Abstract
This study explores the Swedish diversity of the thrips families Aeolothripidae and Melanthripidae. Currently, a total of 12 species in 2 genera of Aeolothripidae occur in Sweden, and 1 in Melanthripidae. The aims of this study include to provide an updated identification key with photographic material and an updated checklist of the country with provincial records. In this study both museum material and new material collected in understudied provinces are included, and a large number of molecular barcodes are produced. The results reveal 26 new provincial records in Sweden, predominantly in northern regions, and 11 provinces in total had new species records. New records of Rhipidothrips brunneus Williams 1913 warranted an examination of distinguishing characters compared to R. niveipennis Reuter, 1899. The original description of R. niveipennis is found to lack sufficient characters to delimit the species, and a redescription based on syntypes is presented.
Keywords:
Thysanoptera; thrips; banded thrips; predatory thrips; checklist; identification key; taxonomy; redescription; sub-arctic; insects 1. Introduction
Aeolothripidae Uzel, 1895 [1], with ca. 200 species, and Melanthripidae Bagnall, 1913 [2], with ca, 70 species, are the two of smallest families of the insect order thrips (Thysanoptera Haliday, 1836 [3]). Species from both Aeolothripidae and Melanthripidae are present in the Palearctic, but species also occur in the Nearctic and more tropical areas worldwide [4,5]. However, research involving the Palaearctic fauna in general, and the Swedish fauna in particular, is scarce.
Most thrips are very small, often not larger than 3 mm. The life histories within this order are very varying, ranging from flower and fungus feeding to predatory, with some species being gall-inducing or dwelling in decaying matter [4]. A few are considered as pests in agriculture [4,6], and invasive species occur.
The family Aeolothripidae is represented by 12 species in Sweden and does not have an accepted English vernacular name other than “banded thrips”, while the translation of the Swedish name is “predatory thrips”. However, most are believed not to be obligate predatory and not all are banded. There are also predatory species and species with a banded pattern in other families. Many seem to feed both on plant tissue and other small arthropods occupying the same habitats [5]. Species in this family often have dark and bright banded (Figure 1A) or spotted wings (Figure 1B), and sometimes brightly colored middle body segments which gives them an ant-like appearance (Figure 1C). Ant mimicry together with fast and agile mobility while hunting often make these thrips easy to recognize in the field.

Figure 1.
Alive specimens of three species in the thrips family Aeolothripidae. (A) Aeolothrips versicolor Uzel, 1895 [1]. (B) A. ericae Bagnall, 1920 [7]. (C) A. albicinctus Haliday, 1836 [3].
The family Melanthripidae is represented only by one species in Sweden. The species in this family are similar in appearance to Aeolothripidae but differ in their sense organs. They are all phytophagous and are often host-specific [5].
Major regional checklist and identification keys focus on Great Britain [4,8] and central and southern Europe [9]. Extensive surveys in Poland have shown that the knowledge of the thrips fauna contains large gaps, with a large amount of new taxonomical and ecological knowledge to be gained from targeted surveys and research projects [10,11,12,13,14].
In the identification key to Norwegian thrips by Kobro [15], only three species of Aeolothripidae were represented, and Melanthripidae is not known in Norway. The most recent checklist including all families in Sweden was provided by Gertsson [16]. The checklist was mainly based on museum specimens. However, new records are likely as few active researchers and inventories focus on the collection of thrips. Since 2015, several additional province records for species in Aeolothripidae have been published [17,18]. Further collection in previously understudied provinces is clearly required. With taxon coverage of Thysanoptera being as low as 18.3% in general Malaise traps [19], it is also crucial to utilize more targeted and diverse sampling methods.
This study is a continuation of the project started in 2021 with the family Phlaeothripidae Uzel, 1895 [1], with the purpose of increasing the knowledge of the taxonomy and biogeography of the Swedish thrips fauna. In total, 15 species were examined, of which 13 occur in Sweden and 2 have the potential of being introduced. The two families were studied together here as they are easily separated from the third family occurring in Sweden, Thripidae Stephens, 1829 [20], by having nine antennal segments. However, this does not imply a close phylogenetic relationship. The goals are to provide a better understanding of the current distribution of all species in the Aeolothripidae and Melanthripidae families in the country, present a complete and updated checklist, provide an updated identification key to all species including potentially introduced species, and contribute to the reference libraries of molecular species barcode sequences.
The development of updated and modern keys for morphological identification is fundamental for gathering data in, e.g., taxonomical, environmental, and ecological studies. The morphological studies are enhanced by molecular data, increasing available genetic barcodes and facilitating further molecular research (e.g., metabarcoding and environmental DNA surveys).
2. Material and Methods
The material for this study was both acquired from museums and also collected during field work in Sweden during the period 2021–2023. The museum collections involved in this study were the Swedish Museum of Natural History (NHRS, Sweden), the Lund Museum of Zoology (MZLU, Sweden), Forschungsinstitut und Naturmuseum Senckenberg (SMF, Germany), and the Finnish Natural History Museum (MZF, Finland), and the private collections of Carl-Axel Gertsson (Sweden), Sverre Kobro (Norway) and Manfred R. Ulitzka (Germany).
Specimens collected in the field were stored in 80% ethanol, and a newly developed method for non-destructive DNA extraction was used [21] as the maceration step before slide mounting. In short, the specimen was placed in lysis buffer with proteinase K and lysed overnight at 56 °C. The specimen was thereafter retrieved from the lysis buffer and subjected to a dehydration and salt cleaning protocol, comprising 72 h in 80% ethanol, followed by an additional 24 h in 80% ethanol if residual salts from the lysis buffer were still present. If no salts were present, the next step was 95% ethanol for 15 min followed by 99.5% ethanol for 5 min. The specimen was inspected, and if additional lightening of the cuticle was needed, the specimen was placed in clove oil for up to 30 min. Mounting was finally carried out by placing the specimen upside down on a cover slip in a drop of Euparal, and a microscope slide was gently lowered until the capillary force of the mounting medium drew the coverslip to the slide. The slide was flipped over and dried at room temperature before microscopic studies.
The lysis buffer without the specimen was used for DNA extraction with a KingFisher™ Flex (Thermo Scientific, MA, USA) robot and Mag-Bind® Blood & Tissue DNA HDQ 96 Kit (Omega Bio-Tek, Georgia, Georgia), according to manufacturer’s protocol. The 658 bp DNA barcode region of COI was amplified using the LCO1490 and HCO2198 primer pair [22]. The PCR reaction was set up with Ready-To-Go PCR beads (Amersham Biosciences, Buckinghamshire, United Kingdom) and 21 µL of H2O, 1 µL of the respective primer and 2 µL of DNA template. The PCR reaction was performed with an initial 95 °C denaturation step (5 min) followed by 40 cycles of 95 °C (30 s), 50 °C (30 s), 72 °C (50 s), and 72 °C (8 min). The sequencing of the barcodes was carried out by Macrogen Inc. (Netherlands). The sequences were inspected and assembled in Geneious 8 and thereafter uploaded to the Barcode of Life Data System (BOLD) [23].
The slide-mounted specimens were studied and photographed using a Zeiss Primostar 3 microscope with a Zeiss Axiocam 208 camera and Zeiss Zen 3.8 software. The images were finalized in Adobe Photoshop 24.7.0. Both general and specific identification literature was used [8,9,24,25,26], as well as original species descriptions. All collected material was deposited at the Swedish Museum of Natural History (NHRS) in Stockholm, Sweden. All metadata and collection data were also reported, in addition to BOLD, to the Swedish Species Observation System (SLU Swedish Species Information Centre), which in turn shares the data to the Global Biodiversity Information Facility (GBIF).
The terminology of morphological characteristics and anatomy mainly followed Schliephake and Klimt [26] and Moritz [27]. Segment numbers were provided in roman numerals. The provincial division of Sweden followed the traditional faunistic provinces, with Lapland divided into smaller regions (Figure 2A).
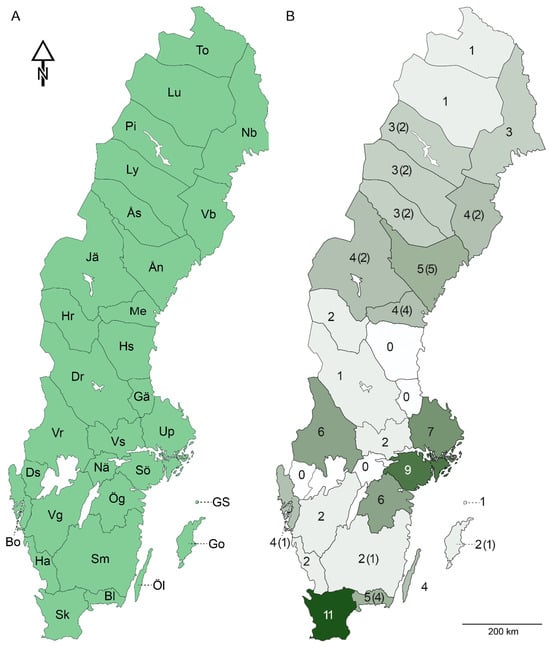
Figure 2.
Map of Sweden. (A) Faunistic provinces. (B) Current species numbers in each Swedish faunistic province, including all new records presented in this study. The number of new records for each province is showed within parentheses. A darker colour tint indicates a higher species count.
| Abbreviations of Swedish provinces (north to south) |
| To = Torne lappmark |
| Lu = Lule Lappmark |
| Pi = Pite lappmark |
| Ly = Lycksele lappmark |
| Ås = Åsele lappmark |
| Nb = Norrbotten |
| Vb = Västerbotten |
| Ån = Ångermanland |
| Jä = Jämtland |
| Hr = Härjedalen |
| Me = Medelpad |
| Hs = Hälsingland |
| Gä = Gästrikland |
| Dr = Dalarna |
| Vr = Värmland |
| Vs = Västmanland |
| Up = Uppland |
| Sö = Södermanland |
| Nä = Närke |
| Ds = Dalsland |
| Bo = Bohuslän |
| Vg = Västergötland |
| Ög = Östergötland |
| GS = Gotska Sandön |
| Go = Gotland |
| Öl = Öland |
| Sm = Småland |
| Ha = Halland |
| Bl = Blekinge |
| Sk = Skåne |
| Morphological abbreviations in figures |
| db = distal band |
| c = clasper |
| is = interocellar seta |
| pas = posteroangular seta |
| s = seta |
| se = sensorium |
3. Results
Targeted field sampling in Sweden resulted in 26 new provincial records of species in the family Aeolothripidae, and among the 30 provinces, 11 had new species records. This represents a large addition to the knowledge of the Swedish distribution of species in this small but understudied family. The current known species richness throughout Sweden is illustrated in Figure 2B, and the largest addition of records is found in the northern regions. In the families Aeolothripidae and Melanthripidae, 13 species are known in Sweden, with the latter being represented by only 1. From the collected material, 164 specimens (145 females, 19 males), representing 7 species, were sequenced for molecular barcodes and were submitted to the BOLD database (https://dx.doi.org/10.5883/DS-SETHY23).
Rhipidothrips niveipennis Reuter, 1899 [28], was originally separated from other species in the genus by its lack of posteroangular bristles on the pronotum [28]. When Williams described R. brunneus Williams, 1913 [29], he further used this characteristic, along with some additional differences in leg and antenna coloration and antennal segment lengths [29]. However, as pointed out in the review of the genus by Bailey, these characteristics are dubious and variable [24]. Speyer and Parr suggested that Reuter’s specimens belonged to multiple species [30]; however, after examining the type material, I cannot support this, but do point out that the specimens are of different quality and some characteristics are difficult to observe. Speyer and Parr further claimed that Reuter described R. niveipennis from Sweden [30], which is not true (the specimens were from Finland, with material studied from multiple localities). From the discussion provided by Speyer and Parr, Williams, and Bailey [24,29,30], it is possible that there have been some confusion and ambiguity about which pronotal seta was observed and to what degree the setae are sclerotized. Speyer and Parr, for example, found a specimen which they claimed had only one pair of “feebly sclerotized” setae and therefore stated that this is consistent with Reuter’s original description. All Reuter’s specimens have a posteroangular seta, both brachypterous and macropterous, but it is faintly visible in some due to its light color and weak sclerotization. Bailey pointed out the ambiguity of the setae but did not study the type specimens in his revision of the genus and highlighted that the brachyopterous form is not fully described. As R. brunneus is now a known part of the Swedish fauna [31] and the separation of the two species is necessary, I present a formal redescription based on Reuter’s type material, including both brachypterous and macropterous forms. It is also included in the identification key with characteristics separating it from R. niveipennis.
The identification key to the 13 known and 2 potentially introduced species in Sweden is followed by the current taxonomical records of Aeolothripidae and Melanthripidae with short descriptions.
3.1. Identification Key to the Adults of Swedish Aeolothripidae and Melanthripidae
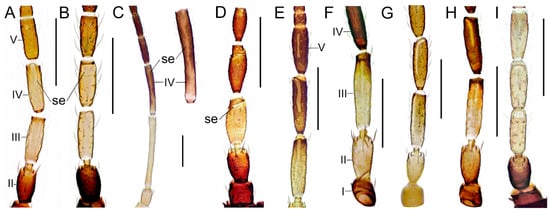
| 1 | Sensory organs on antennal segments III and IV linear and longitudinal (Figure 3A), lens-shaped (Figure 3B) or as irregular blotches (Figure 3C); antennal segments VIII and IX small and broadly joined forming a single conical unit; pronotom with short setae, stouter setae at posterior margin............................. Aeolothripidae 2 |
| Sensory organs linear and transverse (Figure 3D); antennal segments VIII and IX clearly separated; pronotum with many long prominent setae............................................................. Melanthripidae: Melanthrips fuscus | |
| 2(1). | Sensory organs on antennal segments III and IV linear (Figure 3E–H) or lens-shaped (Figure 3B,I)...............3 |
| Antennal sensory organs as long, irregular blotchy area; antennal segment III very elongate (Figure 3C)................................................................................................................................................Franklinothrips vespiformis | |
| 3(2). | Sensory organs on antennal segments III and IV linear (Figure 3A)...................................................Aeolothrips 4 |
| Sensory organs lens-shaped (Figure 3B)........................................................................................... Rhipidothrips 14 | |
| 4(3). | Sensory organ on antennal segment V absent or minute (Figure 3A), at most a tenth of the length of the segment............................................................................................................................................................................5 |
| Sensory organ well developed (Figure 3E), at about half the length of the segment............................A. manteli | |
| 5(4). | Anterior part of head not prolonged in front of eyes (Figure 4A)...........................................................................6 |
| Anterior part of head prolonged in front of eyes (Figure 4B).................................................................A. versicolor | |
| 6(5). | Antennal segment II mainly brown, at most yellow apically (Figure 3A).............................................................7 |
| Antennal segment II mainly yellow (Figure 3F)......................................................................................................12 | |
| 7(6). | Forewing with 2 dark transverse bands (Figure 5A).................................................................................................8 |
| Forewing with 1 dark transverse band and posterior border dark from middle and extending almost all the way to wing apex (Figure 5B)........................................................................................................................A. vittatus | |
| 8(7). | Forewing with posterior border pale in middle (Figure 5A)....................................................................................9 |
| Posterior border dark also in middle area (Figure 5C).........................................................................A. melaleucus | |
| 9(8). | Females with antennal segment III bicolored, apical half brown; sensory organ on segment IV longer than two thirds of segment length in both sexes (Figure 3G).........................................................................................10 |
| Females with antennal segment III yellow with a distinct apical brown band; sensory organ on segment IV about half of segment length in both sexes (Figure 3H).........................................................................................11 | |
| 10(9). | Apical ring vein of forewing brown (Figure 5A)..................................................................................A. propinquus |
| Apical ring vein as pale as wing membrane (Figure 5D)............................................................................A. astutus | |
| 11(9). | Distal dark band on forewing at most 1.5 times long as wide (Figure 6A); male base of bifurcate claspers on tergite IX with setae shorter than claspers (Figure 7A)........................................................................A. intermedius |
| Distal dark band on forewing at least 1.55 times long as wide (Figure 6B); male base of bifurcate claspers on tergite IX with setae longer than claspers (Figure 7B)..............................................................................A. fasciatus | |
| 12(6). | Body (excluding extremities) mainly brown, at most yellow abdominal segments II–III (Figure 6C,E)........13 |
| Head and thorax mainly yellow, brown medially; abdominal segments I–IV (sometimes also V–VI) yellow at least laterally, segments VII–X brown (Figure 6D).............................................................................A. gloriosus | |
| 13(12). | Female tergite I not transversely striated (Figure 7C); always with wings (Figure 6C)..........................A. ericae |
| Female tergite I transversely striated (Figure 7D); usually micropterous (Figure 6E)....................A. albicinctus | |
| 14(3). | Antennal segment V evenly brown (Figure 3B); interocellar setae at least 20 µm (Figure 4C)........R. brunneus |
| Antennal segment V yellow, sometimes fading to brown (Figure 3I); interocellar setae less than 20 µm (Figure 4D)........................................................................................................................................................R. niveipennis |
3.2. Taxonomy
| Order Thysanoptera Haliday, 1836 [3] |
| Suborder Terebrantia Haliday, 1836 [3] |
| Family Aeolothripidae Uzel, 1895 [1] |
The Aeolothripidae are characterized by having nine antennal segments, but the terminal segments can be partly fused and harder to distinguish. The sensorial organs on the antennae are either elongate and longitudinal, or as regular or irregular blotches or lens-shaped structures (Figure 3A–C). Macropterous, brachypterous and micropterous conditions exist, also within species. Males are rarely collected and, in some cases, unknown. Wings in many species are banded, but for some species they are evenly shaded or completely pale. The biology is variable, from obligate predators to omnivores to pollen feeding or feeding on plant tissue and fluids.
| Genus Aeolothrips Haliday, 1836 [3] |
| Aeolothrips albicinctus Haliday, 1836 [3] |
| Figure 1C, Figure 6E and Figure 7D |
| Aeolothrips albicinctus Haliday, 1836: 451 [3]. |
- Material Examined
SWEDEN; 1♀; Södermanland, Tyresta national park; 2–15 July 2000; B. Viklund, L-O Wikars, H. Ahnlund leg.; Malaise trap; NHRS.; 2♂♂; Södra Värmland, Rämmens socken, Fjällrämmens beach, 5 km NNE of Liljendal; 26 May 1974; U. Qvick leg.; in Calamagrostis; MZLU 00151698, 00158168.; 1♀1♂; Uppland, Stockholm, Experimentalfältet; 10 August 1919; O. Ahlberg leg.; NHRS.; 1♀; Värmland, Gustaf Adolfs socken, Geijersholms dam; 8 June 1975; U. Qvick leg.; on Calamagrostis; MZLU 00158166.; 1♀; Blekinge, Karlskrona kommun, Torhamn; N56.092591, E15.853811; 31 May 2023; E. Wahlberg leg.; on Barbarea vulgaris; voucher ID: BK3T; BOLD ID: SETHY263-23; NHRS.; 1♀; Bohuslän, Tjörns kommun, Kebene; N57.977127, E11.605660; 11 June 2023; E. Wahlberg leg.; in mixed herbaceous vegetation; voucher ID: BD2T; BOLD ID: SETHY203-23; NHRS.; 1♀; Jämtland, Strömsunds kommun, Sävselet; N63.538795, E15.444725; 15 June 2023; E. Wahlberg leg.; mixed flowering plants; voucher ID: BQ1T; BOLD ID: SETHY310-23; NHRS.; 1♀; Lycksele lappmark, Sorsele kommun, Saxnäs; N65.455847, E17.572265; 16 June 2023; E. Wahlberg leg.; mixed flowering plants; voucher ID BP2T; BOLD ID: SETHY303-23; NHRS.; 5♀♀; Medelpad, Timrå kommun, Indalälvens delta; N62.508667, E17.448164; 14 June 2023; E. Wahlberg leg.; in mixed herbaceous vegetation; voucher IDs: BD3T, BD4T, BD9T, BE6T, BE9T; BOLD IDs: SETHY204-23, SETHY205-23, SETHY210-23, SETHY216-23, SETHY219-23; NHRS.; 1♀; Småland, Västerviks kommun, Tinderednäs; N57.977431, E16.483873; 1 June 2023; E. Wahlberg leg.; on Ranunculus; voucher ID: BL1T; BOLD ID: SETHY270-23; NHRS.; 1♀; Södermanland, Flens kommun, Malmköpings nature reserve; N59.1458, E16.7205; 7 June 2023; E. Wahlberg leg.; voucher ID BI1T; BOLD ID: SETHY245-23; NHRS.; 1♀; Södermanland, Gnesta kommun, Gåsinge; N59.0855, E17.2140; 14 May 2023; E. Wahlberg leg.; on Prunus spinosa; voucher ID: AZ2T; BOLD ID: SETHY167-23; NHRS.; 3♀♀; Västerbotten, Skellefteå kommun, Nilsliden; N64.944032, E20.367689; 18 June 2023; E. Wahlberg leg.; in mixed herbaceous vegetation; voucher IDs: BO1T, BOT2T, BOT3T; BOLD IDs: SETHY294-23, SETHY295-23, SETHY296-23; NHRS.; 2♀♀; Västerbotten, Skellefteå kommun, Yttervik; N64.680468, E21.095959; 18 June 2023; E. Wahlberg leg.; on Silene dioica; voucher IDs: BN5T, BN6T; BOLD IDs: SETHY289-23, SETHY290-23; NHRS.; 7♀♀; Ångermanland, Kramfors kommun, Västhammar; N63.030171, E17.773730; 19 June 2023; E. Wahlberg leg.; in mixed herbaceous vegetation; voucher IDs: AZ5T, AZ6T, AZ7T, AZ8T, BA6T, BA7T, BA8T; BOLD IDs: SETHY170-23, SETHY171-23, SETHY172-23, SETHY173-23, SETHY180-23, SETHY181-23, SETHY182-23; NHRS.; 1♀; Åsele lappmark, Dorotea kommun, Häggås; N64.402187, E16.434090; 16 June 2023; E. Wahlberg leg.; mixed flowering plants; voucher ID: BR7T; BOLD ID: SETHY322-23; NHRS.
- Description
Body (excl. extremities) bicolored (Figure 6E). Antennal segments I–III yellow; segment IV brown. Sensory organs on antennal segments III and IV linear and directed longitudinally. Head mainly dark. Thorax mainly dark. Anterior and posterior margins of pronotum without long setae. Often micropterous (Figure 6E), if wings present then forewings with two dark transverse bands; apical vein of forewing as pale as wing membrane. Abdominal segments II and III yellow, remaining segments brown (Figure 6E). Female tergite I transversely striated (Figure 7D). Male abdominal segment IX without lateral stout setae.
- Remarks
Found on various herbaceous plants and presumed to be predatory on mites [25] and has an ant-like appearance and behavior. Holarctic distribution. In Fennoscandia, in Sweden, Norway, Denmark, and Finland; in Sweden, previously recorded in Skåne, Öland, Södermanland, Uppland, Värmland, and Lappland (not specified) [16]. Also reported here from Blekinge, Småland, Bohuslän, Medelpad, Jämtland, Ångermanland, and Västerbotten.
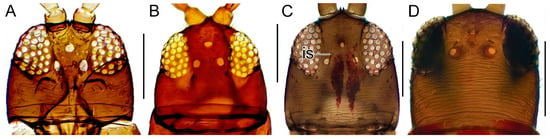
Figure 4.
Head in dorsal view. (A) Aeolothrips ericae, ♂. (B) A. versicolor, ♀. (C) Rhipidothrips brunneus, ♀. (D) R. niveipennis, ♀. Scale bar: 100 µm.
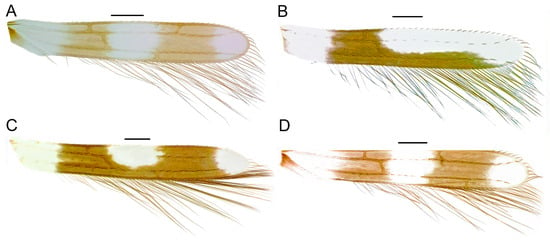
Figure 5.
Forewing. (A) Aeolothrips propinquus, ♀. (B) A. vittatus, ♀. (C) A. melaleucus, ♀. (D) A. astutus, ♀. Scale bar: 100 µm.
| Aeolothrips astutus Priesner, 1926 [32] |
| Figure 5D. |
| Aeolothrips astutus Priesner, 1926: 109 [32]. |
- Material examined
SWEDEN; 2♀♀; Skåne, Maglehem, Spröjtagränd 6; N55.767558, E14.149546; 20 July 2021; C.-A. Gertsson leg.; on Anchusa officinalis; MZLU 00148155, 001583875.; 1♀; Skåne, Saxtorp, Badsjön; 2 July 2021; C.-A. Gertsson leg.; on Anchusa officinalis; MZLU 00158377.
- Description
Body (excl. extremities) wholly brown. Antennal segment I brown; II mainly brown, at most yellow apically; III bicolored, apical half brown; IV brown. Sensory organs on antennal segments III and IV linear and directed longitudinally. Anterior and posterior margins of pronotum without long setae. Forewings present with two dark transverse bands; apical ring vein as pale as wing membrane (Figure 5D).
- Remarks
Found on Anchusa officinalis [18]. Palearctic distribution. In Fennoscandia, in Sweden and Denmark. In Sweden, found in Södermanland and Skåne [18].
| Aeolothrips ericae Bagnall, 1920 [7] |
| Figure 1B, Figure 3F, Figure 4A, Figure 6C and Figure 7C. |
| Aeolothrips ericae Bagnall, 1920: 60 [7]. |
- Material examined
SWEDEN; 1♂; Värmland, Gustaf Adolfs s:n, Geijersholms herrråd; 10 June 1975; U. Qvick leg.; on flowering Orobus tuberosus; MZLU 00158187.; 1♀; Värmland, Gustaf Adolfs s:n, 1 km SE of Mossjön; 18 June 1975; U. Qvick leg.; on flowering Cardaminopsis arenosa; MZLU 00158202.; 2♀♀; Södermanland, Tyresta national park; 5 June–15 July 2000; B. Viklund, L.-O. Wikars, H. Ahnlund leg.; Malaise trap; NHRS.; 1♀1♂; Södermanland, Gnesta kommun, Bryggeriholmen; N59.048168, E17.318564; 23 June 2022; E. Wahlberg leg.; voucher IDs: AV6T, AV7T; BOLD IDs: SETHY140-22, SETHY141-22; NHRS.; 1♀; Jämtland, Östersunds kommun, Södergård; N63.095297, E14.820085; 30 June–13 July 2021; Swedish Insect Inventory Program (SIIP) leg.; mowing meadow next to coniferous forest; Malaise trap; locality ID: 54; collection event ID: M54-003; sample ID: 39_G6; Station Linné.; 1♀; Ångermanland, Härnösand kommun, Lockeby 375; N62.6402228, E17.770841; 15 June–1 July 2021; Swedish Insect Inventory Program (SIIP) leg.; pasture in deciduous forest; Malaise trap; locality ID: 56; collection event ID: M56-001; sample ID: 72_H9; Station Linné.; 1♀; Blekinge, Karlskrona kommun, Gamla reningsverket; N56.189540, E15.622587; 31 May 2023; E. Wahlberg leg.; on Anchusa officinalis; voucher ID: AZ4T; BOLD ID: SETHY169-23; NHRS.; 6♀♀; Blekinge, Karlskrona kommun, Torhamn; N56.092591, E15.853811; 31 May 2023; E. Wahlberg leg.; on Barbarea vulgaris; voucher IDs: BK4T, BK5T, BK6T, BK7T, BK8T, BK9T; BOLD IDs: SETHY264-23, SETHY265-23, SETHY266-23, SETHY267-23, SETHY268-23, SETHY269-23; NHRS.; 3♀♀1♂; Jämtland, Ragunda kommun, Boberg; N63.359057, E15.717947; 15 June 2023; E. Wahlberg leg.; on Astragalus alpinus; voucher IDs: BR1T, BR4T, BR5T, BQ9T; BOLD IDs: SETHY317-23, SETHY319-23, SETHY320-23, SETHY316-23; NHRS.; 3♀♀; Jämtland, Strömsunds kommun, Sävselet; N63.538795, E15.444725; 15 June 2023; E. Wahlberg leg.; mixed flowering plants; voucher IDs: BQ3T, BQ4T, BQ5T; BOLD IDs: SETHY311-23, SETHY312-23, SETHY313-23; NHRS.; 2♀♀1♂; Lycksele lappmark, Sorsele kommun, Saxnäs; N65.455847, E17.572265; 16 June 2023; E. Wahlberg leg.; mixed flowering plants; voucher IDs: BO8T, BP1T, BO9T; BOLD IDs: SETHY300-23, SETHY302-23, SETHY301-23; NHRS.; 6♀♀; Medelpad, Timrå kommun, Indalälvens delta; N62.508667, E17.448164; 14 June 2023; E. Wahlberg leg.; in mixed herbaceous vegetation; voucher IDs: BD5T, BD6T, BD7T, BE4T, BE5T, BF1T; BOLD IDs: SETHY206-23, SETHY207-23, SETHTY208-23, SETHY214-23. SETHY215-23, SETHY220-23; NHRS.; 1♀; Medelpad, Timrå kommun, Svedje; N62.519346, E17.288289; 14 June 2023; E. Wahlberg leg.; in mixed herbaceous vegetation; voucher ID: BN3T; BOLD ID: SETHY287-23; NHRS.; 2♀♀; Pite lappmark, Arjeplogs kommun, Arjeplog; N66.040783, E17.868661; 18 June 2023; E. Wahlberg leg.; on Astragalus alpinus; voucher IDs: BP7T, BP8T; BOLD IDs: SETHY307-23, SETHY308-23; NHRS.; 3♀♀1♂; Västerbotten, Skellefteå kommun, Yttervik; N64.680468, E21.095959; 18 June 2023; E. Wahlberg leg.; on Silene dioica; voucher IDs: BN7T, BN8T, BN9T, BN4T; BOLD IDs: SETHY291-23, SETHY292-23, SETHY293-23, SETHY288-23; NHRS.; 2♀♀; Ångermanland, Kramfors kommun, Västhammar; N63.030171, E17.773730; 19 June 2023; E. Wahlberg leg.; in mixed herbaceous vegetation; voucher IDs: AZ9T, BA2T; BOLD IDs: SETHY174-23, SETHY176-23; NHRS.; 14♀♀4♂♂; Ångermanland, Sollefteå kommun, Märrgård; N63.717364, E16.823417; 15 June 2023; E. Wahlberg leg.; in mixed herbaceous vegetation; voucher IDs: BB2T, BB3T, BB5T, BB7T, BB9T, BC1T, BC2T, BC3T, BC4T, BC5T, BC6T, BC7T, BC8T, BD1T, BB4T, BB6T, BB8T, BC9T; BOLD IDs: SETHY185-23, SETHY186-23, SETHY188-23. SETHY190-23, SETHY192-23, SETHY193-23, SETHY194-23, SETHY195-23, SETHY196-23, SETHY197-23, SETHY198-23, SETHY199-23, SETHY200-23, SETHY202-23, SETHY187-23, SETHY189-23, SETHY191-23, SETHY201-23; NHRS.; 15♀♀4♂♂; Åsele lappmark, Dorotea kommun, Västra Ormsjö; N64.467878, E15.951629; 16 June 2023; E. Wahlberg leg.; on Astragalus alpinus; voucher IDs: BF3T, BF5T, BF6T, BF7T, BF9T, BG1T, BG2T, BG3T, BG4T, BG7T, BG8T, BG9T, BH1T, BH3T, BH4T, BF8T, BG5T, BH2T, BH6T; BOLD IDs: SETHY222-23, SETHY224-23, SETHY225-23, SETHY226-23, SETHY228-23, SETHY229-23, SETHY230-23, SETHY231-23, SETHY232-23, SETHY235-23, SETHY236-23, SETHY237-23, SETHY238-23, SETHY240-23, SETHY241-23, SETHY227-23, SETHY233-23, SETHY239-23, SETHY243-23; NHRS.
- Description
Body (excl. extremities) mostly wholly brown (Figure 6C). Antennal segment I yellow or pale light brown; II mainly yellow; III bicolored, apically darker; IV brown (Figure 3F). Sensory organs on antennal segments III and IV linear and directed longitudinally (Figure 3F). Anterior and posterior margins of pronotum without long setae. Forewings present with two dark transverse bands; apical ring vein of forewing as pale as wing membrane (Figure 6C). Abdominal segments all brown; X often slightly lighter; male abdominal segment IX with sickle-shaped lateral stout setae (Figure 6C).
- Remarks
Found in various herbaceous plants, presumed to be a facultative predator. Palaearctic distribution. In Fennoscandia in Sweden, Norway, and Finland. In Sweden broadly distributed throughout, with previous records from Skåne, Halland, Södermanland, Östergötland, Västergötland, Bohuslän, Västmanland, Dalarna, Värmland, Härjedalen and Jämtland. Also reported here from Blekinge, Medelpad, Ångermanland, Västerbotten, Åsele lappmark, Lycksele lappmark, and Pite lappmark.
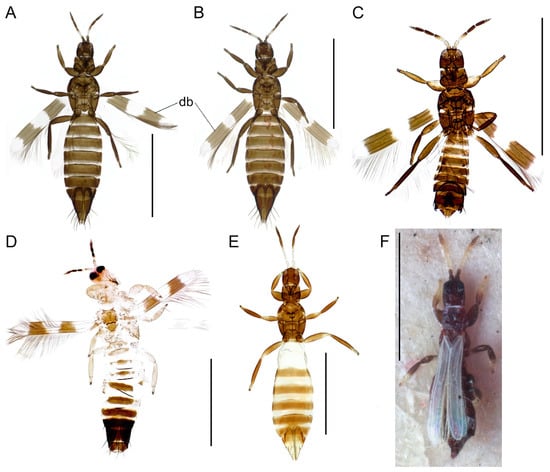
Figure 6.
Habitus in dorsal view. (A) A. intermedius, ♀. (B) A. fasciatus, ♀. (C) A. ericae, ♂. (D) A. gloriosus, ♀. (E) A. albicinctus, ♀. (F) Rhipidothrips niveipennis, ♀, syntype. Scale bar: 1 mm.
| Aeolothrips fasciatus (Linnaeus, 1758) [33] |
| Figure 6B and Figure 7B. |
| Thrips fasciata Linnaeus, 1758: 457 [33]. |
- Material examined
SWEDEN; 1♂1♀; Uppland, Stockholm, Experimentalfältet; 10 June 1919; O. Ahlberg leg.; in grass; MZLU 00158215.; 1♂; Skåne, Lund; 18 July 1957; L. Cederholm leg.; on white filter paper; MZLU.; 1♂; Skåne, Hasslebro; N55.940809, E13.395151; 7–14 July 2020; Y. Cough et al. Leg.; blue pan trap; MZLU 00117239.; 1♂; Skåne, Rörum; N55.622867, E14.245726; 27 July–3 August 2020; white pan trap; Y. Cough et al. leg.; MZLU 00117237.; 1♀; Skåne, Kågerröd; N56.006561, E13.187555; 7–14 July 2020; Blue pant rap; Y. Cough et al. leg.; MZLU 00117238.; 1♀; Norrbotten, Övertorneå kommun, Hanhinvittikko; N66.451386, E23.483197; 7 June–22 June 2021; Swedish Insect Inventory Program (SIIP) leg.; claimed hay meadow; Malaise trap; locality ID: 50; collection event ID: M50-013; sample ID: 7_D10; Station Linné.; 1♀; Östergötland, Linköpings kommun, Västerlösa; N58.42107, E15.33843; 28 July–4 August 2019; Insect Biome Atlas (IBA) leg.; urban; Malaise trap; locality ID: TDUYXP; collection event ID: S7536S; sample ID: 231_C3; Station Linné.; 8♀♀1♂; Blekinge, Karlskrona kommun, Augerum; N56.216929, E15.676876; 1 June 2023; E. Wahlberg leg.; on Anchusa officinalis; voucher IDs: BL9T, BM1T, BM2T, BM3T, BM4T, BM6T, BM7T, BM8T, BK2T; BOLD IDs: SETHY277-23, SETHY278-23, STHY279-23, SETHY280-23, SETHY281-23, SETHY282-23, SETHY283-23, SETHY284-23, SETHY262-23; NHRS.; 1♀; Blekinge, Karlskrona kommun, Gamla reningsverket; N56.189540, E15.622587; 31 May 2023; E. Wahlberg leg.; on Anchusa officinalis; voucher ID: AZ3T; BOLD ID: SETHY168-23; NHRS.; 3♀♀; Blekinge, Karlskrona kommun, Sandhamn; N56.090980, E15.856533; 31 May 2023; E. Wahlberg leg.; in mixed herbaceous vegetation; voucher IDs: BL3T, BL6T, BL8T; BOLD IDs: SETHY272-23, SETHY275-23, SETHY276-23; NHRS.; 2♀♀1♂; Jämtland, Ragunda kommun, Boberg; N63.359057, E15.717947; 15 June 2023; E. Wahlberg leg.; on Astragalus alpinus; voucher IDs: BQ6T, BR3T, BQ8T; BOLD IDs: SETHY314-23, SETHY318-23, SETHY315-23; NHRS.; 1♀; Lycksele lappmark, Sorsele kommun, Saxnäs; N65.455847, E17.572265; 16 June 2023; E. Wahlberg leg.; mixed flowering plants; voucher ID: BO7T; BOLD ID: SETHY299-23; NHRS.; Female 3♀♀3♂♂; Medelpad, Timrå kommun, Indalälvens delta; N62.508667, E17.448164; 14 June 2023; E. Wahlberg leg.; in mixed herbaceous vegetation; voucher IDs: BE1T, BE7T, BE8T, BD8T, BE2T, BE3T; BOLD IDs: SETHY211-23, SETHY217-23, SETHY218-23, SETHY209-23, SETHY212-23, SETHY213-23; NHRS.; 1♀; Medelpad, Timrå kommun, Svedje; N62.519346, E17.288289; 14 June 2023; E. Wahlberg leg.; in mixed herbaceous vegetation; voucher ID: BM9T; BOLD ID: SETHY285-23; NHRS.; 2♀♀2♂♂; Pite lappmark, Arjeplogs kommun, Arjeplog; N66.040783, E17.868661; 18 June 2023; E. Wahlberg leg.; on Astragalus alpinus; voucher IDs: BP6T, BP9T, BP3T, BP5T; BOLD IDs: SETHY306-23, SETHY309-23, SETHY304-23, SETHY305-23; NHRS.; 1♀; Södermanland, Flens kommun, Sparreholms ekhagar; N59.086089, E16.8305657; 7 June 2023; E. Wahlberg leg.; on Ranunculus; voucher ID: BI7T; BOLD ID: SETHY251-23; NHRS.; 5♀♀; Ångermanland, Kramfors kommun, Västhammar; N63.030171, E17.773730; 19 June 2023; E. Wahlberg leg.; in mixed herbaceous vegetation; voucher IDs: BA1T, BA4T, BA5T, BA9T, BB1T; BOLD IDs: SETHY175-23, SETHY178-23, SETHY179-23, SETHY183-23, SETHY184-23; NHRS.; 1♀; Ångermanland, Nordmalings kommun, Torrböle; N63.700503, E19.604039; 19 June 2023; E. Wahlberg leg.; in mixed herbaceous vegetation; voucher ID: BI6T; BOLD ID: SETHY250-23; NHRS.; 2♀♀; Åsele lappmark, Dorotea kommun, Västra Ormsjö; N64.467878, E15.951629; 16 June 2023; E. Wahlberg leg.; on Astragalus alpinus; voucher IDs: BF4T, BH5T; BOLD IDs: SETHY223-23, SETHY242-23; NHRS.
- Description
Body (excl. extremities) wholly dark (Figure 6B). Antennal segment I brown; II mainly brown, at most yellow apically; III yellow with apical brown band; IV brown. Sensory organs on antennal segments III and IV linear and directed longitudinally. Anterior and posterior margins of pronotum without long setae. Forewings present with two dark transverse bands; apical ring vein of forewing as pale as wing membrane (Figure 6B). Male base of bifurcate claspers on tergite IX with setae longer than claspers (Figure 7B).
- Remarks
Found in various herbaceous plants, especially flowers, and presumably a facultative predator [34]. Difficult to separate from A. intermedius Bagnall, 1934, and the distribution is not clearly understood. Records from Palaearctic as well as New Zealand and Tasmania [34]. In all Fennoscandian countries, and widely distributed in Sweden, with previous records from Skåne, Halland, Öland, Gotland, Gotska Sandön, Östergötland, Västergötland, Bohuslän, Södermanland, Uppland, Västmanland, Härjedalen, and Norrbotten. Also reported here from Blekinge, Jämtland, Medelpad, Ångermanland, Åsele lappmark, Lycksele lappmark, and Pite lappmark.
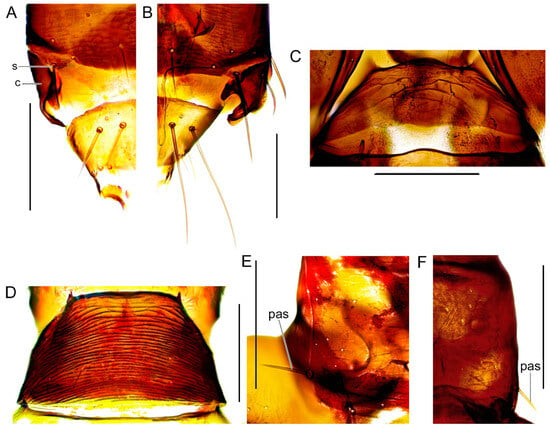
Figure 7.
Tergites IX–X, dorsal view. (A) A. intermedius, ♂. (B) A. fasciatus, ♂. Tergite I, dorsal view. (C) A. ericae, ♀. (D) A. albicinctus, ♀. Pronotum, dorsal view. (E) Rhipidothrips brunneus, ♀. (F) R. niveipennis, ♀. Scale bar: 100 µm.
| Aeolothrips gloriosus Bagnall, 1914 [35] |
| Figure 6D. |
| Aeolothrips gloriosus Bagnall, 1914: 375 [35]. |
- Material examined
SWEDEN; 2♀♀; Skåne, Sandhammaren; 17 July 1997; in Calluna; L. Vasiliu-Oromulu det.; MZLU 00109577.
- Description
Body (excl. extremities) mostly yellow, darker medially and segments VII–X, sometimes also V–VI, brown (Figure 6D). Antennal segment I yellow or pale light brown; II mainly yellow; III bicolored, apical half brown. Sensory organs on antennal segments III and IV linear and directed longitudinally. Anterior and posterior margin of pronotum without long setae. Forewings present with two dark transverse bands; apical ring vein as pale as wing membrane (Figure 6D). Abdominal segments IX without stout lateral setae.
- Remarks
Found on a variety of different flowering trees [9], but in Sweden on Calluna sp., and is probably a facultative predator. Remarkable in comparison with other species in the region with its brightly colored habitus. Palaearctic distribution, in south to northern Africa. In Fennoscandia, only known from a single locality in Sweden, Skåne [16].
| Aeolothrips intermedius Bagnall, 1934 [36] |
| Figure 3H, Figure 6A and Figure 7A. |
| Aeolothrips fasciata var. adusta Uzel, 1895: 73 [36]. |
- Material examined
SWEDEN; 4♀♀; Södermanland, Tyresta national park; 15 July–26 August 2000; Malaise trap; B. Viklund, L.-O. Wikars, H. Ahnlund leg.; NHRS.; 1♂; Värmland, Gustaf Adolfs s:n, 1 km southwest of Mossjön; 18 June 1975; on flowering Cardaminopsis arenosa; U. Qvick leg.; MZLU 00109588.; 1♀; Värmland, Gustaf Adolfs s:n, 1 km southwest of Mossjön; 18 June 1975; on flowering Rumex acetosella; U. Qvick leg.; MZLU 00109590.; 1♀; Värmland, Gustaf Adolfs s:n, 1 km southwest of Mossjön; 18 June 1975; on various spp of gramines; U. Qvick leg.; MZLU 00109586.; 1♀; Ångermanland, Nordmalings kommun, Mullsjö; N63.699004, E19.741838; 19 June 2023; E. Wahlberg leg.; on Crepis and Geranium; voucher ID: BF2T; BOLD ID: SETHY221-23; NHRS.
- Description
Body (excl. extremities) wholly dark (Figure 6A). Antennal segment I brown; II mainly brown, at most yellow apically; III yellow with apical brown band; IV brown (Figure 3H). Sensory organs on antennal segments III and IV linear and directed longitudinally (Figure 3H). Anterior and posterior margins of pronotum without long setae. Forewings present with two dark transverse bands; apical ring vein of forewing as pale as wing membrane (Figure 6A). Male base of bifurcate claspers on tergite IX with setae shorter than claspers (Figure 7A).
- Remarks
As A. fasciatus is found in various herbaceous plants, especially flowers, living as a facultative predator. The difficulties in separating A. intermedius from A. fasciatus makes a complete view of the distribution difficult, but it is presumably mainly Palaearctic [25]. In Fennoscandia it is present in Sweden, Norway, Denmark, and Finland. In Sweden it is limited to the southern half in contrast to the widely spread A. fasciatus, recorded previously in Skåne, Småland, Östergötland, Södermanland, and Värmland. The new record herein is from Ångermanland.
| Aeolothrips manteli Titschack, 1962 [37] |
| Figure 3E. |
| Aeolothrips manteli Titschack, 1962: 133 [37]. |
- Material examined
NETHERLANDS; 1♀; Bloemendaal; 8 June 1962; on Anchusa officinalis; W. P. Mantel leg.; coll. H. Priesner; SMF.
- Description
Body (excl. extremities) wholly dark. Antennal segment I brown; II mainly brown, at most yellow apically; III yellow at base, darker apically, IV brown (Figure 3E). Sensory organs on antennal segments III and IV linear and directed longitudinally, sensory organ on segment V also elongate and about half as long as segment (Figure 3E). Anterior and posterior margin of pronotum without long setae. Forewings present with two dark transverse bands; apical ring vein darker than wing membrane.
- Remarks
Found on Anchusa officinalis, originally described and recorded from the Netherlands, but was also recorded in Norway in 2001 [38]. Kobro discussed the possibility of human-mediated dispersal [38], and Gertsson raised the possibility of occurrence in Sweden due to the geographical proximity [18]. During this study several localities with Anchusa officinalis were sampled without finding A. manteli, but it is included herein as its introduction to Sweden is plausible.
| Aeolothrips melaleucus Haliday, 1852 [39] |
| Figure 5C. |
| Coleothrips melaleuca Haliday, 1852: 1117 [39]. |
- Material examined
SWEDEN; 1♀; Öland, Mörbylånga kommun, Gamla Skogsby (Kalkstad), diversitetesängen; N56.616700, E16.507617; 25 June–13 July 2003; meadow with bushes; Malaise trap; J. Liljeblad leg.; NHRS.; 1♀; Värmland, Kristinehamn, 200 m west of Västra Klintvägen; 17 August 1974; U. Qvick leg.; MZLU 00109594.; 1♀; Skåne, Svedala kommun, Hyby; N55.56882, E13.230962; 27 July–3 August 2019; Insect Biome Atlas (IBA) leg.; grassland; Malaise trap; locality ID: TBPT1B; collection event ID: SXUEFH; sample ID: 247_F12; Station Linné.; 1♀; Blekinge, Karlskrona kommun, Sandhamn; N56.090980, E15.856533; 31 May 2023; E. Wahlberg leg.; in mixed herbaceous vegetation; voucher ID: BA3T; NHRS.; 1♀; Ångermanland, Kramfors kommun, Västhammar; N63.030171, E17.773730; 19 June 2023; E. Wahlberg leg.; in mixed herbaceous vegetation; voucher ID: BA3T; BOLD ID: SETHY177-23; NHRS.
- Description
Body (excl. extremities) wholly dark. Antennal segment I brown; II mainly brown, at most yellow apically; III mainly yellow; IV yellow at base, darker apically. Sensory organs on antennal segments III and IV linear and directed longitudinally. Anterior and posterior margin of pronotum without long setae. Forewings present with two dark transverse bands, middle area between bands also dark posteriorly; apical ring vein as pale as wing membrane (Figure 5C). Male abdominal segment IX with lateral stout and sickle-shaped setae.
- Remarks
Found in various deciduous trees, mainly on leaves and flowers as a predator on e.g., mites [9,25]. Very widespread Holarctic distribution [25,40]. In Fennoscandia reported in Sweden, Norway, and Finland. In Sweden previously recorded in Skåne, Öland, Östergötland, Södermanland, Uppland, and Värmland, and herein also recorded in Blekinge and Ångermanland.
| Aeolothrips propinquus Bagnall, 1924 [41] |
| Figure 3G and Figure 5A. |
| Aeolothrips propinquus Bagnall, 1924: 269 [41]. |
- Material examined
SWEDEN; 7♀♀; Öland, Neptuni åkrar; 14 June 2022; on Echium vulgare; C.-A. Gertsson leg.; MZLU 00158394.
- Description
Body (excl. extremities) wholly dark. Antennal segment I brown; II mainly brown, at most yellow apically; III bicolored, apical half brown; IV brown (Figure 3G). Sensory organs on antennal segments III and IV linear and directed longitudinally, extending basal third and basal quarter of segment, respectively (Figure 3G). Anterior and posterior margin of pronotum without long setae. Forewings present with two dark transverse bands; apical ring vein brown (Figure 5A). Male abdominal segment IX without lateral stout setae.
- Remarks
Found in flowers of Boraginaceae and Scrophulariaceae [9,41], and has large Palaearctic distribution extending in south to northern Africa [9,25]. In Fennoscandia limited to Sweden and Denmark, and in Sweden, recorded in Skåne, Södermanland, Jämtland, Öland, and Bohuslän [16,42].
| Aeolothrips versicolor Uzel, 1895 [1] |
| Figure 1A and Figure 4A. |
| Aeolothrips versicolor Uzel, 1895: 69 [1]. |
- Material examined
AUSTRIA∙ 1♀; Oberösterreich, Linz; 30 May 1919; on Quercus robur; NHRS. SWEDEN; 1♀; Öland, Jordtorpsåsen; 2 July 2014; C. Hansson leg.; MZLU 00109491.; 1♀; Skåne; Kullaberg; 10 June 1954; on oak; L. Cederholm leg.; MZLU 00109500.; 1♀; Gotland, Slite, Filehajdar; N57.706733, E18.712835; 28 June 2023; R. Vicente leg.; rich fen; voucher ID: BR8T; BOLD ID: SETHY323-23; NHRS.; 3♀♀; Södermanland, Flens kommun, Sparreholms ekhagar; N59.086089, E16.8305657; 7 June 2023; E. Wahlberg leg.; on Tilia cordata; voucher IDs: BI3T, BI4T, BI5T; BOLD IDs: SETHY247-23, SETHY248-23, SETHY249-23; NHRS.
- Description
Body (excl. extremities) wholly dark. Antennal segment I brown; II, III and basis of IV mainly yellow. Sensory organs on antennal segments III and IV linear and directed longitudinally Anterior part of head prolonged in front of eyes (Figure 4A). Anterior and posterior margin of pronotum without long setae. Forewings present; forewings with one dark transverse band that is very broad and covering a large part of the middle portion of wing, and seldom has two dark transverse bands in Sweden; apical ring vein as pale as wing membrane. Male abdominal segment IX without lateral stout setae.
- Remarks
This species occurs in two variants, one with two dark bands on the forewings and one with one broad dark band covering almost the complete wing. The Swedish specimens often belong to the first variant, which is also the continental character [25]. Often collected from Pinus, probably a predator on small arthropods, and with a Holarctic distribution [9]. Also widespread in Fennoscandia in Sweden, Norway, Denmark, and Finland as well as within Sweden, with previous records from Skåne, Östergötland, Södermanland, Uppland, Värmland, Västerbotten, and Norrbotten. Here, a new record from Gotland is presented.
| Aeolothrips vittatus Haliday, 1836 [3] |
| Figure 3A and Figure 5B. |
| Aeolothrips (Coleothrips) vittatus Haliday, 1836: 451 [3]. |
- Material examined
SWEDEN; 1♀; Skåne, Kullaberg; 10 June 1954; on oak; L. Cederholm leg.; MZLU 00109500.; 1♀; Värmland, Ekshärads s:n, 500 E of Bergängstjärnen; 3 June 1974; beaten from young Pinus sylvestris; U. Qvick leg.; MZLU 00109501.; 1♀; Värmland, Gustaf Adolfs socken, Geijersholms skola; 2 June 1974; beaten from branches of young Pica abies; U. Qvick leg.; MZLU 00109502.; 1♀; Medelpad, Sundsvalls kommun, Björköviken, Njurunda; N62.2458168, E17.5283155; 21 June–7 July 2021; Swedish Insect Inventory Program (SIIP) leg.; sandy mixed forest; Malaise trap; locality ID: 55; collection event ID: M55-001; sample ID: 104_C6; Station Linné.; 1♀; Norrbotten, Jokkmokks kommun, Kåskats; N66.47580, E20.26901; 8 June–16 June 2019; Insect Biome Atlas (IBA) leg.; wetland; Malaise trap; locality ID: TDGVY8; collection event ID: SVLS5M; sample ID: 223_D4; Station Linné.
- Description
Body (excl. extremities) wholly dark. Antennal segment I brown; II mainly brown, at most yellow apically; III–IV mostly yellow but darker apically (Figure 3A). Sensory organs on antennal segments III and IV linear and directed longitudinally (Figure 3A). Anterior and posterior margin of pronotum without long setae. Forewings present; forewings with one dark transverse band and posterior border dark from the middle and extending almost all the way to wing apex; apical ring vein as pale as wing membrane (Figure 5B).
- Remarks
Mainly found on Pinus and probably a predator of small arthropods such as mites [9,25]. The distribution is Holarctic, in Fennoscandia in Sweden, Norway, Denmark, and Finland. The Swedish distribution includes Skåne, Östergötland, Södermanland, Uppland, Värmland, Västerbotten, and Norrbotten. Here also reported from Medelpad.
| Genus Franklinothrips Back, 1912 [43] |
| Franklinothrips vespiformis (Crawford, 1909) [44] |
| Figure 3C. |
| Aeolothrips vespiformis Crawford, 1909: 109 [44]. |
- Material examined
RÉUNION (FRANCE); 1♀; Piteon de Grande Anse; 20 Jan. 1989; in low roadside vegetation; H. G. Müller leg.; R. zur Strassen det.; SMF T 15992.
- Description
Body (excl. extremities) sharply bicolored with yellow segments. Antennal segment I–III yellow; III eight times as long as wide; IV brown (Figure 3C). Sensory organs on antennal segments III and IV as irregular blotches (Figure 3C). Head and thorax mainly dark. Anterior and posterior margin of pronotum without long setae. Forewings present with two dark transverse bands; apical ring vein brown. Abdominal segments II and III yellow.
- Remarks
A neotropical species but has been introduced in several regions. The use of the species as a biological pest control against other thrips species increases the risk of further introductions around the world. Wild populations have not been recorded in Fennoscandia. The climatic requirements of this species make unintentional introduction and reproduction unlikely at northern latitudes, but with climatic changes the risk increases. Recently F. vespiformis was approved for biological control in Sweden [45] and therefore the species is included here.
| Genus Rhipidothrips Uzel, 1895 [1] |
| Rhipidothrips brunneus Williams, 1913 [29] |
| Figure 3B, Figure 4C and Figure 7E. |
| Rhipidothrips brunneus Williams, 1913: 216 [29]. |
- Material examined
ITALY; 1♀; Catania, 6 km N of Granieri; 10 May 1984; on fresh green grass; A. Vesmanis leg.; R. zur Strassen det.; SMF T 12861. EGYPT; 1♀; Rhipidothrips cahirensis, PARATYPE; pyramids at canal bank; 27 Mar. 1930; H. Priesner leg.; SMF T 19326.; 11♀♀; Blekinge, Karlskrona kommun, Knösö; N56.167848, E15.664502; 18 May 2023; E. Wahlberg leg.; on Poaceae; voucher IDs: BI2T, BI9T, BJ1T, BJ2T, BJ3T, BJ5T, BJ6T, BJ7T, BJ8T, BJ9T, BK1T; BOLD IDs: SETHY246-23, SETHY252-23, SETHY253-23, SETHY354-23, SETHY255-23, SETHY256-23, SETHY257-23, SETHY258-23, SETHY259-23, SETHY260-23, SETHY261-23; NHRS.; 3♀♀; Blekinge, Karlskrona kommun, Sandhamn; N56.090980, E15.856533; 31 May 2023; E. Wahlberg leg.; in mixed herbaceous vegetation; voucher IDs: BL4T, BL5T, BT9T; BOLD IDs: SETHY273-23, SETHY274-23, SETHY325-23; NHRS.
- Description
Body (excl. extremities) wholly dark. Antennal segment I–II brown; III–IV mainly yellow (Figure 3B). Sensory organs on antennal segments III and IV lens-shaped (Figure 3B). Interocellar setae prominent and long (at least 20 μm) and lateral setae posterior to eyes strong (Figure 4C). Anterior margin of pronotum with one pair of stout but short angular bristles; posterior margin with two to three pairs of stout but short setae; posteroangular setae strong and dark (Figure 7E). Forewings sometimes present but often brachypterous, pale; apical ring vein pale as wing membrane.
- Remarks
Mainly found on grasses and has a widespread Palaearctic distribution [24,25] in south to Egypt and Iran. Also found in North America and Australia, and possibly introduced there [46]. In Fennoscandia recorded in Finland and Sweden; in Sweden, found in Blekinge in 2022 [31].
| Rhipidothrips niveipennis Reuter, 1899 [28] |
| Figure 3I, Figure 4D, Figure 6F and Figure 7F. |
| Rhipidothrips niveipennis Reuter, 1899: 30 [28]. |
- Material examined
FINLAND; 1♀; SYNTYPE (macropterous); Pargas, Lofsdal, garden; O. M. Reuter leg.; on Abies; MZF GV.33091; Spec. typ. No. 6058.; 1♀; SYNTYPE (brachypterous); Pargas, Lenholmen; on Covallaria; O. M. Reuter leg.; MZF GV.33092; Spec. typ. No. 6059.; 1♀; SYNTYPE (macropterous); Helsingin maalaiskunta, Vantaa; B. Wasastjärna leg.; MZF GX.6325; Spec. typ. No. 6060. SWEDEN; 1♀; Uppland, Stockholm, Experimentalfältet; 19 May 1919; on Phleum; O. Ahlberg leg.; MZLU 00109507.; 4♀; Uppland, Stockholm, Experimentalfältet; 19 May 1919; on Phleum; O. Ahlberg leg.; NHRS.
- Diagnosis
Similar to R. brunneus with body dark brown with the tips of the tibia and whole tarsi yellow, brown antennal segments I, II, VI–IX, segments III and IV yellow (Figure 3I, 6F). Antennal segment V yellow to bright brown (Figure 3I) in contrast to the darker R. brunneus (Figure 3B). Head striated, with stronger striations posteriorly (Figure 4D), but not as heavy as in R. brunneus (Figure 4C). Laterodorsal bristles behind eyes present, but weak and bright (Figure 4D). Interocellar bristles present, at most 20 μm long (Figure 4D). Posteroangular bristle present on pronotum, stout but pale in colour (Figure 7F). Most often macropterous (Figure 6F), and brachypterous specimens are rare.
- Redescription based on syntypes
Body (excl. extremities) wholly dark (Figure 6F). Antennal segment I–II brown; III–IV mainly yellow, V yellow to bright brown (Figure 3I). Sensory organs on antennal segments III and IV lens-shaped (Figure 3I). Head transversely striated with stronger striations posteriorly, forming a weakly defined collar, lateral sides of the head straight (Figure 4D). Eyes elongate and extend posteriorly on the ventral side. Dorsal setae on head all weak except for the interocellar setae that are stout but short, lateral setae posterior to eyes weak (Figure 4D). Pronotum transversely striated, anterior margin with one pair of stout but short angular bristles; posterior margin with two to three pairs of stout but short setae; posteroangular setae present, stout and yellow (Figure 7F). Forewings often present, but sometimes brachypterous, wings very pale to white; apical ring vein pale as wing membrane (Figure 6F). Mesonotum transversely striated, metanotum longitudinally striated. Abdominal tergites transversely striated; I with 1 pair, II–VII with three pairs of weak median bristles; one to two pairs of lateral bristles on II–VII; sternites II–VII similarly striated but with three pairs of weak setae posteriomarginally. Abdominal segment IX striated with two pairs of strong posteromarginal dorsal bristles; the lateral pair long and median short; three pairs of lateral strong setae, the posterior pair shorter. Terminal segment X with three pairs of strong bristles situated slightly posterior to medially, with the lateral setae the longest.
- Remarks
The characteristics separating this species from other species in Rhipidothrips was, according to Reuter, the lack of pronotal posteroangular setae [28], but these are present on the type specimens but are yellow. This was also noted by Bailey, who added the interocellar setae as a defining character separating R. niveipennis and R. brunneus [24]. In R. niveipennis these are weak and short. Rarely collected, described from Finland but recorded in Norway, Sweden, and France. Type material collected on Convallaria majalis, but also found on grasses and different flowering plants [24]. The only Swedish record is from Uppland.
| Family Melanthripidae Bagnall, 1913 [2] |
The Melanthripidae family are, like the Aeolothripidae, characterized by having nine antennal segments but which are never fused terminally and the sensorial organs on the antennae are elongate and transverse (Figure 3D). Melanthrips fuscus, the only species in Sweden, is macropterous as are all European species. Characterized by having long bristles on the head and pronotum, as well as strong bristles on wings. Biology little studied, but probably feeds on plant tissue.
| Genus Melanthrips Haliday, 1836 [3] |
| Melanthrips fuscus (Sulzer, 1776) [47] |
| Figure 3D. |
| Thrips fuscus Sulzer, 1776: 113 [47]. |
- Material examined
SWEDEN; 1♀; Uppland, Rydbo; 30 June 1919; on Triticum aestivum; O. Ahlberg, leg.; MZLU 00109506.; 2♀♀; Skåne, Alnarp; May 1899; on Euphorbia palustris; F. Trybom leg; NHRS.
- Description
Body (excl. extremities) wholly dark. Antennal segments all more or less brown (Figure 3D). Sensory organs on antennal segments III and IV linear and directed transversely (Figure 3D). Anterior margin of pronotum with two pairs and posterior with at least four pairs of well-developed and long bristles; 1 pair of well-developed and strong posteroangular bristles. Forewings present; mostly shaded, paler at base, never with pale round patterns; apical ring vein of brown.
- Remarks
Found mainly in different species of Brassicaceae [6], but not exclusively, and is Palearctic in its distribution with records in south towards northern Africa. In Fennoscandia recorded in Sweden and Denmark, and in Sweden in Skåne, Östergötland, Södermanland, and Uppland.
4. Discussion and Conclusions
The substantial number of new records is not surprising, as the thrips fauna of Sweden has not been subject to extensive targeted sampling since Quick exerted substantial efforts [48]. His study, however, was mainly centered around Värmland. Ahlberg provided the first catalogue of thrips in Sweden [49], and also provided additional data from his sampling focused on Uppland and Södermanland. Gertsson’s updated list of Swedish thrips in general was a substantial addition to updating the knowledge [16], but mainly focused on material in collections and additional material collected in southern Sweden and Öland. During this study, a focus was placed on the northern provinces, previously a blank spot. Most new records are from these provinces, proving that this area has been previously neglected. However, some recent dispersal cannot be disregarded. Many finds are from flowering plants close to roads, human habitation, and agricultural areas. As many thrips species are connected to their habitats and substrate species, the dispersal of plant material can also be a vector for thrips’ dispersal.
In the northern parts of Sweden, the species richness is in general low, with the few species present commonly found on Fabaceae and a few other plants commonly found at higher latitudes. Species dependent on deciduous forest tree species as well as on more southern herbaceous flora are absent, reflecting the less diverse habitats. One common species is Aeolothrips ericae, often found on Ericaceae and Fabaceae. Another widely spread species is A. fasciatus, a generalist regarding substrate choice. One notable find is that this species is the most common species compared to its close relative A. intermedius, as which it is often misidentified. Due to the difficulties in separating the females of the two species, and females being much more commonly occurring, the distribution of the two species is unclear. Both during the collection in this study and when examining material in collections, it is clear that A. fasciatus is the dominating species in Sweden, while A. intermedius is much rarer. The females in this study were double checked for identification errors with COI barcode data, including both males and females of both species, and the separation of these based on the characteristics in the key was confirmed at least for Swedish populations. Another observation is that the wing colour form of A. versicolor consists of one large broad dark band. This form is most similar to continental populations rather than, for example, populations in Great Britain [25].
The above notes and the overlapping species of the genus Rhipidothrips highlight the necessity of regionally relevant checklists and identification keys, as well as updated literature. Genetic data such as barcode sequences further provide data for the studies of the regional fauna specifically, but also taxonomic issues in general. Genetic barcode data also provide much needed taxonomic references for use in modern metabarcoding studies. There are still blank spots on the map of Sweden and Fennoscandia, and this study proves that there are still more discoveries and records to be made.
Despite efforts to find A. manteli, this species has not been found in Sweden, even though it does occur in Norway. However, as Kobro states, the species could be transported by anthropogenic means [38], and its future possible establishment warrants its inclusion here. This is also the case with Franklinothrips vespiformis, recently approved as a biological control agent in Sweden [45]. With a changing climate with warmer seasons and longer summers, more exotic species can also become established, particularly with the help from human activity.
Funding
This research was funded by the Swedish Taxonomy Initiative (SLU Artdatabanken, Sweden), grant number SLU.dha.2022.4.3-206.
Institutional Review Board Statement
Not applicable.
Data Availability Statement
Molecular barcodes have been submitted to BOLD database (https://dx.doi.org/10.5883/DS-SETHY23). All metadata and collection data were also reported to the Swedish Species Observation System (SLU Artdatabanken, Sweden), which in turn shares the data with the Global Biodiversity Information Facility (GBIF).
Acknowledgments
Heidi Viljanen at the Finnish Natural History Museum, Helsinki, Finland, for help with Reuter’s specimens of R. niveipennis, Laura Marrero Palma at the Senckenberg Research Institute and Natural History Museum, Senckenberg, Germany, for specimens of R. brunneus and several other species. Terrence Bellingan, Department of Entomology and Arachnology Albany Museum, Albany, South Africa, and Robert Douglas, Oxford University Museum of Natural History. Oxford, Great Britain, for their efforts in trying to locate type specimens of R. brunneus. Ellen Sandström and Christoffer Fägerström at the entomological collections at the Biological Museum in Lund, Sweden, for their hospitality and assistance. Mårten Klinth and Dave Karlsson at Station Linné, Öland, Sweden, for lending the specimens from the Swedish Insect Inventory Program (SIIP) and the Insect Biome Atlas (IBA). Carl-Axel Gertsson, Lund, Sweden, has provided invaluable inspiration and knowledge.
Conflicts of Interest
The author declares no conflicts of interest.
References
- Uzel, H. Monographie der Ordnung Thysanoptera; Kvart: Hradec Králové, Czech Republic, 1895. [Google Scholar]
- Bagnall, R.S. Brief Descriptions of New Thysanoptera. Ann. Mag. Nat. Hist. 1913, 8, 290–299. [Google Scholar] [CrossRef]
- Haliday, A.H. An Epitome of the British Genera in the Order Thysanoptera. Entomol. Mag. 1836, 3, 439–451. [Google Scholar]
- Kirk, W.D.J. Thrips, Naturalists’ Handbooks 25; The Richmond Publishing Co., Ltd.: Slough, UK, 1996. [Google Scholar]
- Mound, L.A.; Tree, D.J. Thysanoptera Australiensis—Thrips of Australia; Lucidcentral.org, Identic Pty Ltd.: Queensland, Australia, 2020. [Google Scholar]
- Paine, T.D. Cuban Laurel Thrips (Thysanoptera: Phlaeothripidae) Biology in Southern California: Seasonal Abundance, Temper-Ature Dependent Development, Leaf Suitability, and Predation. Ann. Entomol. Soc. Am. 1992, 85, 164–172. [Google Scholar] [CrossRef]
- Bagnall, R.S. Preliminary Notes and Descriptions of Some European Species of Aeolothrips. Ntomologist’s Mon. Mag. 1920, 56, 60–62. [Google Scholar] [CrossRef]
- Mound, L.A.; Morison, G.D.; Pitikin, B.R.; Palmer, J.M.T. Handbooks for the Identification of British Insects; Royal Entomological Society: London, UK, 1976; Part 1; Volume I. [Google Scholar]
- zur Strassen, R. Die Terebranten Thysanopteren Europas und des Mittelmeer-Gebietes; Goecke & Evers: Keltern, Germany, 2003. [Google Scholar]
- Kucharczyk, H.; Zawirska, I. Study on the Thrips Fauna (Insecta: Thysanoptera) on Xerothermic Grassland of South-East Poland. Sonderdr. Aus CFS-Cour. 1994, 178, 3–7. [Google Scholar]
- Kakol, E.; Kucharczyk, H. Occurrence of Thrips (Thysanoptera, Insecta) on Winter and Spring Wheat in the Chosen Regions of Poland. Acta Phytopathol. Entomol. Hung. 2003, 39, 263–269. [Google Scholar] [CrossRef]
- Kucharczyk, H. Thrips (Insecta: Thysanoptera) as an Element of Ecological Monitoring in Białowieża Primeval Forest. Śne Pract. Badaw. 2004, 3, 85–94. [Google Scholar]
- Kucharczyk, H.; Kucharczyk, M. The Red List of Threatened Thrips Species of Middle-Eastern Poland. Acta Phytopathol. Entomol. Hung. 2008, 43, 297–305. [Google Scholar] [CrossRef]
- Dubovský, M.; Fedor, P.; Kucharczyk, H.; Masarovič, R.; Balkovič, J. Assemblages of Bark-Dwelling Thrips (Thysanoptera) of Uneven-Aged Oak Forests in Slovakia. Sylwan 2010, 154, 659–668. [Google Scholar]
- Kobro, S. Norske Insekttabeller 19. In Trips (Thysanoptera); Norsk Entomologisk Förening: Oslo, Norway, 2013. [Google Scholar]
- Gertsson, C.-A. An Annotated Checklist of Thysanoptera (Thrips) from the Nordic Countries. Entomol. Tidskr. 2015, 136, 185–198. [Google Scholar]
- Gertsson, C.-A. Två för Sverige nya tripsarter: Haplothrips alpicola Priesner, 1950 och Haplothrips utae Klimt, 1970 (Thysanoptera) samt nya provinsfynd. Entomol. Tidskr. 2021, 142, 21–30. [Google Scholar]
- Gertsson, C.-A.; Fägerström, C.; Sjödahl, M. Två för Skandinavien nya tripsarter (Thysanoptera): Hoplothrips caespitis (Uzel, 1895) och Megalothrips bonannii Uzel, 1895 samt nya provinsfynd. Entomol. Tidskr. 2022, 143, 17–24. [Google Scholar]
- Ronquist, F.; Forshage, M.; Häggqvist, S.; Karlsson, D.; Hovmöller, R.; Bergsten, J.; Holston, K.; Britton, T.; Abenius, J.; An-dersson, B.; et al. Completing Linnaeus’s Inventory of the Swedish Insect Fauna: Only 5000 Species Left? PLoS ONE 2020, 15, 0228561. [Google Scholar] [CrossRef] [PubMed]
- Stephens, J.F. A Systematic Catalogue of British Insects; Bladwin and Cradock: London, UK, 1829. [Google Scholar]
- Wahlberg, E. A Modern Workflow for Non-Destructive DNA Extraction and Slide Preparation of Thrips (Insecta, Thysanoptera) for Taxonomic Studies and Collection Deposition. Nor. J. Entomol. 2023, 70, 1–5. [Google Scholar]
- Folmer, O.; Hoeh, W.R.; Black, M.B.; Vrijenhoek, R.C. Conserved Primers for PCR Amplification of Mitochondrial DNA from Different Invertebrate Phyla. Mol. Mar. Biol. Biotechnol. 1994, 3, 294–299. [Google Scholar] [PubMed]
- Ratnasingham, S.; Hebert, P.D. BOLD: The Barcode of Life Data System (http://www.barcodinglife.org). Mol. Ecol. Notes 2007, 7, 355–364. [Google Scholar] [CrossRef]
- Bailey, S.F. A Review of the Genus Rhipidothrips Uzel (Thysanoptera: Aeolothripidae). Pan-Pac. Entomol. 1954, 30, 209–220. [Google Scholar]
- Mound, L.A.; Collins, D.W.; Hastings, A.T.B. Thrips of the British Isles; Lucidcentral.org, Identic Pty Ltd.: Queensland, Australia, 2018. [Google Scholar]
- Schliephake, G.; Klimt, K.T. Fransenflügler Die Tierwelt Deutschlands; Gustav Fisher Verlag: Jena, Germany, 1979. [Google Scholar]
- Moritz, T.G. Thripse—Fransenflügler Pflanzensaftsaugende Insekten Bd. 1; Westarp Wissenschaften: Hohenwarsleben, Germany, 2006. [Google Scholar]
- Reuter, O.M. Thysanoptera fennica: Förteckning och beskrifning öfver finska Thysanoptera. Soc. Fauna Flora Fenn. 1899, 17, 1–69. [Google Scholar]
- Williams, C.B. Records and Descriptions of British Thysanoptera. J. Econ. Biol. 1913, 8, 216–230. [Google Scholar]
- Speyer, E.R.; Parr, W.J. Studies upon Thysanoptera. Nurs. Mark. Gard. Ind. Dev. Soc. Annu. Rep. 1950, 36, 35–37. [Google Scholar]
- Wahlberg, E. Rhipidothrips brunneus (Williams, 1913) (Thysanoptera: Aeolothripidae) ny art för Sverige, samt ett nytt landskapsfynd av Thrips menyanthidis Bagnall, 1923 (Thripidae). Entomol. Tidskr. 2023, 144, 47–51. [Google Scholar]
- Priesner, H. Die Thysanopteren Europas, Abteilung I–II; F. Wagner Verlag: Vienna, Austria, 1926. [Google Scholar]
- Linnaeus, C. Systema Naturae; Laurentii Salvii: Stockholm, Sweden, 1758. [Google Scholar]
- Mound, L.A.; Nielsen, M.; Hastings, A.T.A. Thrips of New Zealand; Lucidcentral.org, Identic Pty Ltd.: Queensland, Australia, 2017. [Google Scholar]
- Bagnall, R.S. Brief Descriptions of New Thysanoptera. Ann. Mag. Nat. Hist. 1914, 73, 22–31. [Google Scholar] [CrossRef]
- Bagnall, R.S. Contributions towards a Knowledge of the European Thysanoptera. Ann. Mag. Nat. Hist. 1934, 83, 481–500. [Google Scholar] [CrossRef]
- Titschack, E. Faunistische Mitteilungen Aus Nordwestdeutschland. Bombus 1962, 2, 133–140. [Google Scholar]
- Kobro, S. The First Record of a Male of Aeolothrips manteli Titschack (Thysanoptera) and Some Characters of the Norwegian Species of the Genus. Nor. J. Entomol. 2005, 52, 65–68. [Google Scholar]
- Haliday, A.H. Order III Physapoda. In List of the Homopterous Insects in the British Museum Part IV; British Museum: London, UK, 1852; pp. 1094–1118. [Google Scholar]
- Stannard, L.J. The Thrips, or Thysanoptera of Illinois. Bull. Ill. Nat. Hist. Surv. 1968, 29, 213–552. [Google Scholar] [CrossRef]
- Bagnall, R.S. New and Rare British Thysanoptera. Entomol. Mon. Mag. 1924, 60, 269–275. [Google Scholar]
- Gertsson, C.-A. Tripsen Aelothrips propinquus (Bagnall, 1924) (Thysanoptera) ny för Öland och Bohuslän. Lucanus 2022, 27, 49–51. [Google Scholar]
- Back, E.A. Notes on Florida Thysanoptera, with Description of a New Genus. Entomol. News 1912, 23, 73–77. [Google Scholar]
- Crawford, D.L. Some Thysanoptera of Mexico and the South, I. Pomona Coll. J. Entomol. 1909, 1, 109–119. [Google Scholar]
- Swedish Environmental Protection Agency. Beslut om Godkännande av Franklinothrips Vespiformis för Användning som Biologiskt Bekämpningsmedel i Sverige; Naturvårdsverket: Stockholm, Sweden, 2023; p. 07608-17. [Google Scholar]
- Hoddle, M.S.; Mound, L.A.; Paris, D. Thrips of California; CBIT Publishing: Queensland, Australia, 2009. [Google Scholar]
- Sulzer, J.H. Abgekürzte Geschichte Der Insecten, Nach Dem Linnaeischen System; Steiner: Winterthur, Germany, 1776. [Google Scholar]
- Qvick, U. New Records and Notes on the Swedish Thrips Fauna Thysanoptera. Entomol. Tidskr. 1977, 98, 127–131. [Google Scholar]
- Ahlberg, O. Svensk Lnsektfauna 6. Tripsar. Thysanoptera; Entomologiska Föreningen i Stockholm: Stockholm, Sweden, 1926. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).