A Comprehensive Approach to Improving Endemic Plant Species Research, Conservation, and Popularization
Abstract
1. Introduction
2. Materials and Methods
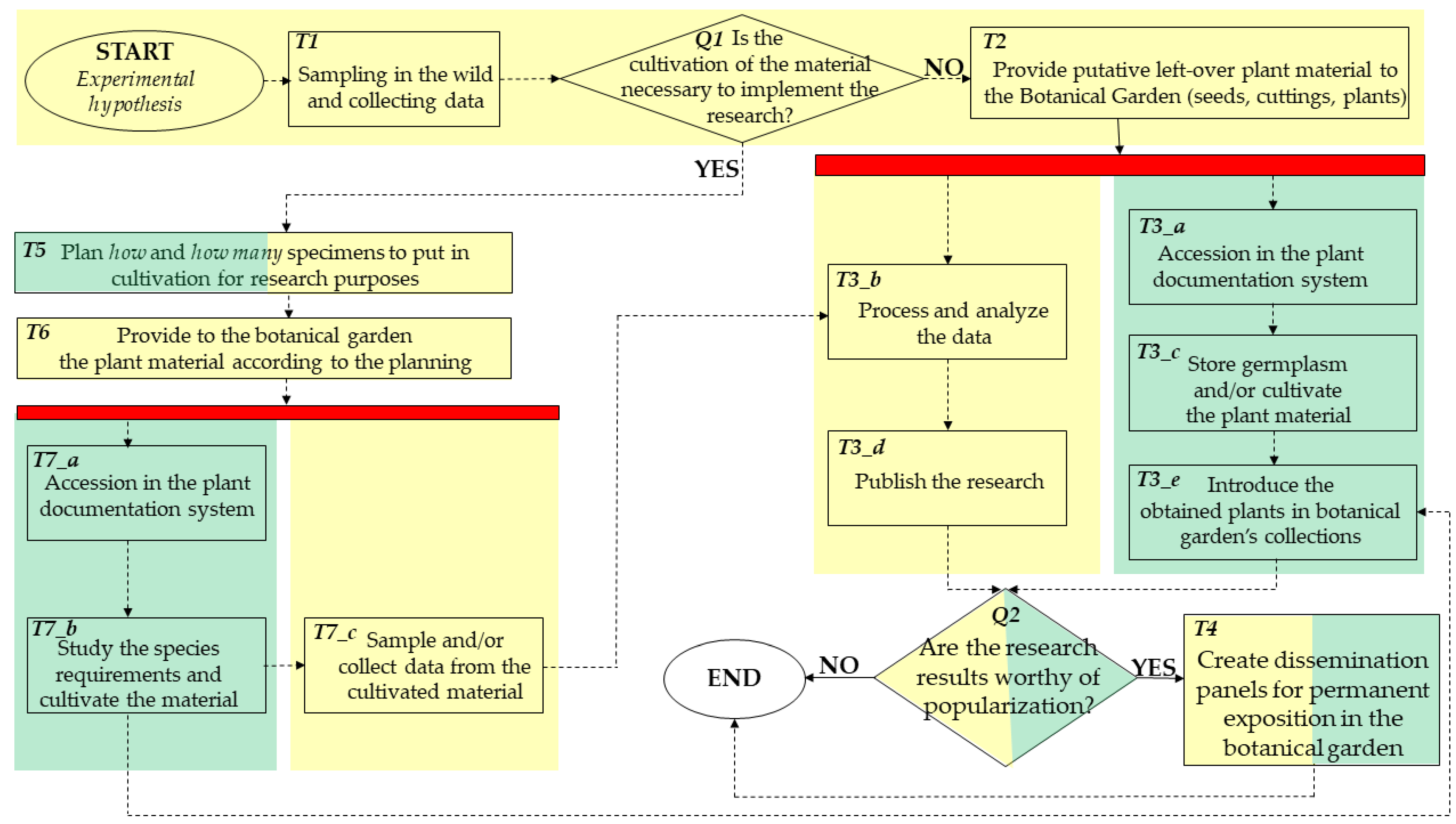
2.1. The Involved Institutions
2.2. General Procedures
2.3. Aquilegia Species from N Apennines and W Alps
2.4. Armeria arenaria Complex
2.5. Bellevalia webbiana
2.6. Crocus etruscus and C. ilvensis
2.7. Dianthus virgineus Complex
2.8. Pulmonaria hirta Complex
2.9. Santolina chamaecyparissus Complex
3. Overview of the Main Results Achieved in Horticulture and Management
4. Discussion
A Workflow for Cooperation between Botanical Institutions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Puttick, M.N.; Morris, J.L.; Williams, T.A.; Cox, C.J.; Edwards, D.; Kenrick, P.; Pressel, S.; Wellman, C.H.; Schneider, H.; Pisani, D.; et al. The interrelationships of land plants and the nature of the ancestral embryophyte. Curr. Biol. 2018, 28, 733–745. [Google Scholar] [CrossRef]
- Ramírez-Barahona, S.; Sauquet, H.; Magallón, S. The delayed and geographically heterogeneous diversification of flowering plant families. Nat. Ecol. Evol. 2020, 4, 1232–1238. [Google Scholar] [CrossRef]
- Carta, A.; Peruzzi, L.; Ramírez-Barahona, S. A global phylogenetic regionalisation of vascular plants reveals a deep split between Gondwanan and Laurasian biotas. New Phytol. 2022, 233, 1494–1504. [Google Scholar] [CrossRef]
- Westwood, M.; Cavender, N.; Meyer, A.; Smith, P. Botanic garden solutions to the plant extinction crisis. Plants People Planet 2021, 3, 22–32. [Google Scholar] [CrossRef]
- Heywood, V.H.; Iriondo, J.M. Plant conservation: Old problems, new perspectives. Biol. Conserv. 2003, 113, 321–335. [Google Scholar] [CrossRef]
- Edwards, C.; Wyse Jackson, P. The development of plant conservation in botanic gardens and the current and future role of conservation genetics for enhancing those conservation efforts. Mol. Front. J. 2019, 3, 44–65. [Google Scholar] [CrossRef]
- Thompson, J.D.; Lavergne, S.; Affre, L.; Gaudeul, M.; Debussche, M. Ecological differentiation of Mediterranean endemic plants. Taxon 2005, 54, 967–976. [Google Scholar] [CrossRef]
- Lavergne, S.; Thompson, J.D.; Garnier, E.; Debussche, M. The biology and ecology of narrow endemic and widespread plants: A comparative study of trait variation in 20 congeneric pairs. Oikos 2004, 107, 505–518. [Google Scholar] [CrossRef]
- Peruzzi, L.; Conti, F.; Bartolucci, F. An inventory of vascular plants endemic to Italy. Phytotaxa 2014, 168, 1–75. [Google Scholar] [CrossRef]
- Orsenigo, S.; Montagnani, C.; Fenu, G.; Gargano, D.; Peruzzi, L.; Abeli, T.; Alessandrini, A.; Bacchetta, G.; Bartolucci, F.; Bovio, M.; et al. Red Listing plants under full national responsibility: Extinction risk and threats in the vascular flora endemic to Italy. Biol. Conserv. 2018, 224, 213–222. [Google Scholar] [CrossRef]
- Salles, D.M.; do Carmo, F.F.; Jacobi, C.M. Habitat loss challenges the conservation of endemic plants in mining-targeted Brazilian mountains. Environ. Conserv. 2019, 46, 140–146. [Google Scholar] [CrossRef]
- Işik, K. Rare and endemic species: Why are they prone to extinction? Turk. J. Bot. 2011, 35, 411–417. [Google Scholar] [CrossRef]
- Chiarugi, A. Le date di fondazione dei primi Orti Botanici del mondo. Giorn. Bot. Ital. 1953, 60, 785–839. [Google Scholar] [CrossRef]
- Chen, G.; Sun, W. The role of botanical gardens in scientific research, conservation, and citizen science. Plant Divers. 2018, 40, 181–188. [Google Scholar] [CrossRef]
- Hohn, T.C. Curatorial Practices for Botanical Gardens; Rowman Altamira: Lanham, MD, USA, 2008; ISBN 978-0-7591-1063-2. [Google Scholar]
- Qumsiyeh, M.; Handal, E.; Chang, J.; Abualia, K.; Najajrah, M.; Abusarhan, M. Role of museums and botanical gardens in ecosystem services in developing countries: Case study and outlook. Int. J. Environ. Sci. 2017, 74, 340–350. [Google Scholar] [CrossRef]
- D’Antraccoli, M.; Weiger, N.; Cocchi, L.; Peruzzi, L. The trees of the Pisa Botanic Garden under climate change scenarios: What are we walking into? Sustainability 2023, 15, 4585. [Google Scholar] [CrossRef]
- Wyse Jackson, P.; Sutherland, L. Role of botanic gardens. In Encyclopedia of Biodiversity; Levin, S.A., Ed.; Academic Press: Waltham, MA, USA, 2017; Volume 6, pp. 504–521. [Google Scholar]
- Mounce, R.; Smith, P.; Brockington, S. Ex situ conservation of plant diversity in the world’s botanic gardens. Nat. Plants 2017, 3, 795–802. [Google Scholar] [CrossRef]
- Philpott, M.; Pence, V.C.; Bassüner, B.; Clayton, A.S.; Coffey, E.E.D.; Downing, J.L.; Edwards, C.E.; Folgado, R.; Ligon, J.J.; Powell, C.; et al. Harnessing the power of botanical gardens: Evaluating the costs and resources needed for exceptional plant conservation. Appl. Plant Sci. 2022, 10, e11495. [Google Scholar] [CrossRef] [PubMed]
- Smith, P. The challenge for botanic garden science. Plants People Planet 2019, 1, 38–43. [Google Scholar] [CrossRef]
- BGCI. BGCI’s Manual on Planning, Developing and Managing Botanic Gardens; Botanic Gardens Conservation International: Richmond, VG, USA, 2022. [Google Scholar]
- Sharrock, S.; Jones, M. Saving Europe’s threatened flora: Progress towards GSPC Target 8 in Europe. Biodivers. Conserv. 2011, 20, 325–333. [Google Scholar] [CrossRef]
- Dayrat, B. Towards integrative taxonomy. Biol. J. Linn. Soc. 2005, 85, 407–417. [Google Scholar] [CrossRef]
- Olson, M.E. Plant evolutionary ecology in the age of the extended evolutionary synthesis. Integr. Comp. Biol. 2019, 59, 493–502. [Google Scholar] [CrossRef]
- Wickham, H. Ggplot2: Elegant Graphics for Data Analysis; Springer-Verlag: New York, NY, USA, 2016. [Google Scholar]
- Enio, N. Il Genere Aquilegia L. (Ranunculaceae) in Italia—The genus Aquilegia L. (Ranunculaceae) in Italy. Aquilegiarum Italicarum in Europaearum Conspectu Descriptio; Polistampa: Firenze, Italy, 2015. [Google Scholar]
- Vandelook, F.; Van Assche, J.A. Temperature requirements for seed germination and seedling development determine timing of seedling emergence of three monocotyledonous temperate forest spring geophytes. Ann. Bot. 2008, 102, 865–875. [Google Scholar] [CrossRef]
- Carta, A.; Hanson, S.; Müller, J.V. Plant regeneration from seeds responds to phylogenetic relatedness and local adaptation in Mediterranean Romulea (Iridaceae) species. Ecol. Evol. 2016, 6, 4166–4178. [Google Scholar] [CrossRef]
- Schütz, W.; Rave, G. The effect of cold stratification and light on the seed germination of temperate sedges (Carex) from various habitats and implications for regenerative strategies. Plant Ecol. 1999, 144, 215–230. [Google Scholar] [CrossRef]
- Nikolaeva, M.G.; Rasumova, M.V.; Gladkova, V.N. Pravocnik po Prorascivanij Pokojascichsja Semjan (Reference Book on Dormant Seed Germination); Nauka, Leningrad Branch: Leningrad, Russia, 1985. [Google Scholar]
- Pinzani, L.; Bacci, S.; Olivieri, F.; Bedini, G.; Carta, A. Comparative Seed Morphology in Related High-Mountain Species of the Genus Aquilegia (Ranunculaceae). Atti Soc. Tosc. Sci. Nat. Mem. Ser. B 2021, 128, 65–71. [Google Scholar] [CrossRef]
- POWO Plants of the World Online. Available online: https://powo.science.kew.org/ (accessed on 19 December 2022).
- Arrigoni, P.V. Contribution to the study of the genus Armeria (Plumbaginaceae) in the Italian peninsula. Flora Medit. 2015, 25, 7–32. [Google Scholar] [CrossRef]
- Aeschimann, D.; Lauber, K.; Martin, M.D.; Theurillat, J.-P. Flora Alpina; Zanichelli: Bologna, Italy, 2004; Volume 3. [Google Scholar]
- Bartolucci, F.; Peruzzi, L.; Galasso, G.; Albano, A.; Alessandrini, A.; Ardenghi, N.M.G.; Astuti, G.; Bacchetta, G.; Ballelli, S.; Banfi, E.; et al. An updated checklist of the vascular flora native to Italy. Plant Biosyst. 2018, 152, 179–303. [Google Scholar] [CrossRef]
- Tiburtini, M.; Astuti, G.; Bartolucci, F.; Casazza, G.; Varaldo, L.; De Luca, D.; Bottigliero, M.V.; Bacchetta, G.; Porceddu, M.; Domina, G.; et al. Integrative taxonomy of Armeria arenaria (Plumbaginaceae), with a special focus on the putative subspecies endemic to the Apennines. Biology 2022, 11, 1060. [Google Scholar] [CrossRef] [PubMed]
- Chiarugi, A. Saggio di una revisione cito-sistematica della flora Italiana. I: Il tetraploidismo della Bellevalia webbiana Parl. e il suo diritto di cittadinanza nella flora italiana. Caryologia 1949, 1, 362–377. [Google Scholar] [CrossRef]
- Borzatti von Loewenstern, A.; Giordani, T.; Astuti, G.; Andreucci, A.; Peruzzi, L. Phylogenetic relationships of Italian Bellevalia species (Asparagaceae), inferred from morphology, karyology and molecular systematics. Plant Biosyst. 2013, 147, 776–787. [Google Scholar] [CrossRef]
- Gestri, G.; Alessandrini, A.; Sirotti, M.; Carta, A.; Peruzzi, L. Contributo alla conoscenza della flora vascolare endemica di Toscana ed aree contermini. 2. Bellevalia webbiana Parl. (Asparagaceae). Inform. Bot. Ital. 2010, 42, 423–429. [Google Scholar]
- Peruzzi, L.; Carta, A. Bellevalia webbiana. The IUCN Red List of Threatened Species 2011 2011, eT195349A8957996. [Google Scholar] [CrossRef]
- Peruzzi, L.; Dolci, D.; Chiarucci, A. Potential climatic and elevational range shifts in the Italian narrow endemic Bellevalia webbiana (Asparagaceae) under climate change scenarios. Nat. Cons. 2022, 50, 145–157. [Google Scholar] [CrossRef]
- Grime, J.P. Vegetation classification by reference to strategies. Nature 1974, 250, 26–31. [Google Scholar] [CrossRef]
- Astuti, G.; Bedini, G.; Carta, A.; Roma-Marzio, F.; Trinco, A.; Peruzzi, L. Comparative assessment of reproductive traits across different habitats in the endangered Webb’s hyacinth (Bellevalia webbiana Parl.). Nat. Cons. 2018, 24, 81–92. [Google Scholar] [CrossRef]
- Peruzzi, L.; Astuti, G.; Algisi, S.; Coppi, A. Genetic differentiation among populations of the threatened Bellevalia webbiana (Asparagaceae) and its consequence on conservation. Plant Biosyst. 2021, 155, 188–193. [Google Scholar] [CrossRef]
- Astuti, G.; Ciccarelli, D.; Roma-Marzio, F.; Trinco, A.; Peruzzi, L. Narrow endemic species Bellevalia webbiana shows significant intraspecific variation in tertiary CSR strategy. Plant Biosyst. 2019, 153, 12–18. [Google Scholar] [CrossRef]
- Sommier, S. La flora dell’Arcipelago Toscano. Nota II. Nuov. Giorn. Bot. Ital. 1903, 10, 133–200. [Google Scholar]
- Pignatti, S. Crocus. In Flora d’Italia; Edagricole: Bologna, Italy, 1982; Volume 3, pp. 419–422. [Google Scholar]
- Fossi Innamorati, T. The vascular flora of the Island of Elba (Tuscan Archipelago). Fourth part. Webbia 1994, 49, 93–123. [Google Scholar] [CrossRef]
- Gamisans, J.; Jeanmonod, D. Flora Corsica; Edisud: Aix-en-Provence, France, 2007. [Google Scholar]
- Carta, A.; Pierini, B.; Alessandrini, A.; Frignani, F.; Peruzzi, L. Contributo alla conoscenza della flora vascolare endemica di Toscana e aree contermini. 1. Crocus etruscus (Iridaceae). Inf. Bot. Ital. 2010, 42, 47–52. [Google Scholar]
- Peruzzi, L.; Carta, A. Crocus ilvensis sp. nov. (sect. Crocus, Iridaceae), endemic to Elba Island (Tuscan Archipelago, Italy). Nord. J. Bot. 2011, 29, 6–13. [Google Scholar] [CrossRef]
- Harpke, D.; Carta, A.; Tomović, G.; Ranđelović, V.; Ranđelović, N.; Blattner, F.R.; Peruzzi, L. Phylogeny, karyotype evolution and taxonomy of Crocus series Verni (Iridaceae). Plant Syst. Evol. 2015, 301, 309–325. [Google Scholar] [CrossRef]
- Carta, A.; Probert, R.; Moretti, M.; Peruzzi, L.; Bedini, G. Seed dormancy and germination in three Crocus ser. Verni species (Iridaceae): Implications for evolution of dormancy within the genus. Plant Biol. 2014, 16, 1065–1074. [Google Scholar] [CrossRef] [PubMed]
- Carta, A.; Flamini, G.; Cioni, P.L.; Pistelli, L.; Peruzzi, L. Flower bouquet variation in four species of Crocus ser. Verni. J. Chem. Ecol. 2015, 41, 105–110. [Google Scholar] [CrossRef]
- Raca, I.; Blattner, F.R.; Waminal, N.E.; Kerndorff, H.; Ranđelović, V.; Harpke, D. Disentangling Crocus series Verni and its polyploids. Biology 2023, 12, 303. [Google Scholar] [CrossRef]
- Domina, G.; Astuti, G.; Barone, G.; Gargano, D.; Minuto, L.; Varaldo, L.; Peruzzi, L. Lectotypification of the Linnaean name Dianthus virgineus (Caryophyllaceae) and its taxonomic consequences. Taxon 2021, 70, 1096–1100. [Google Scholar] [CrossRef]
- Bacchetta, G.; Brullo, S.; Casti, M.; Giusso del Galdo, G.P. Taxonomic revision of the Dianthus sylvestris group (Caryophyllaceae) in central-southern Italy, Sicily and Sardinia. Nord. J. Bot. 2010, 28, 137–173. [Google Scholar] [CrossRef]
- Puppi, G.; Cristofolini, G. Systematics of the complex Pulmonaria saccharata-P. vallarsae and related species (Boraginaceae). Webbia 1996, 51, 1–20. [Google Scholar] [CrossRef]
- Vosa, C.G.; Pistolesi, G. Chromosome Numbers and Distribution of the Genus Pulmonaria (Boraginaceae) in Tuscany and Neighbouring Areas. Caryologia 2004, 57, 121–126. [Google Scholar] [CrossRef]
- Liu, L.; Astuti, G.; Coppi, A.; Peruzzi, L. Different chromosome numbers but slight morphological differentiation and genetic admixture among populations of the Pulmonaria hirta complex (Boraginaceae). Taxon 2022, 71, 1025–1043. [Google Scholar] [CrossRef]
- Astuti, G.; Liu, L.; Peruzzi, L. Chromosome numbers for the Italian flora: 7. Ital. Bot. 2019, 7, 183–187. [Google Scholar] [CrossRef]
- Astuti, G.; Bedini, G.; Ciccarelli, D.; Liu, L.; Tiburtini, M.; Peruzzi, L. Chromosome numbers for the Italian flora: 9. Ital. Bot. 2020, 9, 101–110. [Google Scholar] [CrossRef]
- Kerner, A. Monographia Pulmonariarum; Sumptibus Librariae Academicae Wagnerianae: Innsbruck, Austria, 1878. [Google Scholar]
- Bolliger, M. Die Gattung Pulmonaria in Westeuropa; J. Cramer: Vaduz, Liechtenstein, 1982. [Google Scholar]
- Merxmüller, H.; Sauer, W. Pulmonaria. In Flora Europaea; Cambridge University Press: Cambridge, UK, 1972; Volume 3, pp. 100–102. [Google Scholar]
- Schneider, C.; Rasband, W.; Eliceiri, K. NIH Image to ImageJ: 25 years of image analysis. Nat. Methods 2012, 9, 2089. [Google Scholar] [CrossRef] [PubMed]
- Giacò, A.; Astuti, G.; Peruzzi, L. Typification and nomenclature of the names in the Santolina chamaecyparissus species complex (Asteraceae). Taxon 2021, 70, 189–201. [Google Scholar] [CrossRef]
- Tundis, R.; Loizzo, M. A review of the traditional uses, phytochemistry and biological activities of the genus Santolina. Planta Med. 2018, 84, 627–637. [Google Scholar] [CrossRef]
- De Giorgi, P.; Giacò, A.; Astuti, G.; Minuto, L.; Varaldo, L.; De Luca, D.; De Rosa, A.; Bacchetta, G.; Sarigu, M.; Peruzzi, L. An integrated taxonomic approach points towards a single-species hypothesis for Santolina (Asteraceae) in Corsica and Sardinia. Biology 2022, 11, 356. [Google Scholar] [CrossRef] [PubMed]
- Giacò, A.; De Giorgi, P.; Astuti, G.; Varaldo, L.; Minuto, L.; Peruzzi, L. Taxonomy and distribution of the genus Santolina (Asteraceae) in Italy. Biogeographia 2022, 37, a021. [Google Scholar] [CrossRef]
- Giacò, A.; De Giorgi, P.; Astuti, G.; Caputo, P.; Serrano, M.; Carballal, R.; Sáez, L.; Bacchetta, G.; Peruzzi, L. A morphometric analysis of the Santolina chamaecyparissus complex (Asteraceae). Plants 2022, 11, 3458. [Google Scholar] [CrossRef] [PubMed]
- Giacò, A.; De Giorgi, P.; Astuti, G.; Varaldo, L.; Sáez, L.; Carbajal, R.; Serrano, M.; Casazza, G.; Bacchetta, G.; Caputo, P.; et al. Diploids and polyploids in the Santolina chamaecyparissus complex (Asteraceae) show different karyotype asymmetry. Plant Biosyst. 2022, 156, 1237–1246. [Google Scholar] [CrossRef]
- Giacò, A.; Varaldo, L.; Casazza, G.; De Luca, D.; Caputo, P.; Sarigu, M.; Bacchetta, G.; Sáez, L.; Peruzzi, L. An integrative taxonomic study of Santolina (Asteraceae) from southern France and north-eastern Spain reveals new endemic taxa. J. Syst. Evol. 2022, in press. [Google Scholar] [CrossRef]
- Primack, R.B.; Ellwood, E.R.; Gallinat, A.S.; Miller-Rushing, A.J. The growing and vital role of botanical gardens in climate change research. New Phytol. 2021, 231, 917–932. [Google Scholar] [CrossRef] [PubMed]
- Wandersee, J.H.; Schussler, E.E. Preventing plant blindness. Am. Biol. Teach. 1999, 61, 82–86. [Google Scholar] [CrossRef]
- Peruzzi, L.; Roma-Marzio, F.; Flamini, G. Spontaneous emission of volatiles from the male flowers of the early-branching angiosperm Amborella trichopoda. Planta 2020, 251, 67. [Google Scholar] [CrossRef]
- Ascrizzi, R.; Cioni, P.L.; Amadei, L.; Maccioni, S.; Flamini, G. Geographical patterns of in vivo spontaneously emitted volatile organic compounds in Salvia species. Microchem. J. 2017, 133, 13–21. [Google Scholar] [CrossRef]
- Garnett, S.T.; Christidis, L. Taxonomy anarchy hampers conservation. Nature 2017, 546, 25–27. [Google Scholar] [CrossRef]
- Morrison, W.R.; Lohr, J.L.; Duchen, P.; Wilches, R.; Trujillo, D.; Mair, M.; Renner, S.S. The impact of taxonomic change on conservation: Does it kill, can it save, or is it just irrelevant? Biol. Conserv. 2009, 142, 3201–3206. [Google Scholar] [CrossRef]
- Maunder, M.; Higgens, S.; Culham, A. The effectiveness of botanic garden collections in supporting plant conservation: A European case study. Biodivers. Conserv. 2001, 10, 383–401. [Google Scholar] [CrossRef]
- Ensslin, A.; Godefroid, S. How cultivating wild plants in botanic gardens can change their genetic and phenotypic status and what it means for their conservation value. Sibbaldia 2019, 17, 51–69. [Google Scholar] [CrossRef]
- Maunder, M.; Hawkins, J.; Hughes, C.E.; Culham, A. Hybridisation in Ex Situ Plant Collections: Conservation Liabilities and Opportunities; Guerrant, E.O., Havens, K., Maunder, M., Eds.; Island Press: Washington, DC, USA, 2004; pp. 325–364. [Google Scholar]
- Faraji, L.; Karimi, M. Botanical gardens as valuable resources in plant sciences. Biodivers. Conserv. 2022, 31, 2905–2926. [Google Scholar] [CrossRef]
- Williams, S.J.; Jones, J.P.G.; Gibbons, J.M.; Clubbe, C. Botanic gardens can positively influence visitors’ environmental attitudes. Biodivers. Conserv. 2015, 24, 1609–1620. [Google Scholar] [CrossRef]









| Name before Study | Name after Study (If Different) | Endemic to (Before Study) | Endemic to (After Study, If Different) | Ex Situ Cultivation (IPEN Number) |
|---|---|---|---|---|
| Aquilegia reuteri | SW Alps | IT-0-PI-2021-0242 | ||
| A. ophiolithica | N Apennines | IT-0-PI-2021-0241 | ||
| A. lucensis | N Apennines | IT-0-PI-2021-0238 IT-0-PI-2021-0239 IT-0-PI-2021-0240 | ||
| A. bertolonii | Apuan Alps | IT-0-PI-2021-0235 IT-0-PI-2021-0236 IT-0-PI-2021-0237 | ||
| A. alpina | W Alps | IT-0-PI-2021-0232 IT-0-PI-2021-0233 IT-0-PI-2021-0234 | ||
| Armeria arenaria subsp. apennina | A. arenaria subsp. marginata | N Apennines | IT-0-PI-2021-0041 | |
| A. arenaria subsp. arenaria | France, W Alps | France | FR-0-PI-2021-0043 | |
| A. arenaria subsp. marginata | N Apennines | IT-0-PI-2021-0040 | ||
| A. arenaria subsp. praecox | SW Alps | W Alps | FR-0-PI-2021-0042 | |
| Bellevalia webbiana | N Italian peninsula | IT-0-PI-2020-0728 | ||
| Crocus etruscus | N Italian peninsula | IT-0-PI-2020-0737 | ||
| C. etruscus | C. ilvensis | N Italian peninsula | Elba Island | IT-0-PI-2020-0734 |
| Dianthus virgineus | C Mediterranean | IT-0-PI-2020-2441 | ||
| D. virgineus | C Mediterranean | IT-0-PI-2020-2455 | ||
| D. virgineus | C Mediterranean | IT-0-PI-2020-2451 | ||
| D. virgineus | C Mediterranean | IT-0-PI-2020-2457 | ||
| D. virgineus | C Mediterranean | IT-0-PI-2020-2442 | ||
| D. virgineus | C Mediterranean | IT-0-PI-2020-2450 | ||
| D. virgineus | C Mediterranean | IT-0-PI-2020-2443 | ||
| D. virgineus | C Mediterranean | IT-0-PI-2020-2444 | ||
| D. virgineus | C Mediterranean | IT-0-PI-2020-2452 | ||
| D. virgineus | C Mediterranean | IT-0-PI-2020-2456 | ||
| D. virgineus | C Mediterranean | IT-0-PI-2020-2454 | ||
| D. virgineus | C Mediterranean | IT-0-PI-2020-2453 | ||
| Pulmonaria apennina | P. hirta | Italian peninsula | Italy, SE France | IT-0-PI-2021-0124 |
| P. apennina | P. hirta | Italian peninsula | Italy, SE France | IT-0-PI-2021-0415 |
| P. hirta | NW Italy, SE France | Italy, SE France | IT-0-PI-2021-0426 | |
| P. hirta | NW Italy, SE France | Italy, SE France | IT-0-PI-2021-0416 | |
| P. hirta | NW Italy, SE France | Italy, SE France | IT-0-PI-2021-0125 | |
| P. hirta | NW Italy, SE France | Italy, SE France | IT-0-PI-2021-0127 | |
| P. vallarsae | P. hirta | N Italy | Italy, SE France | IT-0-PI-2021-0126 |
| Santolina benthamiana | E Pyrenees | FR-0-PI-2021-0017 | ||
| S. benthamiana | S. intricata | E Pyrenees | FR-0-PI-2021-0018 | |
| S. chamaecyparissus subsp. tomentosa | S. villosa | Spain | ES-0-PI-2021-0211 ES-0-PI-2021-0403 | |
| S. corsica | Sardinia, Corsica | FR-0-PI-2021-0035 | ||
| S. decumbens | S. decumbens subsp. decumbens | SE France | FR-0-PI-2021-0034 | |
| S. decumbens | S. decumbens subsp. diversifolia | SE France | FR-0-PI-2021-0402 | |
| S. decumbens | S. decumbens subsp. tisoniana | SE France | FR-0-PI-2022-0024 | |
| S. ericoides | S France, Spain | ES-0-PI-2021-0212 FR-0-PI-2021-0230 | ||
| S. etrusca | N Italian peninsula | IT-0-PI-2020-2517 IT-0-PI-2020-2518 | ||
| S. insularis | S. corsica | Sardinia | Sardinia, Corsica | IT-0-PI-2021-0003 IT-0-PI-2022-0025 |
| S. ligustica | NW Italy | IT-0-PI-2021-0033 | ||
| S. magonica | Baleares | ES-0-PI-2021-0015 ES-0-PI-2021-0016 | ||
| S. neapolitana | S Italy | IT-0-PI-2020-2539 | ||
| S. pinnata | Apuan Alps | IT-0-PI-2020-1776 IT-0-PI-2021-0231 | ||
| S. vedranensis | Baleares | ES-0-PI-2021-0002 | ||
| S. virens | N Spain | ES-0-PI-2022-0023 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
D’Antraccoli, M.; Carta, A.; Astuti, G.; Franzoni, J.; Giacò, A.; Tiburtini, M.; Pinzani, L.; Peruzzi, L. A Comprehensive Approach to Improving Endemic Plant Species Research, Conservation, and Popularization. J. Zool. Bot. Gard. 2023, 4, 490-506. https://doi.org/10.3390/jzbg4020036
D’Antraccoli M, Carta A, Astuti G, Franzoni J, Giacò A, Tiburtini M, Pinzani L, Peruzzi L. A Comprehensive Approach to Improving Endemic Plant Species Research, Conservation, and Popularization. Journal of Zoological and Botanical Gardens. 2023; 4(2):490-506. https://doi.org/10.3390/jzbg4020036
Chicago/Turabian StyleD’Antraccoli, Marco, Angelino Carta, Giovanni Astuti, Jacopo Franzoni, Antonio Giacò, Manuel Tiburtini, Lorenzo Pinzani, and Lorenzo Peruzzi. 2023. "A Comprehensive Approach to Improving Endemic Plant Species Research, Conservation, and Popularization" Journal of Zoological and Botanical Gardens 4, no. 2: 490-506. https://doi.org/10.3390/jzbg4020036
APA StyleD’Antraccoli, M., Carta, A., Astuti, G., Franzoni, J., Giacò, A., Tiburtini, M., Pinzani, L., & Peruzzi, L. (2023). A Comprehensive Approach to Improving Endemic Plant Species Research, Conservation, and Popularization. Journal of Zoological and Botanical Gardens, 4(2), 490-506. https://doi.org/10.3390/jzbg4020036








