Abstract
Linear atrophoderma of Moulin (LAM) is a rare, benign dermatosis characterized by unilateral, hyperpigmented, slightly atrophic plaques distributed along Blaschko’s lines. We report the case of an 18-year-old woman with a four-year history of asymptomatic lesions on the right abdomen, thigh, and foot. Histopathology revealed basal layer hyperpigmentation, mild collagen thickening, and a sparse perivascular lymphocytic infiltrate, without sclerosis. The clinical and histological findings confirmed the diagnosis of LAM. Given the stable course and absence of symptoms, a conservative approach with follow-up was adopted. This case underscores the importance of recognizing LAM’s distinctive presentation to avoid unnecessary treatments.
1. Introduction
Linear atrophoderma of Moulin (LAM) is an uncommon, acquired dermatosis initially recognized by Moulin et al. in 1992 [1]. It manifests as unilateral, hyperpigmented, slightly atrophic plaques distributed along Blaschko’s lines, typically appearing in childhood or adolescence. Lesions progress over several months before stabilizing, with no preceding inflammation or subsequent sclerosis [2,3].
The pathogenesis remains unclear, though somatic mosaicism from a postzygotic mutation is the most widely accepted hypothesis [4]. An immune-mediated mechanism has also been suggested, supported by reports describing histological features consistent with connective tissue disease in some patients with LAM [5]. Clinical variants include forms with a preceding inflammatory phase [6], telangiectatic erythema [7], or atypical, bilateral, or multiple band-like blaschkoid patterns [8].
Given its rarity and clinical overlap with other linear dermatoses, recognition of its distinctive blaschkoid distribution is essential. We present the case of an adolescent showing the characteristic clinical and histological features of LAM, aiming to highlight diagnostic considerations and management approach.
2. Case Report
An otherwise healthy 18-year-old female presented with a four-year history of asymptomatic cutaneous lesions on the right side of her body. The first lesion appeared as a small, well-defined, brownish macule on the right abdomen, which gradually enlarged and became slightly depressed. Over time, additional similar lesions developed on the right posterior thigh and the dorsum of the right foot. No preceding erythema, induration, or inflammatory symptoms were reported. The patient denied trauma, preceding skin disease, systemic complaints, or constitutional symptoms.
2.1. Dermatological Examination
On clinical examination, multiple, well-demarcated, hyperpigmented plaques were observed in a linear arrangement strictly following Blaschko’s lines. On the right flank and abdomen, the plaques showed a slightly atrophic, smooth surface (Figure 1). On the posterior aspect of the right thigh, similar lesions extended longitudinally in a blaschkoid pattern (Figure 2). The surrounding skin appeared normal, without signs of sclerosis, induration, or active inflammation. Hair, nails, and mucous membranes were spared. Neurological and musculoskeletal examinations revealed no abnormalities.
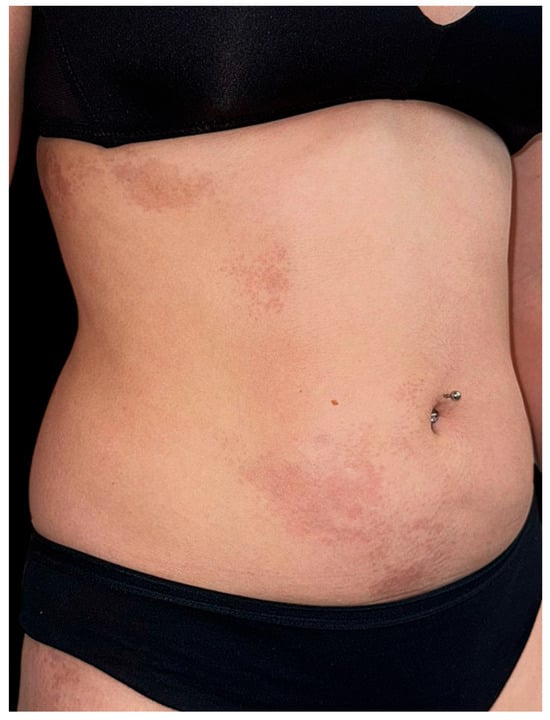
Figure 1.
Hyperpigmented, well-demarcated atrophic plaques distributed linearly along Blaschko’s lines on the right flank and abdomen.
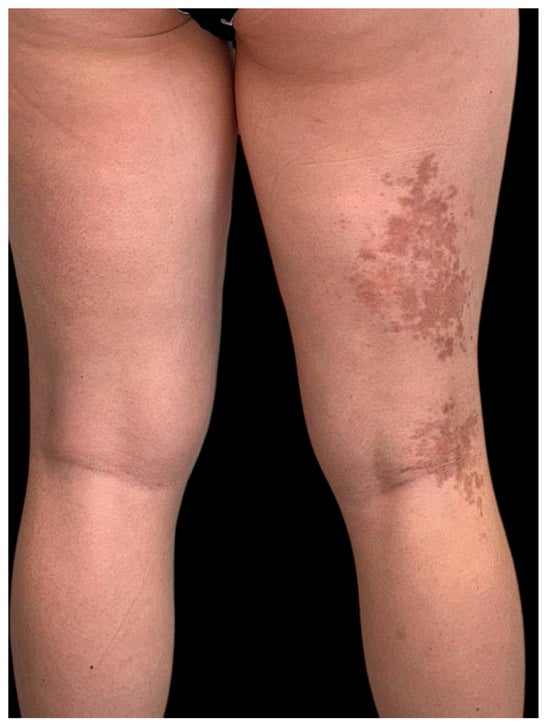
Figure 2.
Similar atrophic hyperpigmented plaques following a blaschkoid pattern on the posterior region of the right thigh.
2.2. Complementary Tests
Routine laboratory tests were within normal limits. A 4 mm punch biopsy taken from the abdominal lesion was formalin-fixed, paraffin-embedded, and stained with H&E; no immunohistochemical studies were performed. It showed basal layer hyperpigmentation, mild epidermal ridge flattening, and slightly thickened collagen bundles in the superficial dermis, with preserved elastic fibers. A sparse superficial perivascular lymphocytic infiltrate was present, without evidence of dermal sclerosis or significant inflammatory activity. These findings were consistent with the diagnosis of LAM.
2.3. Management and Evolution
Given the absence of symptoms and the limited extent of the lesions, no systemic treatment was initiated. The patient was counselled on the benign and non-progressive nature of the condition. At 12 months of follow-up, lesions remained clinically stable without new plaques, induration, or functional impact.
3. Discussion
LAM is an uncommon dermatological condition characterized by the sudden onset of unilateral, hyperpigmented, atrophic plaques that follow Blaschko’s lines, typically arising during childhood or adolescence and stabilizing without spontaneous remission. The absence of preceding inflammation or subsequent sclerosis distinguishes it from other linear dermatoses [1,2,3].
Beyond the classic presentation, rarer variants have been described, including an inflammatory-onset form that begins with erythematous or bruise-like plaques and evolves into hyperpigmented, slightly atrophic bands [6]; a telangiectatic variant marked by persistent telangiectatic erythema within blaschkoid bands with little or no induration [7]; and atypical distributions characterized by bilateral or symmetric involvement [9], multiple parallel bands [10], cervical or facial localization [8,11], and occasional adult-onset courses [12], sometimes accompanied by pigmentary associations such as lentiginosis [13].
The etiology remains incompletely understood. The most widely accepted hypothesis involves somatic mosaicism affecting keratinocytes or fibroblasts. In this model, an early postzygotic mutation generates a genetically distinct keratinocyte or fibroblast clone that expands along embryonic cell migration lines, producing the strictly blaschkoid, unilateral distribution and midline respect; the earlier the mutational event, the broader the segment involved. Because the mutation is confined to somatic tissue (not the germline), familial recurrence is not expected [4]. An autoimmune mechanism has also been suggested, supported by reports of positive autoantibodies in some patients. Specifically, isolated cases have documented systemic autoimmunity with positive antinuclear antibodies (ANA) and anti-ribonucleoprotein (RNP), and immunophenotypic shifts (low IgM and a reduced CD4/CD8 ratio), together with histologic features that overlap with connective-tissue disease and support a localized immune-driven matrix-remodeling process without frank sclerosis [5]. In our case, ANA testing was negative, and there were no systemic symptoms or laboratory findings suggestive of autoimmune disease, underscoring that any immune contribution is likely heterogeneous and confined to a subset of patients.
Histopathologically, LAM typically demonstrates hyperpigmentation of the basal layer, mild thickening or preservation of dermal collagen bundles, and a scant perivascular lymphocytic infiltrate, with preservation of elastic fibers [3,7]. Dermal atrophy is not usually observed; the apparent clinical depression is thought to result from localized loss of subcutaneous tissue [14]. Our patient’s biopsy findings matched this description, supporting the diagnosis.
The principal differential diagnoses are idiopathic atrophoderma of Pasini and Pierini (APP) and linear morphea. APP typically presents in teenagers or young adults with bilateral, often symmetric, slate-gray depressed patches on the lower back and trunk that have an abrupt “step-off” edge but never follow Blaschko’s lines; histology in early lesions shows broadened collagen bundles with only a scant, chronic perivascular infiltrate, whereas older plaques evolve to tightly packed, sometimes hyalinized collagen without true sclerosis of deeper tissues and with little epidermal change beyond mild basal hyperpigmentation. By contrast, morphea shows firm, ivory-white sclerosis (often with a violaceous rim when active), can involve virtually any site, and on histology transitions from an inflammatory border (thickened/edematous collagen, a moderate lymphocytic infiltrate, endothelial swelling and perivascular edema) to late-stage dense, homogenized dermal collagen with adnexal atrophy and replacement of subcutaneous fat by sclerotic collagen; vessel wall fibrosis/narrowing and even fascial involvement may occur in advanced disease. LAM is distinguished from both by its strictly blaschkoid, usually unilateral, hyperpigmented band-like plaques with minimal or no induration and a course that stabilizes after a brief spread [2,4,15,16]. Table 1 summarizes the main differences between these three entities. Other Blaschko-linear dermatoses, such as incontinentia pigmenti, focal dermal hypoplasia, lichen striatus, and epidermal nevi, can generally be ruled out through careful clinicopathologic correlation [2].

Table 1.
Comparative clinical and histopathological characteristics of LAM, APP, and morphea.
Although LAM is benign and self-limiting in progression, its cosmetic impact can be significant, especially when affecting visible areas. Multiple therapeutic approaches have been attempted with variable results. In practice, the absence of a proven standard of care and the typically stable natural history make conservative observation appropriate for asymptomatic, cosmetically acceptable disease, while treatment is individualized to activity, extent, and patient preference. Topical corticosteroids (with or without tacrolimus) have not shown consistent benefit, whereas topical calcipotriol has achieved partial improvement in single-case reports, particularly when used early [3]; hydroquinone and topical retinoids have generally failed to meaningfully change established plaques. Among systemic options, methotrexate has produced clinical improvement in pigmentation and shallow atrophy in a published case, supporting the idea that immunomodulation may help selected, more extensive presentations [17], while colchicine combined with calcipotriol and vitamin E yielded partial repigmentation in a recent report [8]. Historical therapies such as potassium para-aminobenzoate (Potaba), high-dose penicillin, and heparin are inconsistently helpful—one older case suggested stabilization with Potaba, but later summaries judge these options broadly ineffective [3,16,18]. As a procedural option, intralesional platelet-rich plasma (PRP) has been reported to lessen plaque depression without meaningful improvement in pigmentation or surface texture, and may be considered only for cosmetic contouring in selected patients [10]. Overall, management is best framed as: observation and counselling for stable disease; a trial of topical calcipotriol for early or active lesions; PRP for cosmetic contour only; and systemic methotrexate as an individualized option for extensive or progressive disease, recognizing that current evidence rests on single cases and small series.
Raising awareness of LAM’s distinct clinical pattern is essential to prevent misdiagnosis and avoid unnecessary or ineffective treatments. Future prospective studies and case series may help clarify its pathogenesis, define its nosological position relative to APP and morphea, and evaluate potential targeted therapies.
4. Conclusions
LAM is an uncommon, benign dermatosis with a characteristic blaschkoid distribution and distinctive histopathological features. Recognition of its stable, non-progressive course is crucial to differentiate it from other linear dermatoses, such as APP or linear morphea, thereby avoiding unnecessary investigations or treatments. Although several therapeutic options have been reported with variable outcomes, there is no established standard of care, as the available evidence remains limited to isolated case reports. Our case reinforces the importance of accurate diagnosis, appropriate patient counselling, and a conservative approach in asymptomatic, stable presentations. Further studies are needed to elucidate its pathogenesis and to evaluate the efficacy of emerging therapeutic strategies.
Author Contributions
Conceptualization, L.A.-M.d.S. and E.B.-R.; methodology, L.A.-M.d.S. and E.B.-R.; validation, L.A.-M.d.S. and E.B.-R.; formal analysis, L.A.-M.d.S. and E.B.-R.; investigation, L.A.-M.d.S.; resources, L.A.-M.d.S. and E.B.-R.; data curation, E.B.-R.; writing—original draft preparation, L.A.-M.d.S.; writing—review and editing, L.A.-M.d.S. and E.B.-R.; visualization, L.A.-M.d.S. and E.B.-R.; supervision, L.A.-M.d.S. and E.B.-R.; project administration, L.A.-M.d.S. and E.B.-R. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Ethical review and approval were waived for this study, due to this case report involved a single patient and did not constitute a study requiring ethics committee or IRB approval.
Informed Consent Statement
Written informed consent was obtained from the patient.
Data Availability Statement
The original contributions presented in this study are included in the article. Further inquiries can be directed to the corresponding author.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| LAM | Linear atrophoderma of Moulin |
| APP | Atrophoderma of Pasini and Pierini |
References
- Moulin, G.; Hill, M.P.; Guillaud, V.; Barrut, D.; Chevallier, J.; Thomas, L. Bandes pigmentées atrophiques acquises suivant les lignes de Blaschko [Acquired atrophic pigmented band-like lesions following Blaschko’s lines]. Ann. Dermatol. Venereol. 1992, 119, 729–736. [Google Scholar] [PubMed]
- Wollenberg, A.; Baumann, L.; Plewig, G. Linear atrophoderma of Moulin: A disease which follows Blaschko’s lines. Br. J. Dermatol. 1996, 135, 277–279. [Google Scholar] [CrossRef] [PubMed]
- Wongkietkachorn, K.; Intarasupht, J.; Srisuttiyakorn, C.; Aunhachoke, K.; Nakakes, A.; Niumpradit, N. Linear atrophoderma of moulin: A case report and review of the literature. Case Rep. Dermatol. 2013, 5, 11–14. [Google Scholar] [CrossRef] [PubMed]
- Danarti, R.; Bittar, M.; Happle, R.; König, A. Linear atrophoderma of Moulin: Postulation of mosaicism for a predisposing gene. J. Am. Acad. Dermatol. 2003, 49, 492–498. [Google Scholar] [CrossRef] [PubMed]
- Yan, W.; Wang, S.; Liu, H.; Wang, L.; Li, W.; Ran, Y.; Zhang, M. Linear atrophoderma of Moulin: A disease related to immunity or a kind of connective tissue disease? Australas. J. Dermatol. 2017, 58, e126–e128. [Google Scholar] [CrossRef] [PubMed]
- Browne, C.; Fisher, B.K. Atrophoderma of Moulin with preceding inflammation. Int. J. Dermatol. 2000, 39, 850–852. [Google Scholar] [CrossRef] [PubMed]
- Utikal, J.; Keil, D.; Klemke, C.-D.; Bayerl, C.; Goerdt, S. Predominant telangiectatic erythema in linear atrophoderma of Moulin: Novel variant or separate entity? Dermatology 2003, 207, 310–315. [Google Scholar] [CrossRef] [PubMed]
- Al-Janabi, M.H.; Diab, S.; Aljammal, G.; Kassab, L.; Al-Shehabi, Z.; Al-Soiufi, L. Linear atrophoderma of Moulin: A rare case report and review of the literature. Ski. Heal. Dis. 2024, 4, ski2.424. [Google Scholar] [CrossRef] [PubMed]
- Lis-Świety, A.; Bierzyńska-Macyszyn, G.; Arasiewicz, H.; Brzezińska-Wcisło, L. Bilateral atrophoderma linearis: A relationship between atrophoderma linearis Moulin and atrophoderma Pasini–Pierini? Int. J. Dermatol. 2016, 55, 339–341. [Google Scholar] [CrossRef] [PubMed]
- Kharkar, V.D.; Abak, B.A.; Mahajan, S.A. Linear atrophoderma of Moulin: A rare entity. Indian J. Dermatol. Venereol. Leprol. 2018, 84, 591–594. [Google Scholar] [CrossRef] [PubMed]
- Darung, I.; Rudra, O.; Samanta, A.; Agarwal, M.; Ghosh, A. Linear Atrophoderma of Moulin over Face: An Exceedingly Rare Entity. Indian J. Dermatol. 2017, 62, 214–216. [Google Scholar] [CrossRef] [PubMed]
- Ahearn, I.M.; Gittler, J.; Shvartsbeyn, M.; Meehan, S.A.; Pomeranz, M.K. Linear atrophoderma of Moulin. Dermatol. Online J. 2015, 21, 13030. [Google Scholar] [CrossRef]
- Yücel, S.; Özcan, D.; Seçkin, D. Lentiginosis within plaques of hypopigmented linear atrophoderma of Moulin: Another example of twin spotting? Int. J. Dermatol. 2013, 52, 1427–1429. [Google Scholar] [CrossRef] [PubMed]
- Norisugi, O.; Makino, T.; Hara, H.; Matsui, K.; Furuichi, M.; Shimizu, T. Evaluation of skin atrophy associated with linear atrophoderma of Moulin by ultrasound imaging. J. Am. Acad. Dermatol. 2011, 65, 232–233. [Google Scholar] [CrossRef] [PubMed]
- López, N.; Gallardo, M.A.; Mendiola, M.; Bosch, R.; Herrera, E. Atrofodermia lineal de Moulin: Presentación de un caso [A case of linear atrophoderma of Moulin]. Actas Dermosifiliogr. 2008, 99, 165–167. (In Spanish) [Google Scholar] [CrossRef] [PubMed]
- Artola Igarza, J.L.; Sánchez Conejo-Mir, J.; Corbi Llopis, M.R.; Linares Barrios, M.; Casals Andreu, M.; Navarrete Ortega, M. Linear atrophoderma of Moulin: Treatment with Potaba. Dermatology 1996, 193, 345–347. [Google Scholar] [CrossRef] [PubMed]
- Zaouak, A.; Ghorbel, H.H.; Benmously-Mlika, R.; Koubaa, W.; Badri, T.; Fenniche, S.; Mokhtar, I. A case of linear atrophoderma of Moulin successfully treated with methotrexate. Dermatol. Ther. 2014, 27, 153–155. [Google Scholar] [CrossRef] [PubMed]
- Rompel, R.; Mischke, A.L.; Langner, C.; Happle, R. Linear atrophoderma of Moulin. Eur. J. Dermatol. 2000, 10, 611–613. [Google Scholar] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).