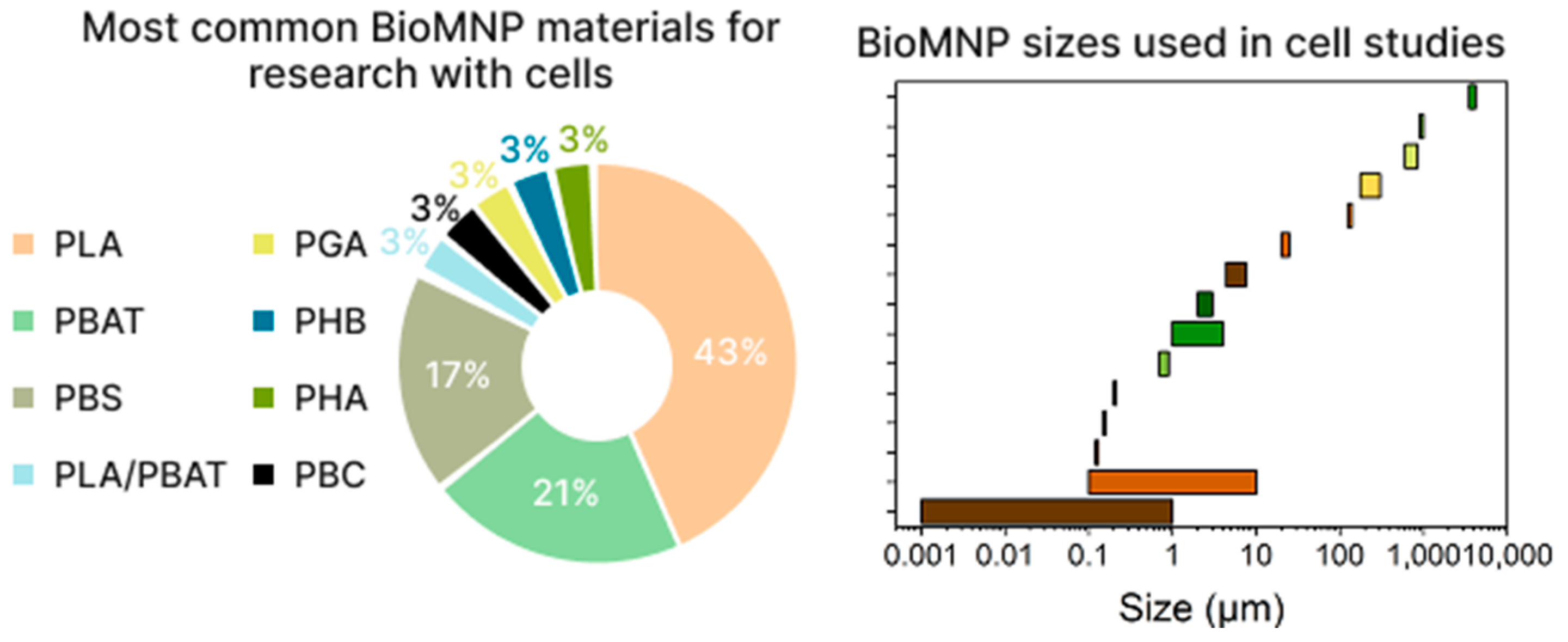
3. Cytotoxicity of Biodegradable Micro- and Nanoplastics
| Materials | Size | Concentration | Cells/Animals | Results | Reference |
|---|---|---|---|---|---|
| Commercial products based on PLA and PHB | 100 nm–10 µm | 0.78125, 1.5625, 3.125, 6.25, 12.5, 25, 50, and 100 µg/L | A549 HepG2 | Plastic particles and their extracts did not reduce cell viability but induced oxidative stress, most notably with PLA. | [23] |
| PLA PBS | 4.41 µm 7.64 µm | 6.25, 12.5, 25, 50, and 100 µg/mL | Vero NHDF HaCaT | Microplastic particles had substantially less toxic to practically non-toxic effects on the cells. Plastics potentially have more alteration effect in epithelial kidney cells (Vero) than fibroblasts (NHDF) and keratinocytes (HaCaT) Microplastic particles were non-toxic or harmless to animal cell lines and altered some intracellular biomolecule profiles. | [24] |
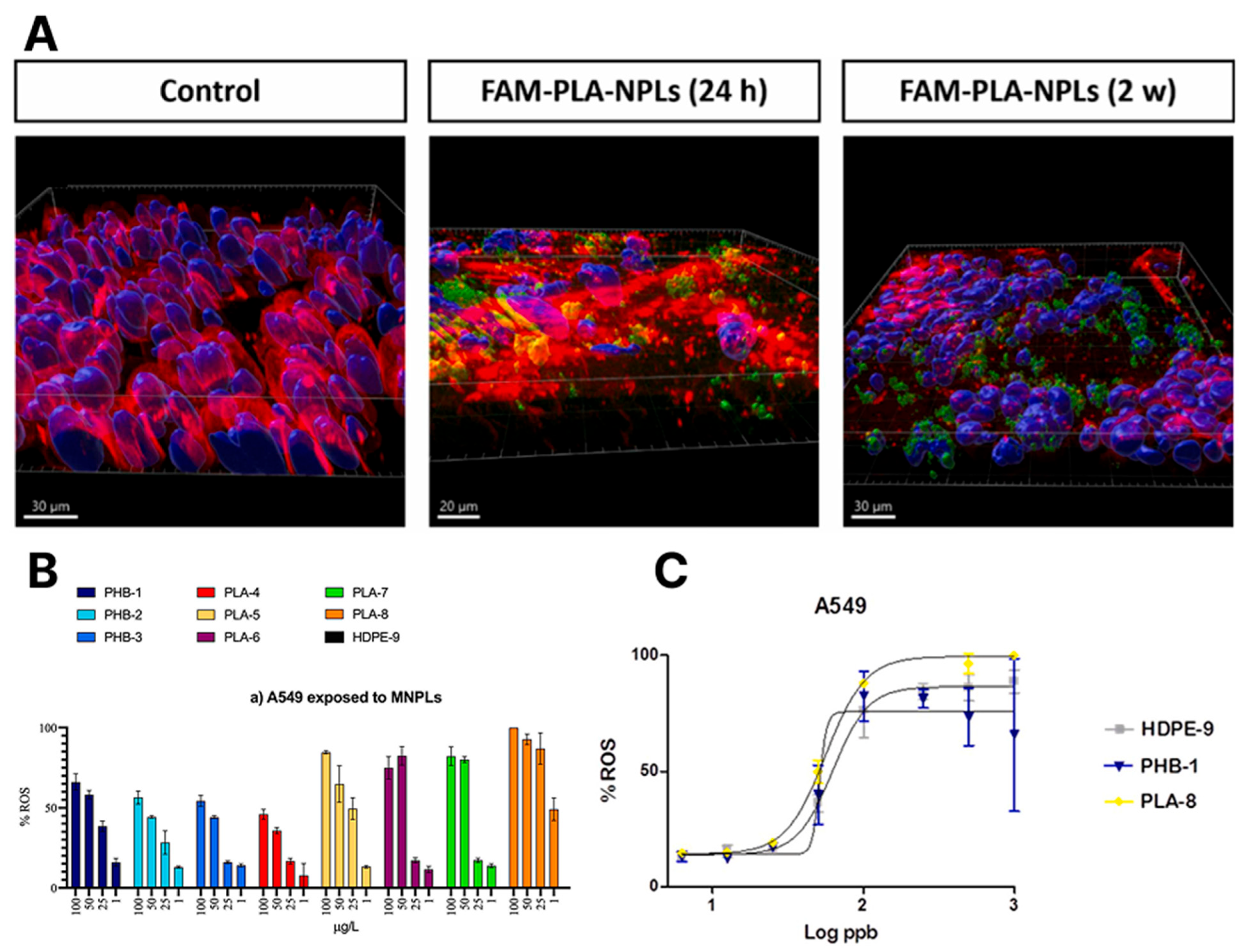
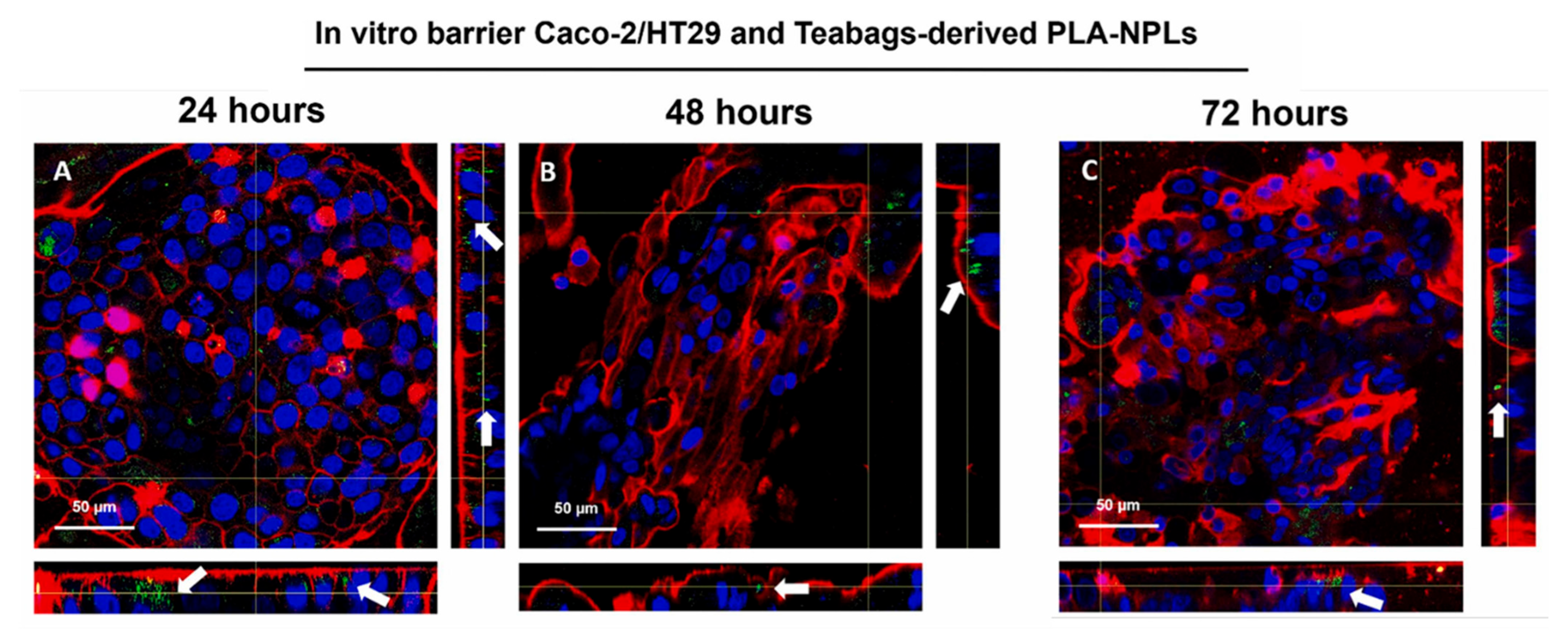
| PLA | 160 nm | 50, 100 µg/ml | Caco-2 HT29 | PLA-NPLs (≤100 μg/mL, 48 h) caused no cytotoxicity or barrier disruption in Caco-2/HT29 cells. A slight, temporary TEER drop occurred at 3 h. Uptake was high, especially in HT29, with retention up to 72 h. | [25] |
| PLA | 130 nm | 2.5, 10, and 20 µg/cm2 | Calu-3 | PLA-NPLs were internalised by 10–70% of Calu-3 cells (dose- and time-dependent), localising near and inside nuclei. Exposure (2.5 µg/cm2) reduced ZO-1 expression and slightly increased mucus secretion. Genotoxicity increased with exposure time (~7% DNA damage at 2 weeks); not linked to oxidative stress. Prolonged exposure triggered strong inflammatory responses, altering expression of up to 20 cytokines and related proteins. | [26] |
| PGA PBSG | 50 µm | 50 and 500 mg/kg | Wistar rats | High doses of all three polyesters resulted in decreased body weight, tissue necrosis, and inflammation in rats. PGA, having the fastest degradation rate, showed the least physiologic toxicity. Low doses of biodegradable plastics did not cause significant toxicity | [27] |
| PLA | 25 μm | 0.0008, 0.004, 0.02, 0.1, and 0.5 mg/ml | HepG2 Caco-2 HUVEC RAW 264.7 | In vitro, PLA nanoparticles and oligomers penetrated HepG2, Caco-2, and HUVEC cells, with higher uptake of oligomers. In vivo, orally administered PLA oligomers and degradation products accumulated in the liver, intestine, and brain. Both PLA MPs (even at 0.01 mg/day) and oligomers induced acute liver and intestinal inflammation and impaired the intestinal barrier, with stronger effects from oligomers. | [28] |
| Polymer MP PLA (~40 kDa) Oligomer MP PLA (~2 kDa) | ~2.5 μm | 2.5 or 25 mg/kg | in vivo 28-day oral administration to mice | Particles from both groups were found in major organs, with polymeric MP accumulating more in the brain, liver, spleen, and lungs, and oligomeric MP—in blood and kidneys. Oligomeric MP also showed higher intestinal accumulation. Nanoparticles crossed the blood-brain barrier without disrupting its integrity. Both PLA MP forms (2.5 and 25 mg/kg/day, 28 days) induced Parkinson-like neurotoxicity, with polymeric MP causing stronger effects. | [29] |
| PLA | 1–1000 nm | - | Phospholipid bilayers | Nanoplastic–lipid bilayer interaction was mainly driven by van der Waals forces, strongest for PLA and weakest for PP. All nanoplastics increased bilayer surface roughness and decreased its thickness. These changes may impair membrane function and cause cytotoxicity, including hemolysis. | [30] |
| PLA | 1–4 µm | 20 particles on cell | ImKC (Kupffer cells) J774A.1 STC1 BNL CL.2 | Microplastic particles showed no significant short-term cytotoxicity against macrophages and epithelial cells. | [31] |
| PGA PLA PBS, aged PBS PBC PBAT | ~800 nm | 1, 25, 100 mg/L | LO2 THP-1 HUVEC Caco-2 | PLA and PBS showed significant toxicity to human cell lines, while PGA had low cytotoxicity and little antiproliferative effect. THP-1 cells internalized PGA microplastics. Aging reduced PGA toxicity, particularly in HUVEC. | [32] |
| PBAT | Extracts from films | L-02 | Decreased cell viability and increased liver damage markers (AST, ALT). Oxidative stress indicated by higher ROS and lower SOD, GSH levels. Inflammation with elevated TNF-α, IL-6, and IL-1β. Reduced ATP levels. Altered AMPK signalling: increased p-AMPK/AMPK ratio and decreased p-mTOR/mTOR, SIRT1, PGC-1α, NRF1, and TFAM. | [33] | |
| PLA | 1mm 240 ± 65 µm | 0.166 g | Gut microbiota | PLA MPs did not significantly disrupt microbial community homeostasis, with bifidobacteria levels tending to increase. Functional shifts suggest altered microbial metabolism and possible PLA biotransformation by colon microbiota. Raman spectroscopy and FESEM showed PLA MPs’ morphological changes after gastric digestion and surface biodegradation with microbial biofilm after intestinal and colonic phases. | [34] |
| PBS PBS+0.1% Cellulose nanocrystals | 125–140 µm | 0, 10, 100, 500 and 1000 µg/mL | MDA-MB-231 CHO-K1 KB RAW 264.7 | Incubation of CHO-K1 cells with agar-fixed particles (up to 1000 μg/mL, 72 h) caused no significant cell death. Direct exposure to KRICT-PBS and CNC-PBS particles (up to 1000 μg/mL) for 72 h had minimal effect on viability of MDA-MB-231, CHO-K1, KB, and RAW 264.7 cells. | [35] |
| PLA | 600–850 µm | 3 and 8 particles per well | Mouse macrophages of the RAW264.7 | Low microplastic dose (3 particles/well) had no effect on cell viability. High dose (8 particles/well) treated at 120–130 °C slightly increased viability, while treatment at 140 °C significantly reduced it (~83.9%). | [36] |
| Aged PLA/PBAT | 5 mm 863 nm 608 nm | 0, 0.1, 1, 10, 100, 200, 500, and 1000 mg/L | THP-1 | UV aging of PLA/PBAT films increased surface roughness and triggered MNP release. These MNPs reduced THP-1 cell viability dose-dependently, with ultrafiltration-derived MNPs showing higher toxicity. PLA/PBAT MNPs posed toxicity risks equal to or greater than conventional plastic MNPs. | [37] |
| PLA | 211.78 nm | from 6.25 to 200 µg/mL | Bhas 42 | It did not show the ability to transform cells under either initiation or promotion conditions. Nanoplastics effectively penetrated inside the cells Bhas 42 | [38] |
| PBAT PBAT+1.5%CB+1,5% HALS PBAT+1.5%CB+1,5% VitE | Aqueous extract of soil | 100, 250, 500, 750 and 1000 µL/mL | HepG2/C3A | MTT assay showed decreased HepG2/C3A viability with higher aqueous soil extract concentrations, but viability remained above 80% at 250 μL/mL. Micronucleus test revealed no cytotoxicity at this concentration (CBPI comparable to control). No genotoxic effects were detected in soil extracts with films before or after photo- and biodegradation (Tail DNA % and Olive tail moment unchanged). | [39] |
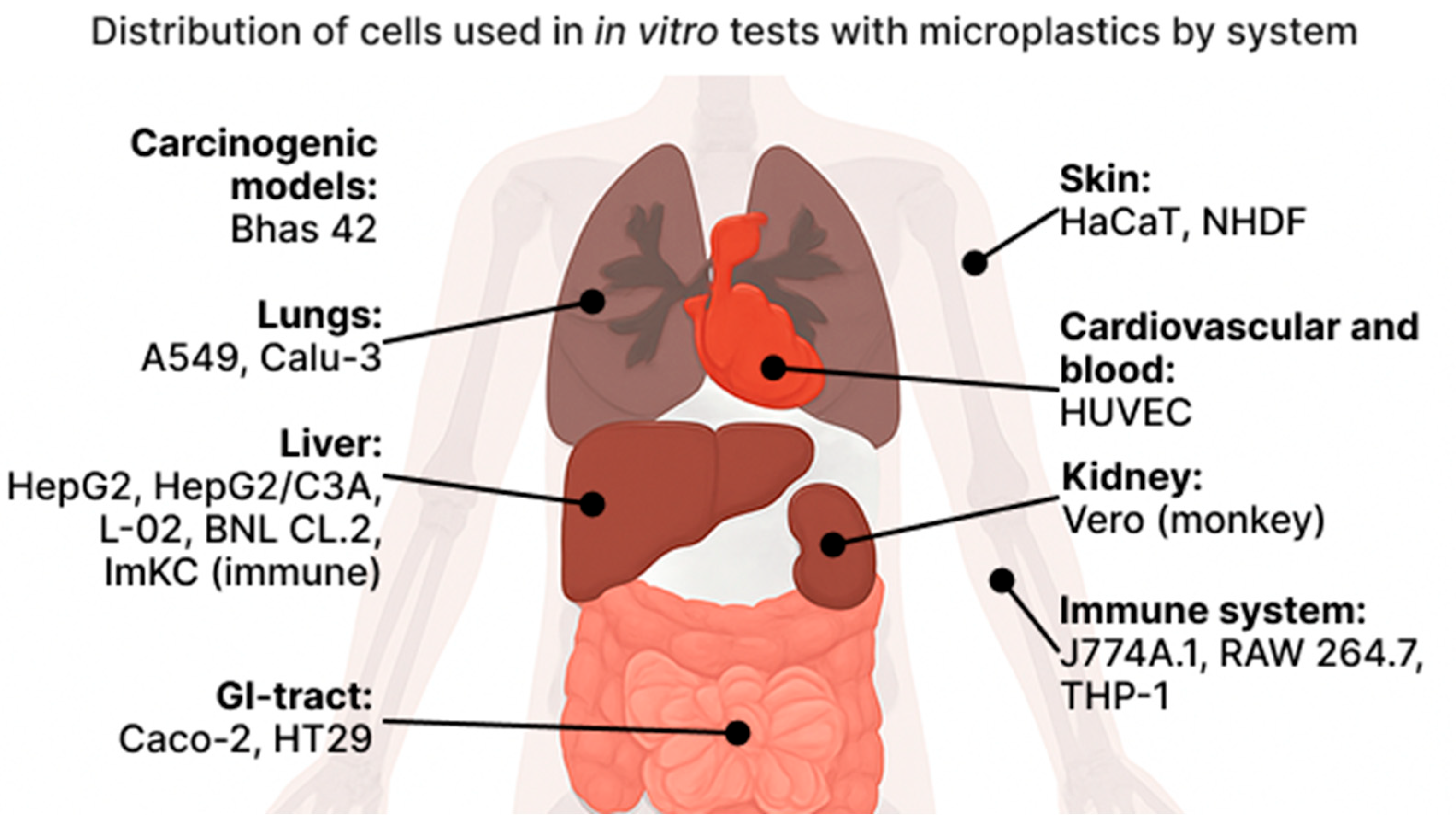
3.1. Organism
3.2. Interaction Between NP and Membrane
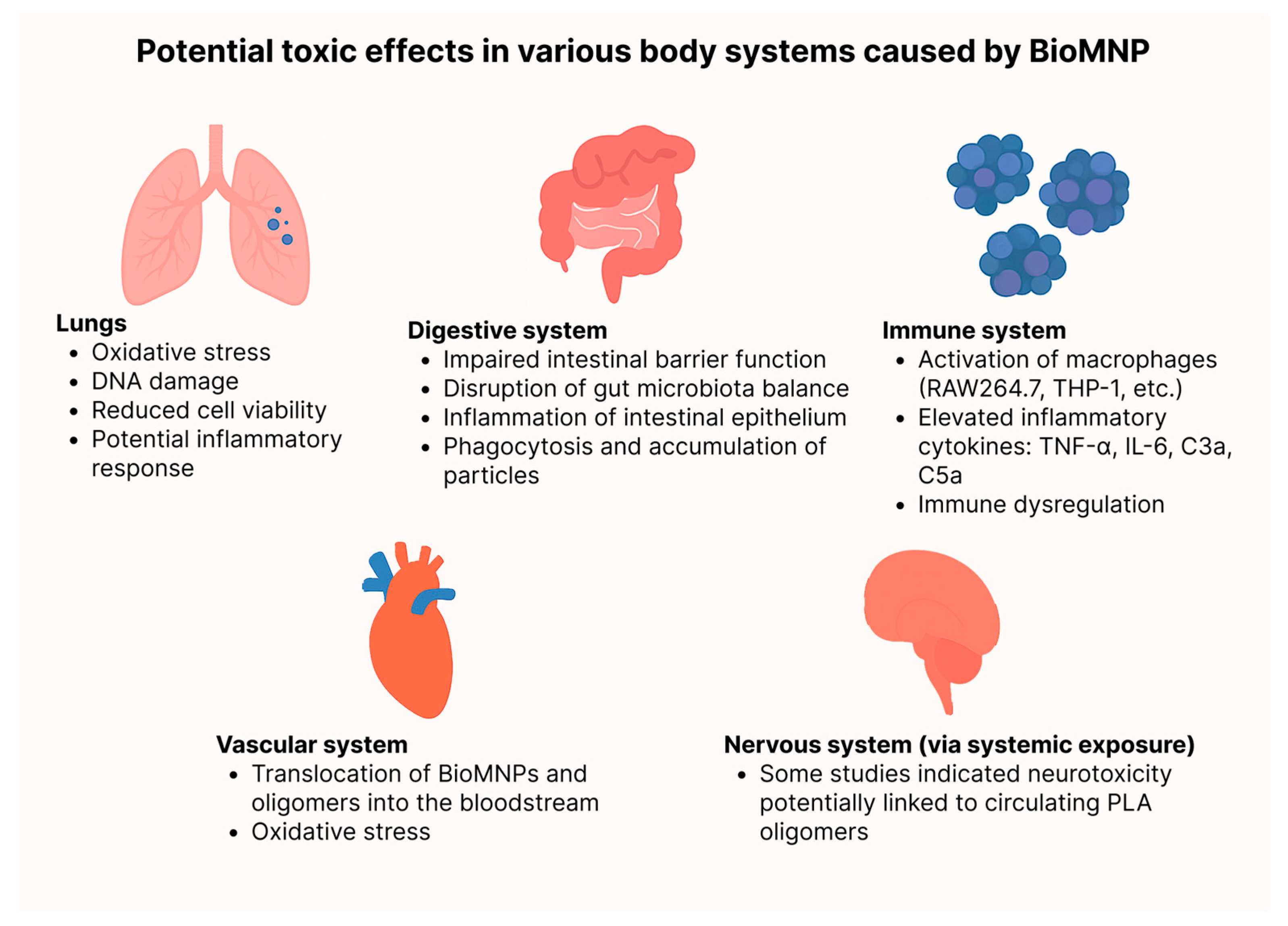
3.3. Lungs
3.4. Digestion
3.5. Immune
3.6. Vascular System
3.7. Kidney
3.8. Skin
4. Conclusions
5. Recommendations for Future Research and Regulation
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| A549 | Human alveolar basal epithelial cells |
| ALI | Air–Liquid Interface (lung model) |
| AMPK | AMP-activated Protein Kinase |
| AST/ALT | Aspartate/Alanine Aminotransferase |
| Bhas 42 | Mouse fibroblast transformation assay cells |
| BioMNP | Biodegradable Micro- and Nanoplastics |
| BioMP | Biodegradable Microplastics |
| BNL CL.2 | Mouse liver epithelial cells |
| Caco-2 | Human colorectal adenocarcinoma cells |
| Calu-3 | Human bronchial epithelial cells |
| CBPI | Cytokinesis-Block Proliferation Index |
| CHO-K1 | Chinese hamster ovary cells |
| EC50 | Half maximal effective concentration |
| ECHA | European Chemicals Agency |
| FESEM | Field Emission Scanning Electron Microscopy |
| FITC | Fluorescein isothiocyanate |
| GSH | Glutathione |
| HaCaT | Human keratinocytes |
| HepG2 | Human hepatocellular carcinoma cells |
| HepG2/C3A | Hepatocellular carcinoma derivative |
| HT29 | Human colorectal adenocarcinoma cells |
| HUVEC | Human umbilical vein endothelial cells |
| IL-6/IL-1β | Interleukins |
| ImKC | Immortalized mouse Kupffer cells |
| J774A.1 | Mouse macrophages |
| KB | Human epidermoid carcinoma cells |
| L-02/LO2 | Normal human liver cells |
| MDA-MB-231 | Human breast cancer cells |
| MMP12 | Matrix Metallopeptidase 12 |
| MNP | Micro- and Nanoplastics |
| MP | Microplastics |
| mTOR | Mechanistic Target of Rapamycin |
| NHDF | Normal human dermal fibroblasts |
| NPL | Nanoplastics |
| NRF1 | Nuclear respiratory factor 1 |
| PBAT | Polybutylene Adipate Terephthalate |
| PBC | Poly(butylene carbonate) |
| PBS | Polybutylene Succinate |
| PBSG | Poly(butylene succinate-co-glycolate) |
| PGA | Polyglycolic Acid |
| PGC-1α | Peroxisome proliferator-activated receptor gamma coactivator 1α |
| PHA | Polyhydroxyalkanoate |
| PHB | Polyhydroxybutyrate |
| PLA | Polylactic Acid |
| RAW 264.7 | Mouse macrophages |
| ROS | Reactive Oxygen Species |
| SimGi® | Simulated Gastrointestinal System |
| SIRT1 | Sirtuin 1 |
| SOD | Superoxide Dismutase |
| STC-1 | Mouse intestinal endocrine cells |
| TEER | Transepithelial Electrical Resistance |
| TFAM | Mitochondrial transcription factor A |
| THP-1 | Human monocytic leukemia cells |
| TNF-α | Tumor Necrosis Factor-alpha |
| Vero | African green monkey kidney cells |
| ZO-1 | Zona Occludens-1 (tight junction protein) |
References
- Hale, R.C.; Seeley, M.E.; La Guardia, M.J.; Mai, L.; Zeng, E.Y. A Global Perspective on Microplastics. J. Geophys. Res. Ocean. 2020, 125, e2018JC014719. [Google Scholar] [CrossRef]
- Gigault, J.; El Hadri, H.; Nguyen, B.; Grassl, B.; Rowenczyk, L.; Tufenkji, N.; Feng, S.; Wiesner, M. Nanoplastics Are Neither Microplastics nor Engineered Nanoparticles. Nat. Nanotechnol. 2021, 16, 501–507. [Google Scholar] [CrossRef] [PubMed]
- Waller, C.L.; Griffiths, H.J.; Waluda, C.M.; Thorpe, S.E.; Loaiza, I.; Moreno, B.; Pacherres, C.O.; Hughes, K.A. Microplastics in the Antarctic Marine System: An Emerging Area of Research. Sci. Total Environ. 2017, 598, 220–227. [Google Scholar] [CrossRef] [PubMed]
- Ragusa, A.; Svelato, A.; Santacroce, C.; Catalano, P.; Notarstefano, V.; Carnevali, O.; Papa, F.; Rongioletti, M.C.A.; Baiocco, F.; Draghi, S.; et al. Plasticenta: First Evidence of Microplastics in Human Placenta. Environ. Int. 2021, 146, 106274. [Google Scholar] [CrossRef] [PubMed]
- Song, J.H.; Murphy, R.J.; Narayan, R.; Davies, G.B.H. Biodegradable and Compostable Alternatives to Conventional Plastics. Philos. Trans. R. Soc. B Biol. Sci. 2009, 364, 2127–2139. [Google Scholar] [CrossRef]
- Dilkes-Hoffman, L.S.; Lant, P.A.; Laycock, B.; Pratt, S. The Rate of Biodegradation of PHA Bioplastics in the Marine Environment: A Meta-Study. Mar. Pollut. Bull. 2019, 142, 15–24. [Google Scholar] [CrossRef]
- Cao, Z.; Kim, C.; Li, Z.; Jung, J. Comparing Environmental Fate and Ecotoxicity of Conventional and Biodegradable Plastics: A Critical Review. Sci. Total Environ. 2024, 951, 175735. [Google Scholar] [CrossRef]
- Haider, T.P.; Völker, C.; Kramm, J.; Landfester, K.; Wurm, F.R. Plastics of the Future? The Impact of Biodegradable Polymers on the Environment and on Society. Angew. Chem. Int. Ed. 2019, 58, 50–62. [Google Scholar] [CrossRef]
- Fu, Y.; Wu, G.; Bian, X.; Zeng, J.; Weng, Y. Biodegradation Behavior of Poly(Butylene Adipate-Co-Terephthalate) (PBAT), Poly(Lactic Acid) (PLA), and Their Blend in Freshwater with Sediment. Molecules 2020, 25, 3946. [Google Scholar] [CrossRef]
- Silva, R.R.A.; Marques, C.S.; Arruda, T.R.; Teixeira, S.C.; de Oliveira, T.V. Biodegradation of Polymers: Stages, Measurement, Standards and Prospects. Macromol 2023, 3, 371–399. [Google Scholar] [CrossRef]
- Shi, M.; Xie, Q.; Li, Z.-L.; Pan, Y.-F.; Yuan, Z.; Lin, L.; Xu, X.-R.; Li, H.-X. Adsorption of Heavy Metals on Biodegradable and Conventional Microplastics in the Pearl River Estuary, China. Environ. Pollut. 2023, 322, 121158. [Google Scholar] [CrossRef]
- Jiang, Z.; Zeng, J.; Wang, X.; Yu, H.; Yue, L.; Wang, C.; Chen, F.; Wang, Z. Biodegradable Microplastics and Dissemination of Antibiotic Resistance Genes: An Undeniable Risk Associated with Plastic Additives. Environ. Pollut. 2025, 372, 125952. [Google Scholar] [CrossRef]
- Tang, K.H.D.; Zhou, J. Ecotoxicity of Biodegradable Microplastics and Bio-Based Microplastics: A Review of in Vitro and in Vivo Studies. Environ. Manag. 2025, 75, 663–679. [Google Scholar] [CrossRef]
- Tao, S.; Li, T.; Li, M.; Yang, S.; Shen, M.; Liu, H. Research Advances on the Toxicity of Biodegradable Plastics Derived Micro/Nanoplastics in the Environment: A Review. Sci. Total Environ. 2024, 916, 170299. [Google Scholar] [CrossRef]
- Malafeev, K.V.; Apicella, A.; Incarnato, L.; Scarfato, P. Understanding the Impact of Biodegradable Microplastics on Living Organisms Entering the Food Chain: A Review. Polymers 2023, 15, 3680. [Google Scholar] [CrossRef]
- He, T.; Qu, Y.; Yang, X.; Liu, L.; Xiong, F.; Wang, D.; Liu, M.; Sun, R. Research Progress on the Cellular Toxicity Caused by Microplastics and Nanoplastics. J. Appl. Toxicol. 2023, 43, 1576–1593. [Google Scholar] [CrossRef] [PubMed]
- Yong, C.Q.Y.; Valiyaveettil, S.; Tang, B.L. Toxicity of Microplastics and Nanoplastics in Mammalian Systems. Int. J. Environ. Res. Public Health 2020, 17, 1509. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Ling, W.; Yang, J.; Xing, Y. Risk Assessment of Microplastics in Humans: Distribution, Exposure, and Toxicological Effects. Polymers 2025, 17, 1699. [Google Scholar] [CrossRef]
- Bai, C.-L.; Liu, L.-Y.; Guo, J.-L.; Zeng, L.-X.; Guo, Y. Microplastics in Take-out Food: Are We over Taking It? Environ. Res. 2022, 215, 114390. [Google Scholar] [CrossRef]
- Pham, D.T.; Kim, J.; Lee, S.-H.; Kim, J.; Kim, D.; Hong, S.; Jung, J.; Kwon, J.-H. Analysis of Microplastics in Various Foods and Assessment of Aggregate Human Exposure via Food Consumption in Korea. Environ. Pollut. 2023, 322, 121153. [Google Scholar] [CrossRef]
- Du, F.; Cai, H.; Zhang, Q.; Chen, Q.; Shi, H. Microplastics in Take-out Food Containers. J. Hazard. Mater. 2020, 399, 122969. [Google Scholar] [CrossRef]
- EUBIO_Admin Market. European Bioplastics e.V. Available online: https://www.european-bioplastics.org/market/ (accessed on 18 July 2025).
- Savva, K.; Borrell, X.; Moreno, T.; Pérez-Pomeda, I.; Barata, C.; Llorca, M.; Farré, M. Cytotoxicity Assessment and Suspected Screening of PLASTIC ADDITIVES in Bioplastics of Single-Use Household Items. Chemosphere 2023, 313, 137494. [Google Scholar] [CrossRef] [PubMed]
- Charoeythornkhajhornchai, P.; Kunjiek, T.; Chaipayang, S.; Phosri, S. Toxicity Assessment of Bioplastics on Brine Shrimp (Artemia franciscana) and Cell Lines. Emerg. Contam. 2023, 9, 100253. [Google Scholar] [CrossRef]
- Banaei, G.; García-Rodríguez, A.; Tavakolpournegari, A.; Martín-Pérez, J.; Villacorta, A.; Marcos, R.; Hernández, A. The Release of Polylactic Acid Nanoplastics (PLA-NPLs) from Commercial Teabags. Obtention, Characterization, and Hazard Effects of True-to-Life PLA-NPLs. J. Hazard. Mater. 2023, 458, 131899. [Google Scholar] [CrossRef] [PubMed]
- García-Rodríguez, A.; Gutiérrez, J.; Villacorta, A.; Arribas Arranz, J.; Romero-Andrada, I.; Lacoma, A.; Marcos, R.; Hernández, A.; Rubio, L. Polylactic Acid Nanoplastics (PLA-NPLs) Induce Adverse Effects on an in Vitro Model of the Human Lung Epithelium: The Calu-3 Air-Liquid Interface (ALI) Barrier. J. Hazard. Mater. 2024, 475, 134900. [Google Scholar] [CrossRef]
- Zhang, Z.-Y.; Zheng, W.-Z.; Zhen, Z.-C.; Li, X.; Wang, P.-L.; Lu, B.; Yang, X.; Huang, D.; Ji, J.-H.; Wang, G.-X. In Vivo and in Vitro Degradation and Biological Toxicity Studies of Polyesters with Varying Degradation Rates. J. Hazard. Mater. 2025, 492, 138196. [Google Scholar] [CrossRef]
- Wang, M.; Li, Q.; Shi, C.; Lv, J.; Xu, Y.; Yang, J.; Chua, S.L.; Jia, L.; Chen, H.; Liu, Q.; et al. Oligomer Nanoparticle Release from Polylactic Acid Plastics Catalysed by Gut Enzymes Triggers Acute Inflammation. Nat. Nanotechnol. 2023, 18, 403–411. [Google Scholar] [CrossRef]
- Liang, B.; Deng, Y.; Zhong, Y.; Chen, X.; Huang, Y.; Li, Z.; Huang, X.; Yang, X.; Du, J.; Ye, R.; et al. Gastrointestinal Incomplete Degradation Exacerbates Neurotoxic Effects of PLA Microplastics via Oligomer Nanoplastics Formation. Adv. Sci. 2024, 11, 2401009. [Google Scholar] [CrossRef]
- Yuan, S.; Zhang, H.; Yuan, S. Understanding the Transformations of Nanoplastic onto Phospholipid Bilayers: Mechanism, Microscopic Interaction and Cytotoxicity Assessment. Sci. Total Environ. 2023, 859, 160388. [Google Scholar] [CrossRef]
- Jasinski, J.; Völkl, M.; Wilde, M.V.; Jérôme, V.; Fröhlich, T.; Freitag, R.; Scheibel, T. Influence of the Polymer Type of a Microplastic Challenge on the Reaction of Murine Cells. J. Hazard. Mater. 2024, 465, 133280. [Google Scholar] [CrossRef]
- Wen, L.; Hu, Q.; Lv, Y.; Ding, W.; Yin, T.; Mao, H.; Wang, T. Environmental Release Behavior, Cell Toxicity and Intracellular Distribution of Novel Biodegradable Plastic Materials. Environ. Pollut. 2025, 367, 125554. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Xiao, X.; Rang, Y.; Li, W.; Huang, H.; Ou, G.; Liu, C. PBAT-Modified Starch Blended Film Extract Induces in Vitro Toxicity in L-02 Cells: Induction of Oxidative Stress, Inflammation, and Modulation of AMPK Pathway. Drug Chem. Toxicol. 2024, 47, 1139–1154. [Google Scholar] [CrossRef] [PubMed]
- Jiménez-Arroyo, C.; Tamargo, A.; Molinero, N.; Reinosa, J.J.; Alcolea-Rodriguez, V.; Portela, R.; Bañares, M.A.; Fernández, J.F.; Moreno-Arribas, M.V. Simulated Gastrointestinal Digestion of Polylactic Acid (PLA) Biodegradable Microplastics and Their Interaction with the Gut Microbiota. Sci. Total Environ. 2023, 902, 166003. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.; Lee, E.; Kim, J.; Shin, G.; Oh, D.X.; Park, S.B.; Park, J.; Lee, E.S. Biotoxicity Evaluation of Biodegradable Polymeric Particles: Exploring the Possible Adverse Impacts on Biological Systems. Polym. Adv. Technol. 2024, 35, e6631. [Google Scholar] [CrossRef]
- Yu, Y.; Liu, Y.; Hao, L.; Zhu, B.; Yang, X.; Ge, S. Enhanced Adsorption and Cytotoxicity of Hydrothermally Aged Microplastics for Volatile Organic Compounds in Sludge. J. Environ. Chem. Eng. 2024, 12, 112104. [Google Scholar] [CrossRef]
- Wu, X.; Zhang, H.; Chen, J.; Tan, F.; Cai, R.; Wang, Y. Photoaging Promotes Toxic Micro/Nanoplastics Release from PLA/PBAT Biodegradable Plastic in Gastrointestinal Condition. Environ. Health 2025, 3, 446–457. [Google Scholar] [CrossRef]
- Domenech, J.; Villacorta, A.; Ferrer, J.F.; Llorens-Chiralt, R.; Marcos, R.; Hernández, A.; Catalán, J. In Vitro Cell-Transforming Potential of Secondary Polyethylene Terephthalate and Polylactic Acid Nanoplastics. J. Hazard. Mater. 2024, 469, 134030. [Google Scholar] [CrossRef]
- Souza, P.M.S.; Sommaggio, L.R.D.; Marin-Morales, M.A.; Morales, A.R. PBAT Biodegradable Mulch Films: Study of Ecotoxicological Impacts Using Allium Cepa, Lactuca Sativa and HepG2/C3A Cell Culture. Chemosphere 2020, 256, 126985. [Google Scholar] [CrossRef]
- Sridharan, S.; Kumar, M.; Singh, L.; Bolan, N.S.; Saha, M. Microplastics as an Emerging Source of Particulate Air Pollution: A Critical Review. J. Hazard. Mater. 2021, 418, 126245. [Google Scholar] [CrossRef]
- Gasperi, J.; Wright, S.L.; Dris, R.; Collard, F.; Mandin, C.; Guerrouache, M.; Langlois, V.; Kelly, F.J.; Tassin, B. Microplastics in Air: Are We Breathing It In? Curr. Opin. Environ. Sci. Health 2018, 1, 1–5. [Google Scholar] [CrossRef]
- Allen, D.; Allen, S.; Abbasi, S.; Baker, A.; Bergmann, M.; Brahney, J.; Butler, T.; Duce, R.A.; Eckhardt, S.; Evangeliou, N.; et al. Microplastics and Nanoplastics in the Marine-Atmosphere Environment. Nat. Rev. Earth Environ. 2022, 3, 393–405. [Google Scholar] [CrossRef]
- Jenner, L.C.; Rotchell, J.M.; Bennett, R.T.; Cowen, M.; Tentzeris, V.; Sadofsky, L.R. Detection of Microplastics in Human Lung Tissue Using μFTIR Spectroscopy. Sci. Total Environ. 2022, 831, 154907. [Google Scholar] [CrossRef]
- Joksimovic, N.; Selakovic, D.; Jovicic, N.; Jankovic, N.; Pradeepkumar, P.; Eftekhari, A.; Rosic, G. Nanoplastics as an Invisible Threat to Humans and the Environment. J. Nanomater. 2022, 2022, 6707819. [Google Scholar] [CrossRef]
- Musch, M.W.; Walsh-Reitz, M.M.; Chang, E.B. Roles of ZO-1, Occludin, and Actin in Oxidant-Induced Barrier Disruption. Am. J. Physiol. Gastrointest. Liver Physiol. 2006, 290, G222–G231. [Google Scholar] [CrossRef] [PubMed]
- Zuri, G.; Karanasiou, A.; Lacorte, S. Microplastics: Human Exposure Assessment through Air, Water, and Food. Environ. Int. 2023, 179, 108150. [Google Scholar] [CrossRef] [PubMed]
- Muhib, M.I.; Uddin, M.K.; Rahman, M.M.; Malafaia, G. Occurrence of Microplastics in Tap and Bottled Water, and Food Packaging: A Narrative Review on Current Knowledge. Sci. Total Environ. 2023, 865, 161274. [Google Scholar] [CrossRef] [PubMed]
- Cox, K.D.; Covernton, G.A.; Davies, H.L.; Dower, J.F.; Juanes, F.; Dudas, S.E. Human Consumption of Microplastics. Environ. Sci. Technol. 2019, 53, 7068–7074. [Google Scholar] [CrossRef]
- Kouketsu, A.; Shimizu, Y.; Nogami, S.; Yamada-Fujiwara, M.; Nagai, H.; Yamauchi, K.; Miyashita, H.; Saito, H.; Odashima, K.; Yanagisawa, Y.; et al. Wound Healing Effect of Autologous Fibrin Glue and Polyglycolic Acid Sheets in a Rat Back Skin Defect Model. Transfus. Apher. Sci. 2021, 60, 103144. [Google Scholar] [CrossRef]
- Wang, H.; Huddleston, S.; Yang, J.; Ameer, G.A. Enabling Proregenerative Medical Devices via Citrate-Based Biomaterials: Transitioning from Inert to Regenerative Biomaterials. Adv. Mater. 2024, 36, 2306326. [Google Scholar] [CrossRef]
- Yang, F.; Zhang, C.; Ma, Z.; Weng, Y. In Situ Formation of Microfibrillar PBAT in PGA Films: An Effective Way to Robust Barrier and Mechanical Properties for Fully Biodegradable Packaging Films. ACS Omega 2022, 7, 21280–21290. [Google Scholar] [CrossRef]
- Stock, V.; Fahrenson, C.; Thuenemann, A.; Dönmez, M.H.; Voss, L.; Böhmert, L.; Braeuning, A.; Lampen, A.; Sieg, H. Impact of Artificial Digestion on the Sizes and Shapes of Microplastic Particles. Food Chem. Toxicol. 2020, 135, 111010. [Google Scholar] [CrossRef]
- Wang, J.; Cong, J.; Wu, J.; Chen, Y.; Fan, H.; Wang, X.; Duan, Z.; Wang, L. Nanoplastic-Protein Corona Interactions and Their Biological Effects: A Review of Recent Advances and Trends. TrAC Trends Anal. Chem. 2023, 166, 117206. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Malafeev, K. The Cytotoxicity of Biodegradable Microplastics and Nanoplastics: Current Status and Research Prospects. Microplastics 2025, 4, 58. https://doi.org/10.3390/microplastics4030058
Malafeev K. The Cytotoxicity of Biodegradable Microplastics and Nanoplastics: Current Status and Research Prospects. Microplastics. 2025; 4(3):58. https://doi.org/10.3390/microplastics4030058
Chicago/Turabian StyleMalafeev, Konstantin. 2025. "The Cytotoxicity of Biodegradable Microplastics and Nanoplastics: Current Status and Research Prospects" Microplastics 4, no. 3: 58. https://doi.org/10.3390/microplastics4030058
APA StyleMalafeev, K. (2025). The Cytotoxicity of Biodegradable Microplastics and Nanoplastics: Current Status and Research Prospects. Microplastics, 4(3), 58. https://doi.org/10.3390/microplastics4030058






