Cutaneous Marginal Zone Lymphoproliferation Arising from Circumorificial Plasmacytosis During Nivolumab Therapy for Urothelial Carcinoma
Abstract
1. Introduction
2. Case Presentation
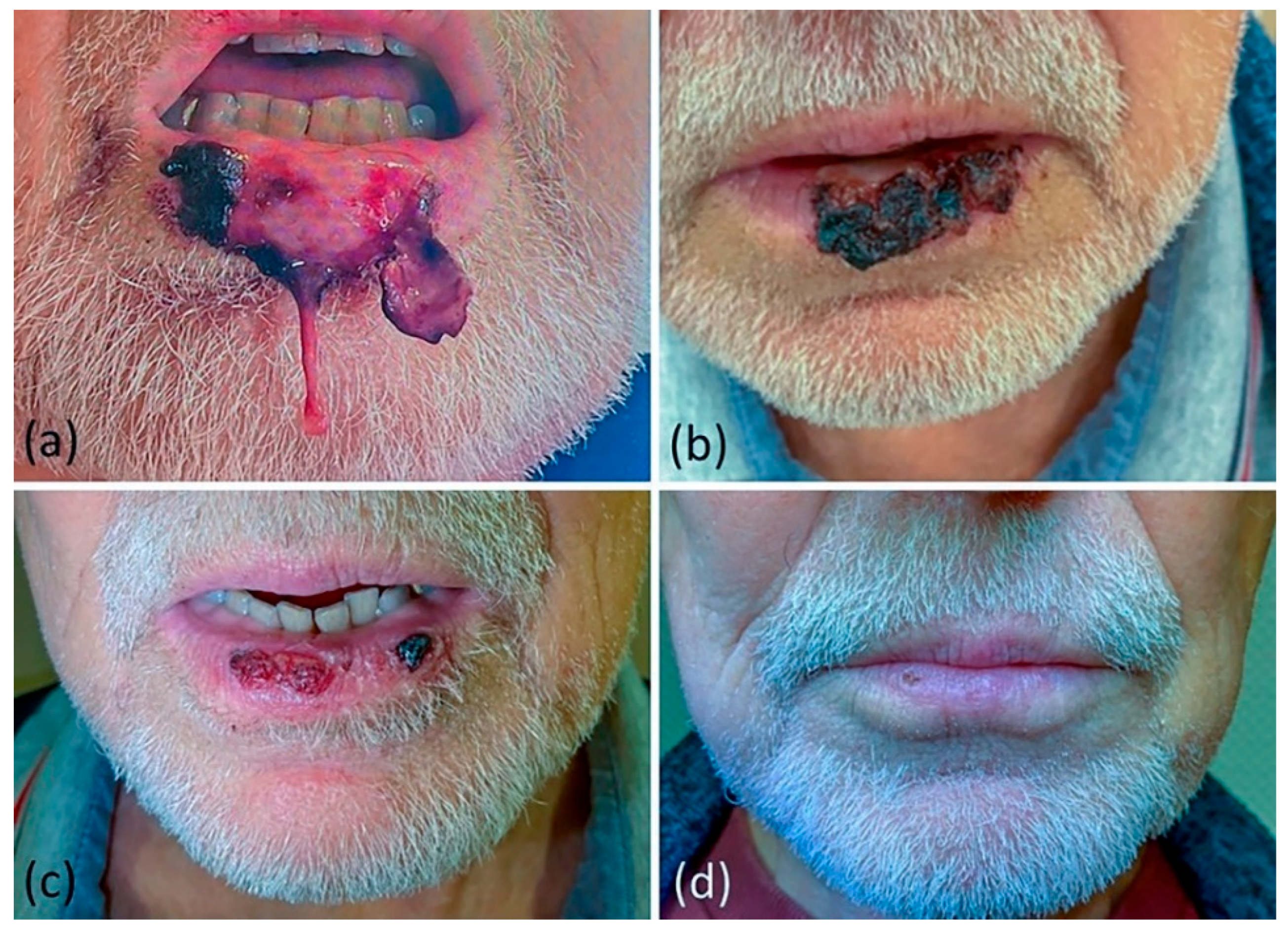
2.1. Clinical Presentation
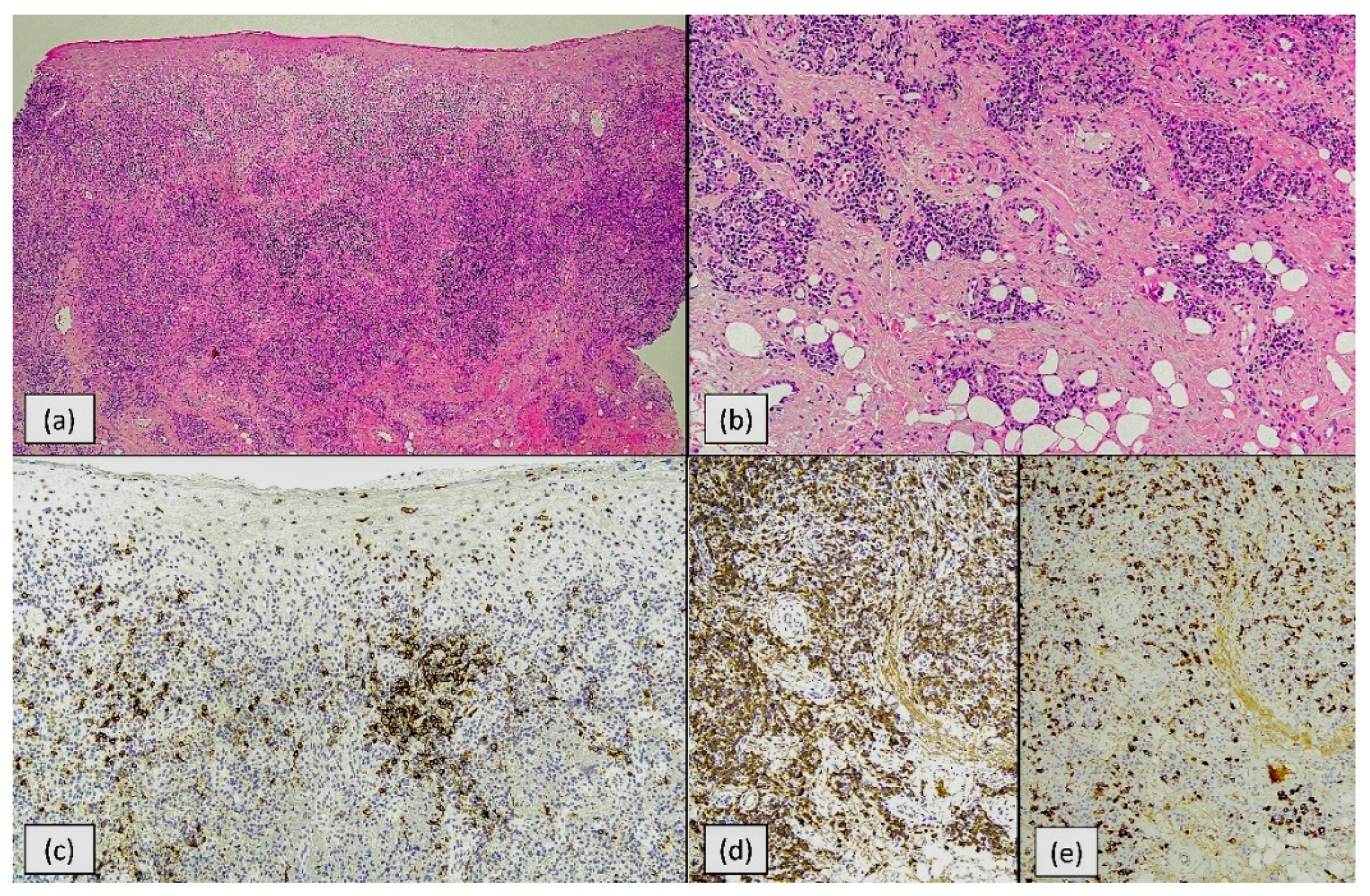
2.2. Routine Histology
2.3. Indirect and Direct Immunofluorescence
2.4. Immunohistochemistry
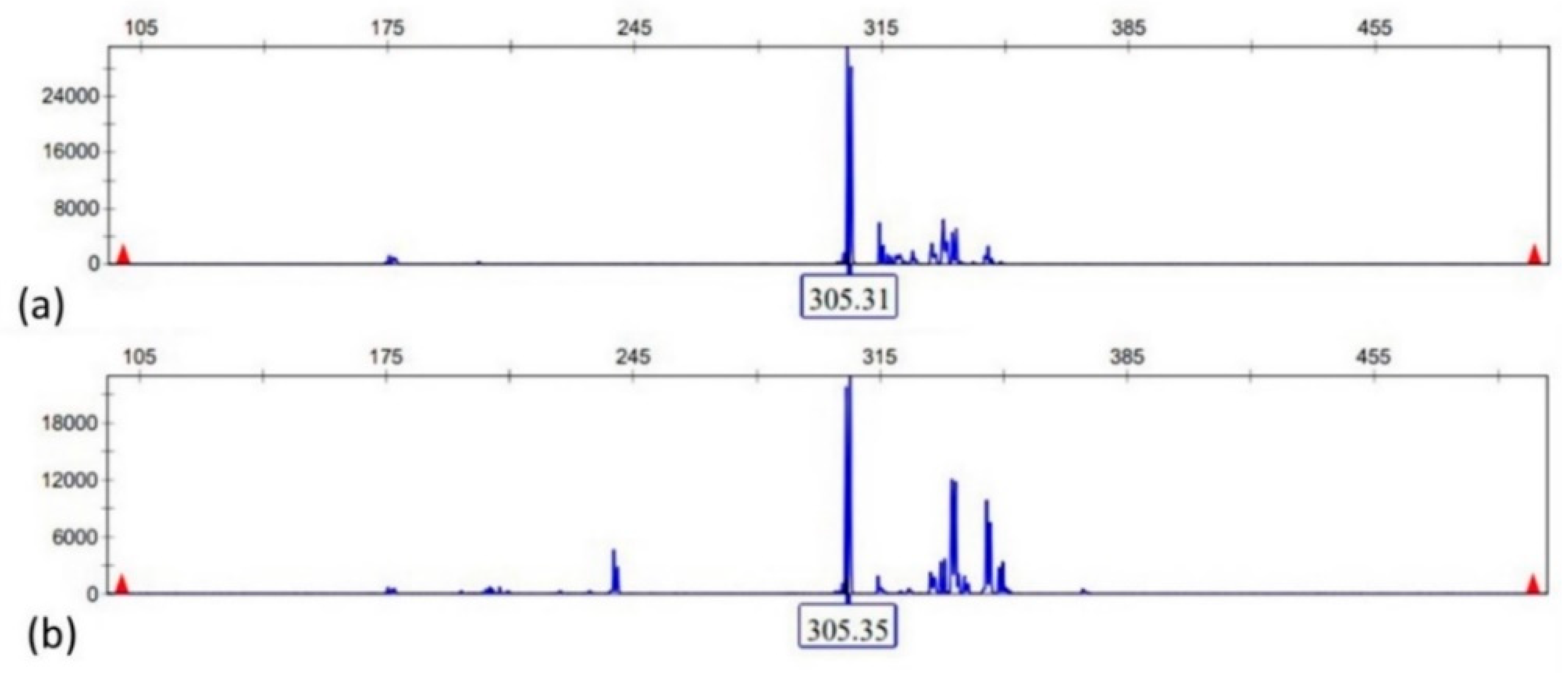
2.5. Multiplex-PCR and EBER In Situ Hybridization
2.6. Additional Laboratory Tests
2.7. Computer Tomography (CT) and Lymph Node Ultrasound
2.8. Treatment
3. Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Eshaq, A.M.; Flanagan, T.W.; Abbad, A.A.B.; Makarem, Z.A.A.; Bokir, M.S.; Alasheq, A.K.; Al Asheikh, S.A.; Almashhor, A.M.; Binyamani, F.; Al-Amoudi, W.A.; et al. Immune Checkpoint Inhibitor-Associated Cutaneous Adverse Events: Mechanisms of Occurrence. Int. J. Mol. Sci. 2024, 26, 88. [Google Scholar] [CrossRef]
- Pacha, O.; Patel, A.B.; Curry, J.L.; Haymaker, C.L.; Ozirmak Lermi, N.; Duose, D.Y.; Chen, K.; Hajjar, J.; Naing, A. Histologic and immune characterization of cutaneous immune-related adverse events induced by immune checkpoint inhibitors. J. Immunother. Cancer. 2025, 13, e011401. [Google Scholar] [CrossRef]
- Oke, I.; Lenert, A.; Swick, B.L.; Lenert, P. Eosinophilic Fasciitis in a 78-Year-Old Man Following Pembrolizumab Treatment for Bladder Cancer. Am. J. Case Rep. 2025, 26, e948323. [Google Scholar] [CrossRef]
- Kachlan, M.O.; Brooks, J.K.; Fantozzi, P.J.; Tenore, G.; Romeo, U.; Pergolini, D.; Sultan, A.S. Oral mucosal adverse events following administration of an immune checkpoint inhibitor: A case report. Gen. Dent. 2025, 73, 10–14. [Google Scholar]
- Venturi, F.; Melotti, B.; Lambertini, M.; Alessandrini, A.; Ardizzoni, A.; Dika, E. Pembrolizumab-Induced Erosive Lichenoid Reaction in a Patient with Metastatic Lung Adenocarcinoma: A Case Report. Int. J. Dermatol. Venereol. 2025, 8, 175–177. [Google Scholar] [CrossRef]
- Korman, A.M.; Zyniewicz, K.; Tinoco, G.; Allen, C.M.; Briody, A. Association of Programmed Cell Death 1 Inhibitor with Circumorificial Plasmacytosis. JAMA Dermatol. 2021, 157, 237–238. [Google Scholar] [CrossRef]
- Tanimu, Y.; Coombs, R.; Tanimu, S.; Onitilo, A. Association of Programmed Cell Death 1 Inhibitor with Circumorificial Plasmacytosis. Case Rep. Oncol. 2024, 17, 33–38. [Google Scholar] [CrossRef]
- Zoon, J.J. Chronic benign circumscripta plasmacytic balanoposthitis. Dermatologica 1952, 105, 1–7. [Google Scholar] [CrossRef]
- Solomon, L.W.; Wein, R.O.; Rosenwald, I.; Laver, N. Plasma cell mucositis of the oral cavity: Report of a case and review of the literature. Oral. Surg. Oral. Med. Oral. Pathol. Oral. Radiol. Endod. 2008, 106, 853–860. [Google Scholar] [CrossRef]
- Bharti, R.; Smith, D.R. Mucous membrane plasmacytosis: A case report and review of the literature. Dermatol. Online J. 2003, 9, 15. [Google Scholar] [CrossRef]
- Gerardi, D.; Torge, D.; Bernardi, S.; Burdo, P.; Piattelli, M.; Varvara, G. Plasma Cell Gingivitis: Clinical Presentation, Histopathologic Correlation, and Therapeutic Challenges. Clin. Pract. 2025, 15, 158. [Google Scholar] [CrossRef]
- Nakamura, M.; Anzai, T.; Arakawa, A.; Takata, Y.; Sonoda, K.; Ishimizu, E.; Matsumoto, F. Upper and Lower Respiratory Mucous Membrane Plasmacytosis with a Cobblestone Appearance: A Case Report. Ear Nose Throat J. 2025, 104 (Suppl. S1), 271S–274S. [Google Scholar] [CrossRef]
- Farraj, K.L.; Kaliounji, A.; Kagolanu, D.; Khan, N. A Rare Case of Extramedullary Plasmacytosis. J. Investig. Med. High. Impact Case Rep. 2021, 9, 23247096211040657. [Google Scholar] [CrossRef]
- Schuermann, H. Plasmacytosis circumorificialis. Dtsch. Zahnarztl. 1960, 15, 601–610. [Google Scholar]
- White, J.W.; Olsen, K.D.; Banks, P.M. Plasma cell orificial mucositis: Report of a case and review of the literature. Arch. Dermatol. 1986, 122, 1321–1324. [Google Scholar] [CrossRef]
- Galvin, S.; Bowe, C.; ORegan, E.M.; Conlon, N.; Flint, S.R.; Healy, C.M. Circumorificial plasmacytosis/plasma cell orificial mucositis: A case series and a review of the literature. Oral. Surg. Oral. Med. Oral. Pathol. Oral. Radiol. 2016, 122, e77–e81. [Google Scholar] [CrossRef]
- Kuriyama, Y.; Shimizu, A.; Toki, S.; Endo, Y.; Yasuda, M.; Motegi, S.I.; Ishikawa, O. Two cases of chronic oral ulcers effectively treated with systemic corticosteroid therapy: Circumorificial plasmacytosis and traumatic ulcerative granuloma with stromal eosinophilia. J. Dermatol. 2019, 46, 48–51. [Google Scholar] [CrossRef]
- Bilgihan, M.T.; Eryigit, A.N.; Ciftciler, R. Efficacy and Safety of Immune Checkpoint Inhibitors in Hematologic Malignancies. Clin. Lymphoma Myeloma Leuk. 2024, 24, 23–31. [Google Scholar] [CrossRef]
- Karki, N.R.; McElhone, P.; Savage, N.; Abdel Karim, N. Diagnosis and management of cold agglutinin disease associated with low-grade B-cell lymphoma in a patient receiving pembrolizumab for lung cancer. BMJ Case Rep. 2021, 14, e243751. [Google Scholar] [CrossRef]
- Kumar, V.; Montgomery, N.D.; van Deventer, H.W.; Whang, Y.E. Waldenström macroglobulinemia with secondary pure red cell aplasia in a patient with metastatic castrate resistant prostate cancer receiving an immune checkpoint inhibitor: A case report. J. Med. Case Rep. 2023, 17, 220. [Google Scholar] [CrossRef]
- Hafian, H.; Schvartz, H.; Patey, M.; Quinquenel, A. Primary oral mucosa-associated lymphoid tissue (MALT) lymphoma in patient with monoclonale gammopathy: A rare case report. BMC Oral Health 2021, 21, 597. [Google Scholar] [CrossRef] [PubMed]
- Al-Aroomi, M.A.; Baihua, L.; Chen, J.; Li, N.; Jiang, C.; Wang, J. Primary giant mucosa-associated lymphoid tissue lymphoma of the lower lip: A case report and literature review. BMC Oral Health 2025, 25, 530. [Google Scholar] [CrossRef] [PubMed]
- Gambichler, T.; Jridi, R.; Bernd, H.-W.; von Stemm, A.; Boms, S. Unusual Presentation of Acrodermatitis Chronica Atrophicans Resulting in Delay of Diagnosis and Inappropriate Treatment in Three Cases. Dermato 2024, 4, 37–45. [Google Scholar] [CrossRef]
- Fărcaş-Berechet, C.M.; Berechet, E.M.; Crăiţoiu, Ş.; Mehedinţi, M.C.; Osman, A.; Eremia, I.A.; Popescu, C.; Iacov-Crăiţoiu, M.M. Clinical, statistical, histological and immunohistochemical aspects of periodontal changes in patients with diabetes mellitus. Rom. J. Morphol. Embryol. 2019, 60, 1191–1198. [Google Scholar] [PubMed]
| Parameter | Present Case | Korman et al. [7] | Tanimu et al. [8] |
|---|---|---|---|
| Underlying malignancy and ICI | Urothelial carcinoma; nivolumab (adjuvant) | Undifferentiated pleomorphic sarcoma; pembrolizumab (neoadjuvant) + radiation; surgery | Stage IVB thymic carcinoma; pembrolizumab q3 wk (53 cycles/39 mo) |
| Age/Sex | 86/male | Elderly male (age not stated) | 27/male |
| Latency from ICI start to COP | ≈6 months | ≈6 months | ≈38 months |
| Anatomic site(s) | Lower lip (mucosal transition zone) | Diffuse gingiva (maxillary/mandibular buccal); buccal/labial mucosa | Left inner cheek, lower inner lip; 4 sublingual lesions |
| Histology/light-chain pattern | Dense plasma cell infiltrate; κ-skew with residual λ; CD56/Cyclin-D1/CD117 negative | CD138+ plasma cells; polyclonal κ/λ; ulcerated multinodular tissue | Sheets of mature plasma cells; polytypic light-chains by CISH |
| Molecular clonality | IgH clonal peak (FR1 305 bp) with polyclonal background | Not reported | Not reported |
| EBV in lesion | Scattered EBER+ small cells | Not reported | Not reported |
| ICI management | Stopped after 11 cycles (generalized maculopapular rash) | Not specified (lesions managed symptomatically) | Continued pembrolizumab |
| COP treatment | Prednisolone taper → topical triamcinolone 0.1% | Topical anesthetics only; no steroids | Dexamethasone mouthwash → 1-wk oral steroids |
| Outcome | Complete regression | Marked regression 1 yr after completing oncologic therapy | Clinical improvement; no further oral lesions |
| Entity | Why Considered | Why Excluded in This Case |
|---|---|---|
| Primary cutaneous marginal zone lymphoma (PCMZL) | Plasma-cell-rich infiltrates; detectable IgH clone | Site (mucosal lip COP), limited B-cell aggregates, EBV-positive bystanders, negative imaging, no paraprotein, complete steroid response |
| Extramedullary plasmacytoma | Dense plasma cells; ulceration | No strict light-chain restriction, no serum/urine monoclonal protein, negative imaging; no destructive mass |
| IgG4-related disease | Plasma-cell-rich lesions; mucosal involvement possible | Few IgG4+ cells, no storiform fibrosis or obliterative phlebitis; clinical/lab features absent |
| Castleman disease (unicentric/multicentric) | Plasma cells; systemic immune activation | No systemic symptoms, nodes not pathologically enlarged; histology lacks Castleman architecture |
| Borrelial lymphocytoma/Lyme-associated MZL mimic | MZL-like infiltrates in endemic regions | Repeated negative serology; anatomic site and histology not classic |
| Syphilis (plasma-cell-rich) | Oral lesions with plasma cells | Negative serology; no spirochetes on special stains |
| Autoimmune blistering diseases (pemphigus/pemphigoid; lichenoid) | Oral erosions under ICI | Direct/indirect immunofluorescence negative; histology lacks diagnostic features |
| Traumatic ulcerative granuloma with stromal eosinophilia (TUGSE) | Painful, persistent oral ulcer; eosinophils | Limited eosinophils; dense plasma-cell predominance; lacks characteristic deep CD30+ myofibroblastic reaction |
| Cheilitis granulomatosa | Lip swelling | Granulomas absent |
| Oral lichen planus/lichenoid mucositis | Common oral irAE | Band-like lymphocytic interface dermatitis absent; plasma cell predominance argues against |
| Plasmacanthoma/Zoon-like processes | Plasma-cell-rich mucositis at orifices | Considered part of the COP spectrum; encompassed by final diagnosis |
| Candidiasis/rhinoscleroma/leishmaniasis | Infectious mimics with plasma cells | No organisms on PAS; clinical/epidemiologic context absent |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gambichler, T.; Bernd, H.-W.; Weyer-Fahlbusch, S.; Lücke, A.; Lorenzen, J.; Susok, L. Cutaneous Marginal Zone Lymphoproliferation Arising from Circumorificial Plasmacytosis During Nivolumab Therapy for Urothelial Carcinoma. Dermato 2025, 5, 23. https://doi.org/10.3390/dermato5040023
Gambichler T, Bernd H-W, Weyer-Fahlbusch S, Lücke A, Lorenzen J, Susok L. Cutaneous Marginal Zone Lymphoproliferation Arising from Circumorificial Plasmacytosis During Nivolumab Therapy for Urothelial Carcinoma. Dermato. 2025; 5(4):23. https://doi.org/10.3390/dermato5040023
Chicago/Turabian StyleGambichler, Thilo, Heinz-Wolfram Bernd, Sera Weyer-Fahlbusch, Anke Lücke, Johann Lorenzen, and Laura Susok. 2025. "Cutaneous Marginal Zone Lymphoproliferation Arising from Circumorificial Plasmacytosis During Nivolumab Therapy for Urothelial Carcinoma" Dermato 5, no. 4: 23. https://doi.org/10.3390/dermato5040023
APA StyleGambichler, T., Bernd, H.-W., Weyer-Fahlbusch, S., Lücke, A., Lorenzen, J., & Susok, L. (2025). Cutaneous Marginal Zone Lymphoproliferation Arising from Circumorificial Plasmacytosis During Nivolumab Therapy for Urothelial Carcinoma. Dermato, 5(4), 23. https://doi.org/10.3390/dermato5040023






