Abstract
Globally, the frequency of fishkill episodes is increasing, owing to natural and human-induced modification of aquatic ecosystems. A massive fishkill took place on 22 October 2017 along an approximately 1.5 km stretch of the Jhelum River in Srinagar City, India. Thousands of fish died during this specific event, not lasting more than three hours, creating chaos and panic among the local population and government circles. In this context, affected fish were assessed for three morphological parameters, which include skin color, eye appearance, and skin texture. To back our findings, three critical water-quality parameters, including pH, water temperature, and dissolved oxygen essential for the survival of fishes were assessed in the affected river stretch. This study assumes importance given that water-quality observation stations for monitoring the health of the Jhelum River are lacking in the highly urbanized Srinagar City. The morphological examination of fish samples revealed discoloration, bulging eyes, and rough skin texture, indicating chemical contamination of waters in the affected river stretch. The water quality analysis revealed neutral pH (7.2), normal temperature (15.6 °C), and mildly depleted dissolved oxygen (6 mg L−1) levels. While the morphological examination of the affected fish indicated chemical contamination, the physicochemical parameters exhibited a typical scenario of river water. For avoiding any such further incident and to precisely ascertain the cause of such fishkill episodes in future, it is suggested that a few continuous water-quality monitoring sites along Jhelum River should be set up, supplemented with robust ecological modeling simulations.
1. Introduction
There is a plethora of literature that suggests both human-induced [1,2,3] and natural causes [4,5,6] for the fishkill. While the human-induced kills include known or accidental additions of harmful chemicals, sewage ingress, and fertilizers from agricultural fields into natural waters [7,8,9,10], the natural causes on the contrary are attributed to temperature fluctuations [11,12], anoxic conditions [13,14], cyanobacterial blooms [15,16], and disease outbreak [17,18]. These episodes sometimes tend to impact the entire lake or river stretch [19] or at times are extremely localized [20]. Table 1 provides the details of the prominently reported episodes of fishkill in India for the last three decades.

Table 1.
Details of recent fishkill episodes in various aquatic ecosystems across India.
Various factors have been observed to induce fishkill in the aquatic ecosystems of Kashmir valley. These include electric shocks [21], use of chemicals [22], anoxic conditions [23], and cyanobacterial blooms [24]. Managing the natural causes of fishkill requires a long-term ecosystem management framework; however, the anthropogenically induced fishkill could largely be controlled through strict vigil and a legal framework. While the mechanism of checking fishkill in the valley plains appears satisfactory, the alpine areas (>3000 m asl) are devoid of effective and robust vigil, which has promoted wanton use of chemicals and electric current—resulting in various unreported fishkill events from these wilderness areas. The anthropogenically driven fish-kill could result from a point source such as sewage ingress and chemical application, or a non-point source such as agricultural runoff. However, owing to the strict legal framework both globally and nationally, the former causes for fishkill have been reduced—although not completely.
Whether anthropogenically driven or otherwise, fishkill always generates public outcry as the consumption of such catch could sometimes be lethal. Timely assessment of the water quality at the time of fishkill is very important to ascertain the root cause for such ecological devastation. Mostly in the developing and underdeveloped world, the limnologists arrive at the site of fishkill after a time lag, after which there is a likelihood that the entire water quality has switched back to normal; especially in case of flowing systems. It hence becomes imperative to use robust models for predicting the incidences of fishkill so that planners and policymakers take scientifically-informed decisions to mitigate such events [37,38]. Furthermore, the use of geospatial [39] and environmental flow [40] models could help in providing key insights about the health of aquatic ecosystems. In addition, the potential of microalgae can be explored for improving the water quality [41].
In this context, the current study investigated the causes of the 22 October 2017 mass fishkill episode in the affected stretch of the Jhelum River in Srinagar City, using water-quality parameters and morphological examination of the affected fish. It is relevant to point out that an earlier study investigated the fishkill in a lentic ecosystem of Kashmir Himalaya using similar set of water quality and morphological characteristics [42].
2. Materials and Methods
2.1. Study Area
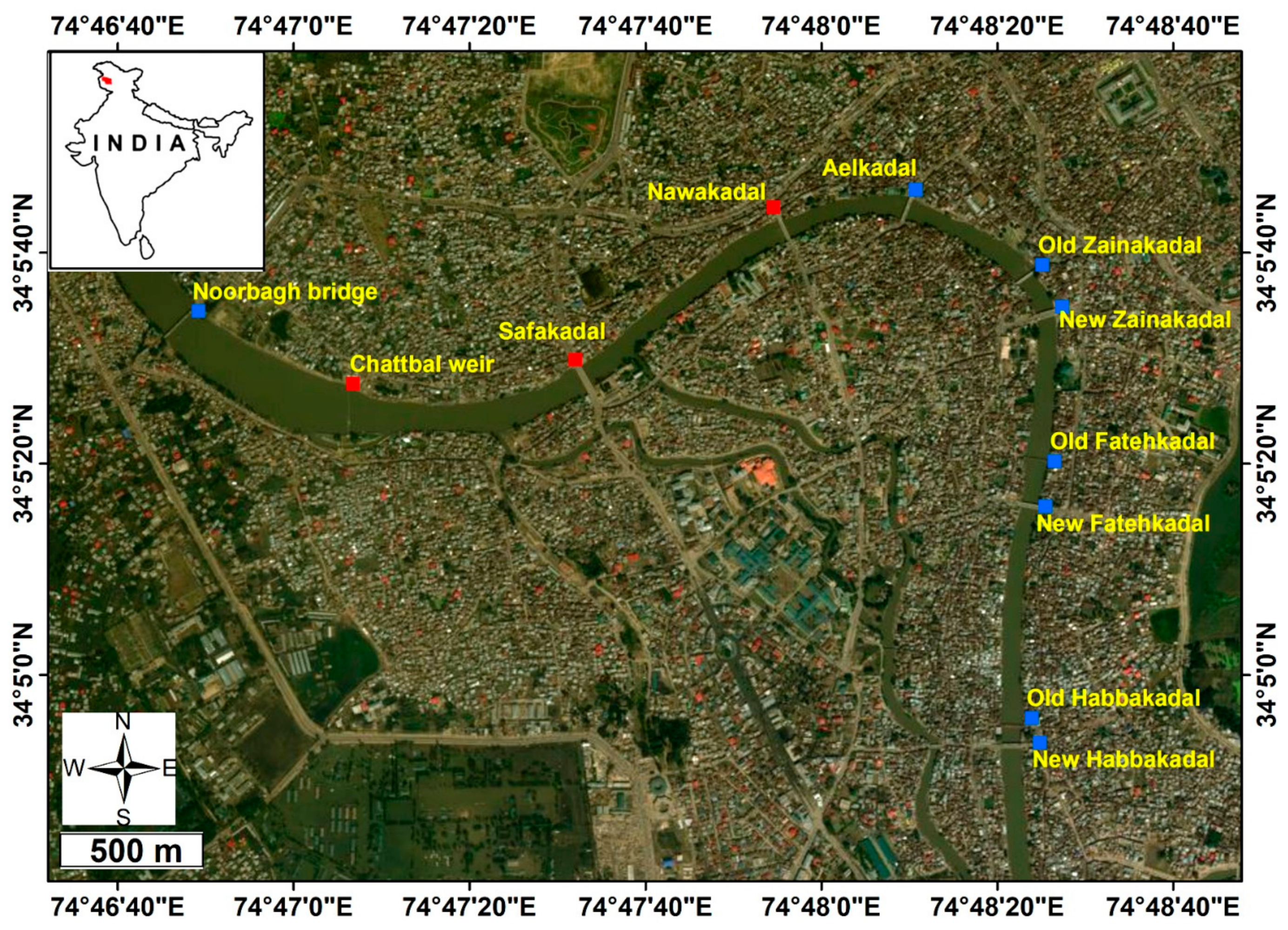
The Jhelum River draining through Srinagar City is an important source of domestic water supply, including drinking water and irrigation [43]. Besides this, the river channel is being exploited for sand utilized in construction purposes [44]. Traditionally, the river has been used as an alternative means of navigation in the city and beyond. The river harbors a rich resource of fisheries that are a source of livelihood to the fishermen living in the vicinity [45]. This research focuses on a 1.5 km stretch of Jhelum from Nawakadal to Chattabal Weir (Figure 1) in the heart of Srinagar, the capital city of Jammu and Kashmir, India. It is in this portion of the river that a massive fishkill occurred in the afternoon of 22 October 2017. It is pertinent to mention that water-quality monitoring stations are nonexistent in the entire stretch of the Jhelum River, which hampers analyzing and forecasting any ecological disaster. The pollution of the river ecosystem is further exacerbated due to the lack of a scientifically-based comprehensive river management plan. The study area lies between the geographical coordinates of 34°5′30″–34°5′45″ latitude and 74°47′8″–74°47′55″ longitude, located at an altitude of 1580 m above sea level. The climate of the area is temperate with four distinct seasons. The major portion of rainfall is received from March to May (spring), and the period from November to February receives heavy snowfall (extreme winters). Geologically, the study area is surrounded by alluvium [46].
Figure 1.
Location of the affected stretch of the Jhelum River, indicated by red dots. Blue dots indicate prominent locations along the unaffected portion of Jhelum River, Srinagar City. Background image: IKONOS GeoEye, dated 21 September 2018.
2.2. Methodology
Since the 2017 fishkill episode was an erratic event, the focus of this study was to get firsthand insight into the causes of mass mortality. This was accomplished by on-the-spot visit to the affected river stretch for ascertaining changes, if any, in the water quality and morphological characteristics of the dead fish. The samples of Shizothorax curvifrons and Cyprinus carpio were collected within approximately 24 h of the reported fishkill episode [47,48]. The affected fish were immediately examined in the laboratory for three important morphological parameters, which include skin color [49], eye appearance [50], and skin texture [51] to infer the cause of fishkill. Since the real-time water quality data is not collected anywhere across the length of Jhelum by any government department, an on-site assessment of water quality was carried out in the afternoon of 23 October 2017 (approximately 24 h after the fishkill episode) to look into the basic limnological characteristics of water in the affected stretch [52]. Three basic water-quality parameters: pH, dissolved oxygen, and water temperature, were assessed at three sites (indicated as red dots in Figure 1) in the affected river stretch using Hanna pHep Tester-HI98107 [53] and Lutron PDO-519 [54] probes.
3. Results and Discussion
The affected fish in the river stretch were crowding at the surface and exhibited problems with buoyancy indicated by their inability to swim. The crowded fish predominantly showed anomalous signs indicated by sluggishness and lack of activity; however, flashing and twisting movements were also witnessed in a few individuals. The morphological examination of the affected fish indicated discoloration, open sores, and black and white spots. Moreover, abnormal shape, swollen areas, and bulging eyes were observed in the affected fish. All the fishes sampled from the river stretch had unusually rough skin texture (Figure 2).

Figure 2.
Morphological changes in Shizothorax curvifrons: (a) bulged-out eyes, black and white spots, (b) pigment discoloration, abnormal shape.
The Department of Fisheries report suggested that the fish were stressed and washing ashore the Jhelum owing to anoxic conditions in the water column. The report further suggested that low oxygen levels were corroborated by the fact that only large-sized Shizothorax curvifrons (kaeshir gaad) was showing such distress, whereas the other fish species—principally Cyprinus carpio (Panjaeb gaad)—did not show any mortality. However, there is no scientifically credible data, either instrumental or lab-based, that supports the contention of depleted oxygen levels in this stretch of Jhelum. The lab-based quality assessment of the water samples that were collected on 22 October 2017 were never made public to back this assumption. It is pertinent to mention that a doctors’ association of Kashmir advised people against consuming fish and fish products [55]. Rather than collecting the samples and getting them analyzed in the lab, it would have been more prudent on the part of fisheries department to assess the samples on site, using electronic field kits.
The water-quality analysis revealed a neutral pH of the Jhelum water ranging from 7.2–7.3. The water temperature varied between 15.8 °C and 16 °C with a mean of 15.9 °C. Mildly depleted dissolved oxygen levels varying between 5.8 mg L−1 and 6.2 mg L−1 were noticed on site (Table 2). A similar fishkill was reported from the Nigeen lake in 2012 [42]; however, this fishkill event, the first of its kind, took place in the flowing Jhelum river, the water quality of which is normally considered to be very good in the upper stretches and poor in the middle stretches falling in the main city area [56].

Table 2.
Physico-chemical analysis of water samples in the affected tract of the Jhelum River.
Additionally, the use of freely available high-resolution satellite remote sensing data such as Planet Cubesat images available at daily timesteps [57,58,59] could help in mapping the urban centres/towns located in the vicinity of river courses that may act as potential sources of water pollution [56]. It becomes equally important to monitor the pace of land-system changes that are affecting the physicochemical and biological characteristics of aquatic ecosystems across Kashmir. This is pertinent since massive land system changes and urbanization have modified the otherwise pristine landscapes around the aquatic ecosystems across Kashmir [60,61,62,63].
Analysis of water-quality parameters at these three sites does not bear weight regarding the assumption of fishkill being attributable to sudden hypoxia. It is pertinent to mention that in very rare circumstances, microclimate can play a significant role in triggering anoxic conditions. Furthermore, the secretion of toxins by harmful algal blooms (HAB) can prove lethal to the fish community [16]. The waters along the Nawakadal–Chattabal stretch did not reveal any anoxic behavior as reflected by normal dissolved oxygen values of around 6 mg L−1. Water temperatures were also usual (15.9 °C), considered normal during this time of the year. To our knowledge, it is for the first time that such a massive fishkill has been reported and documented in the Jhelum in Srinagar. The morphological examination of the dead fish indicated chemical contamination of waters in this stretch of Jhelum.
4. Conclusions
There is no documented information about the fishkill episodes in the pristine river ecosystems of Kashmir Himalaya. The present study was a rapid assessment to look into the causes of fishkill that took place on 22 October 2017. Our preliminary water chemistry analysis does not indicate any natural causative factors for this fishkill episode. However, the morphological analysis of fishes plausibly indicated chemical contamination. A detailed histopathological examination of fishes, which has not been carried out, might have corroborated the human-induced theory, or suggest a natural cause for this particular fishkill. The ideal way to analyze the fishkill episodes would require setting up real-time water-quality monitoring stations at selected urban centres across the Jhelum River. To supplement the water-quality observations, the use of robust models for predicting the incidences of fishkill in the aquatic ecosystems of the Kashmir region need to be explored. This will go a long way in eliminating the otherwise unscientific theories that are later being put forth as the reasons for such ecological disasters. Besides this, awareness among people about the causes and timings of such catastrophes needs to be disseminated. Furthermore, the river courses across sensitive places such as major towns and cities need to be earmarked as no-interference zones, which would enable prevention of chemical contamination and untreated sewage ingress into aquatic ecosystems.
Author Contributions
Conceptualization, I.R. and M.I.R.; methodology, I.R., M.I.R. and S.A.K.; formal analysis, I.R. and M.I.R.; investigation, I.R. and S.A.K.; resources, I.R.; data curation, I.R. and M.I.R.; writing—original draft preparation, I.R.; writing—review and editing, I.R. and M.I.R.; supervision, I.R.; project administration, I.R. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Acknowledgments
The critical comments and suggestions from the two anonymous reviewers and Academic Editor greatly helped in improving the manuscript content and structure.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Olmsted, L.L.; Cloutman, D.G. Repopulation After a Fish Kill in Mud Creek, Washington County, Arkansas following Pesticide Pollution. Trans. Am. Fish. Soc. 1974, 103, 79–87. [Google Scholar] [CrossRef]
- Kragh, T.; Martinsen, K.T.; Kristensen, E.; Sand-Jensen, K. From drought to flood: Sudden carbon inflow causes whole-lake anoxia and massive fish kill in a large shallow lake. Sci. Total Environ. 2020, 739, 140072. [Google Scholar] [CrossRef] [PubMed]
- Rodgers, E.M.; Opinion, A.G.R.; Gomez Isaza, D.F.; Rašković, B.; Poleksić, V.; De Boeck, G. Double whammy: Nitrate pollution heightens susceptibility to both hypoxia and heat in a freshwater salmonid. Sci. Total Environ. 2021, 765, 142777. [Google Scholar] [CrossRef] [PubMed]
- Venugopalan, V.P.; Nandakumar, K.; Rajamohan, R.; Sekar, R.; Nair, K.V.K. Natural eutrophication and fish kill in a shallow freshwater lake. Curr. Sci. 1998, 74, 915–917. [Google Scholar]
- Mohd-Din, M.; Abdul-Wahab, M.F.; Mohamad, S.E.; Jamaluddin, H.; Shahir, S.; Ibrahim, Z.; Hii, K.S.; Tan, S.N.; Leaw, C.P.; Gu, H.; et al. Prolonged high biomass diatom blooms induced formation of hypoxic-anoxic zones in the inner part of Johor Strait. Environ. Sci. Pollut. Res. 2020, 27, 42948–42959. [Google Scholar] [CrossRef]
- Karlson, B.; Andersen, P.; Arneborg, L.; Cembella, A.; Eikrem, W.; John, U.; West, J.J.; Klemm, K.; Kobos, J.; Lehtinen, S.; et al. Harmful algal blooms and their effects in coastal seas of Northern Europe. Harmful Algae 2021, 102, 101989. [Google Scholar] [CrossRef]
- Ram, K.J.; Rao, G.R.M.; Ayyappan, S.; Purushothaman, C.S.; Saha, P.K.; Pani, K.C.; Muduli, H.K. A combination of commercial bleaching powder and urea as a potential piscicide. Aquaculture 1988, 72, 287–293. [Google Scholar] [CrossRef]
- Jones-Lepp, T.L.; Taguchi, V.; Sovocool, W.; Betowski, D.; DeArmond, P.; Schumacher, B.; Winnik, W.; McMillin, R.; Armstrong, C. Novel contaminants identified in fish kills in the Red River watershed, 2011–2013. Environ. Toxicol. Chem. 2018, 37, 336–344. [Google Scholar] [CrossRef]
- Barica, J. Extreme fluctuations in water quality of eutrophic fish kill lakes: Effect of sediment mixing. Water Res. 1974, 8, 881–888. [Google Scholar] [CrossRef]
- Navarrete, I.A.; Tee, K.A.M.; Unson, J.R.S.; Hallare, A.V. Organochlorine pesticide residues in surface water and groundwater along Pampanga River, Philippines. Environ. Monit. Assess. 2018, 190, 1–8. [Google Scholar] [CrossRef]
- Kangur, A.; Kangur, P.; Kangur, K.; Möls, T. The role of temperature in the population dynamics of smelt Osmerus eperlanus eperlanus m. spirinchus Pallas in Lake Peipsi (Estonia/Russia). In Shallow Lakes in a Changing World; Springer: Dordrecht, The Netherlands, 2007; pp. 433–441. [Google Scholar] [CrossRef]
- Wong, V.N.L.; Walsh, S.; Morris, S. Climate affects fish-kill events in subtropical estuaries of eastern Australia. Mar. Freshw. Res. 2018, 69, 1641–1648. [Google Scholar] [CrossRef]
- Townsend, S.A.; Boland, K.T.; Wrigley, T.J. Factors contributing to a fish kill in the Australian wet/dry tropics. Water Res. 1992, 26, 1039–1044. [Google Scholar] [CrossRef]
- Hayami, Y.; Wada, M.; Umezawa, Y.; Fujii, N.; Nakamura, A.; Mori, F. Hypoxic water mass in the highly turbid well-mixed macrotidal Rokkaku River Estuary, Ariake Sea, Japan. Estuar. Coast. Shelf Sci. 2019, 219, 210–222. [Google Scholar] [CrossRef]
- Rodger, H.D.; Turnbull, T.; Edwards, C.; Codd, G.A. Cyanobacterial (blue-green algal) bloom associated pathology in brown trout, Salmo trutta L., in Loch Leven, Scotland. J. Fish Dis. 1994, 17, 177–181. [Google Scholar] [CrossRef]
- Huisman, J.; Codd, G.A.; Paerl, H.W.; Ibelings, B.W.; Verspagen, J.M.H.; Visser, P.M. Cyanobacterial blooms. Nat. Rev. Microbiol. 2018, 16, 471–483. [Google Scholar] [CrossRef]
- Mohan, C.V.; Shankar, K.M. Epidemiological analysis of epizootic ulcerative syndrome of fresh and brackishwater fishes of Karnataka, India. Curr. Sci. 1994, 66, 656–658. [Google Scholar]
- Chitmanat, C.; Lebel, P.; Whangchai, N.; Promya, J.; Lebel, L. Tilapia diseases and management in river-based cage aquaculture in northern Thailand. J. Appl. Aquac. 2016, 28, 9–16. [Google Scholar] [CrossRef]
- Naqvi, S.; George, M.; Narvekar, P.; Jayakumar, D.; Shailaja, M.S.; Sardessai, S.; Sarma, V.V.S.S.; Shenoy, D.M.; Naik, H.; Maheshwaran, P.A.; et al. Severe fish mortality associated with ‘red tide’observed in the sea off Cochin. Curr. Sci. 1998, 75, 543–544. [Google Scholar]
- Parvez, S.; Pandey, S.; Ali, M.; Raisuddin, S. Biomarkers of oxidative stress in Wallago attu (Bl. and Sch.) during and after a fish-kill episode at Panipat, India. Sci. Total Environ. 2006, 368, 627–636. [Google Scholar] [CrossRef]
- Godfrey, H. Mortalities Among Developing Trout and Salmon Ova Following Shock by Direct-Current Electrical Fishing Gear. J. Fish. Res. Board Canada 1957, 14, 153–164. [Google Scholar] [CrossRef]
- Nanda, N.B.P.; Das, P.C.; Jena, J. Use of Rotenone as Piscicide: Toxicity Levels in a Few Common Freshwater Predatory and Weed Fishes. J. Appl. Aquac. 2009, 21, 241–249. [Google Scholar] [CrossRef]
- Godinho, F.N.; Segurado, P.; Franco, A.; Pinheiro, P.; Pádua, J.; Rivaes, R.; Ramos, P. Factors related to fish kill events in Mediterranean reservoirs. Water Res. 2019, 158, 280–290. [Google Scholar] [CrossRef]
- Harrison, P.J.; Piontkovski, S.; Al-Hashmi, K. Understanding how physical-biological coupling influences harmful algal blooms, low oxygen and fish kills in the Sea of Oman and the Western Arabian Sea. Mar. Pollut. Bull. 2017, 114, 25–34. [Google Scholar] [CrossRef] [PubMed]
- Ram, A.; Jaiswar, J.R.M.; Rokade, M.A.; Bharti, S.; Vishwasrao, C.; Majithiya, D. Nutrients, hypoxia and Mass Fishkill events in Tapi Estuary, India. Estuar. Coast. Shelf Sci. 2014, 148, 48–58. [Google Scholar] [CrossRef]
- Sheikh, A.; Slathia, D. Water quality changes and winter mortality of major carps (Cyprinus carpio communis and Cyprinus carpio specularis) in Mansar Lake (a Ramsar Site), Jammu, J&K, India. Environ. Conserv. J. 2018, 19, 51–58. [Google Scholar] [CrossRef]
- Dutta, S.P.S.; Slathia, D.; Slathia, D.; Chandbala, K. Incidences of winter fish kill in subtropical Surinsar Lake (Ramsar Site) in Shivalik hills of Jammu (J&K). Environ. Conserv. J. 2014, 15, 35–40. [Google Scholar] [CrossRef]
- Benjamin, R.; Chakrapani, B.K.; Devashish, K.; Nagarathna, A.V.; Ramachandra, T.V. Fish Mortality in Bangalore Lakes, India. Electron. Green J. 1996, 1, 123–131. [Google Scholar] [CrossRef]
- Pejaver, M.K.; Somani, V. A study on fish kill in Railadevi lake, Thane Maharashtra. J. Aquat. Biol. 2000, 15, 47–49. [Google Scholar]
- Maheshwari, R. Fish death in lakes. Curr. Sci. 2005, 88, 1719–1721. [Google Scholar]
- Patil, G.S.; Patil, S.B. Environmental case study of Water Quality and Climate Change resulting a mass Mortality of Fish at Taj Boudi of Bijapur. IOSR J. Environ. Sci. 2015, 9, 1–7. [Google Scholar]
- Nagdali, S.S.; Gupta, P.K. Impact of mass mortality of a mosquito fish, Gambusia affinis on the ecology of a fresh water eutrophic lake (Lake Naini Tal, India). Hydrobiologia 2002, 468, 45–51. [Google Scholar] [CrossRef]
- Bhatnagar, A.; Devi, P. Water quality guidelines for the management of pond fish culture. Int. J. Environ. Sci. 2013, 3, 1980–2009. [Google Scholar]
- Poodari, S.; Golla, S.; Himabindu, V. Assessment of Water Quality of Peddacheruvu, Sambaiah Cheruvu, Gaddapotharam and Rudraram Cheruvu of Medak District. J. Aquat. Biol. 2014, 7, 137–142. [Google Scholar]
- Murkute, V.B.; Chavan, A.W. Algal blooms and its impact on status of lendra pond at Brahmapuri, dist. Chandrapur. (MS), India. Int. J. Life Sci. 2018, 12, 254–258. [Google Scholar]
- Chauhan, R. Fungal attack on Tilapia mossambicus in culture pond, leading to mass mortality of fishes. Int. J. Pharm. Sci. Res. 2014, 5, 425–428. [Google Scholar]
- Mericas, C.; Malone, R.F. A Phosphorus-Based Fish Kill Response Function for use with Stochastic Lake Models. N. Am. J. Fish. Manag. 1984, 4, 556–565. [Google Scholar] [CrossRef]
- Borsuk, M.E.; Stow, C.A.; Reckhow, K.H. A Bayesian network of eutrophication models for synthesis, prediction, and uncertainty analysis. Ecol. Modell. 2004, 173, 219–239. [Google Scholar] [CrossRef]
- Guiamel, I.A.; Lee, H.S. Watershed modelling of the mindanao river basin in the philippines using the SWAT for water resource management. Civ. Eng. J. 2020, 6, 626–648. [Google Scholar] [CrossRef]
- Ćosić-Flajsig, G.; Vučković, I.; Karleuša, B. An innovative holistic approach to an e-flow assessment model. Civ. Eng. J. 2020, 6, 2188–2202. [Google Scholar] [CrossRef]
- Kim, K.; Jung, J.Y.; Han, H.S. Utilization of microalgae in aquaculture system: Biological wastewater treatment. Emerg. Sci. J. 2019, 3, 209–221. [Google Scholar] [CrossRef]
- Rather, M.I.; Yousuf, A.R.; Shahi, N.; Mehraj, M.; Khanday, S.A. Understanding the cause of fish kill in Nigeen Lake. J. Res. Dev. 2013, 13, 20–32. [Google Scholar]
- Romshoo, S.A.; Rashid, I.; Altaf, S.; Dar, G.H. Jammu and Kashmir State: An Overview. In Biodiversity of the Himalaya: Jammu and Kashmir State. Topics in Biodiversity and Conservation; Dar, G., Khuroo, A., Eds.; Springer: Singapore, 2020; Volume 18, pp. 129–166. [Google Scholar]
- Hassan, K.; Yousuf, A.R.; Zargar, U.; Jamila, I.; Rehman, M. Distributional Pattern of Macro Invertebrates across River Jhelum in Kashmir Valley. Int. J. Fish. Aquat. Stud. 2014, 1, 113–120. [Google Scholar]
- Ahmed, I.; Ahmad, Z.; Ahmad, I. Current status of fish fauna of river jhelum and dal lake of Kashmir Valley. Bull. Pure Appl. Sci. Zool. 2017, 36, 85–92. [Google Scholar] [CrossRef]
- Dar, R.A.; Romshoo, S.A.; Chandra, R.; Ahmad, I. Tectono-geomorphic study of the Karewa Basin of Kashmir Valley. J. Asian Earth Sci. 2014, 92, 143–156. [Google Scholar] [CrossRef]
- La, V.T.; Cooke, S.J. Advancing the science and practice of fish kill investigations. Rev. Fish. Sci. 2011, 19, 21–33. [Google Scholar] [CrossRef]
- Roberts, S.D.; Van Ruth, P.D.; Wilkinson, C.; Bastianello, S.S.; Bansemer, M.S. Marine Heatwave, Harmful Algae Blooms and an Extensive Fish Kill Event During 2013 in South Australia. Front. Mar. Sci. 2019, 6, 610. [Google Scholar] [CrossRef]
- Padhi, S.K.; Tolo, I.; McEachran, M.; Primus, A.; Mor, S.K.; Phelps, N.B.D. Koi herpesvirus and carp oedema virus: Infections and coinfections during mortality events of wild common carp in the United States. J. Fish Dis. 2019, 42, 1609–1621. [Google Scholar] [CrossRef] [PubMed]
- Kilikidis, S.D.; Kamarianos, A.P.; Kousouris, T.; Tsingkounakis, I. Investigation on the cause of a fish-kill (Epinephelus) in the Kisamos Gulf, Crete. Bull. Environ. Contam. Toxicol. 1981, 26, 453–460. [Google Scholar] [CrossRef]
- Kane, A.S.; Dykstra, M.J.; Noga, E.J.; Reimschuessel, R.; Baya, A.; Driscoll, C.; Paerl, H.W.; Landsberg, J. Etiologies, observations and reporting of estuarine finfish lesions. Mar. Environ. Res. 2000, 50, 473–477. [Google Scholar] [CrossRef]
- Duff, K.E.; Laing, T.E.; Smol, J.P.; Lean, D.R.S. Limnological characteristics of lakes located across arctic treeline in northern Russia. Hydrobiologia 1998, 391, 205–222. [Google Scholar] [CrossRef]
- Supardi, E.; Yusuf, S.; Massi, M.N.; Haeruddin, H. Evaluation of different type of electrolyzed water against bacterial colonization of diabetic foot ulcers: Study in vitro. Med. Clin. Pract. 2020, 3, 100090. [Google Scholar] [CrossRef]
- Kabir, K.A.; Schrama, J.W.; Verreth, J.A.J.; Phillips, M.J.; Verdegem, M.C.J. Effect of dietary protein to energy ratio on performance of nile tilapia and food web enhancement in semi-intensive pond aquaculture. Aquaculture 2019, 499, 235–242. [Google Scholar] [CrossRef]
- DAK Advises Not to Consume Fish Washed Ashore in Jhelum River-Cross Town News, a Leading Newspaper of J&K. Available online: https://www.crosstownnews.in/post/19056/dak-advises-not-to-consume-fish-washed-ashore-in-jhelum-river.html (accessed on 7 May 2021).
- Rather, M.I.; Rashid, I.; Shahi, N.; Murtaza, K.O.; Hassan, K.; Yousuf, A.R.; Romshoo, S.A.; Shah, I.Y. Massive land system changes impact water quality of the Jhelum River in Kashmir Himalaya. Environ. Monit. Assess. 2016, 188, 185. [Google Scholar] [CrossRef]
- Rashid, I.; Majeed, U.; Jan, A.; Glasser, N.F. The January 2018 to September 2019 surge of Shisper Glacier, Pakistan, detected from remote sensing observations. Geomorphology 2020, 351, 106957. [Google Scholar] [CrossRef]
- Majeed, U.; Rashid, I.; Sattar, A.; Allen, S.; Stoffel, M.; Nüsser, M.; Schmidt, S. Recession of Gya Glacier and the 2014 glacial lake outburst flood in the Trans-Himalayan region of Ladakh, India. Sci. Total Environ. 2021, 756, 144008. [Google Scholar] [CrossRef] [PubMed]
- Rashid, I.; Majeed, U.; Najar, N.A.; Bhat, I.A. Retreat of Machoi Glacier, Kashmir Himalaya between 1972 and 2019 using remote sensing methods and field observations. Sci. Total Environ. 2021, 785, 147376. [Google Scholar] [CrossRef]
- Rashid, I.; Romshoo, S.A.; Amin, M.; Khanday, S.A.; Chauhan, P. Linking human-biophysical interactions with the trophic status of Dal Lake, Kashmir Himalaya, India. Limnologica 2017, 62, 84–96. [Google Scholar] [CrossRef]
- Rashid, I.; Aneaus, S. High-resolution earth observation data for assessing the impact of land system changes on wetland health in Kashmir Himalaya, India. Arab. J. Geosci. 2019, 12, 453. [Google Scholar] [CrossRef]
- Rashid, I.; Aneaus, S. Landscape transformation of an urban wetland in Kashmir Himalaya, India using high-resolution remote sensing data, geospatial modeling, and ground observations over the last 5 decades (1965–2018). Environ. Monit. Assess. 2020, 192, 635. [Google Scholar] [CrossRef]
- Dar, S.A.; Bhat, S.U.; Aneaus, S.; Rashid, I. A geospatial approach for limnological characterization of Nigeen Lake, Kashmir Himalaya. Environ. Monit. Assess. 2020, 192, 12. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).