Nafion Solvated by Ethylene Carbonate, Dimethyl Carbonate and Dimethylacetamide as Electrolyte for Lithium Metal Batteries †
Abstract
:1. Introduction
2. Materials and Methods
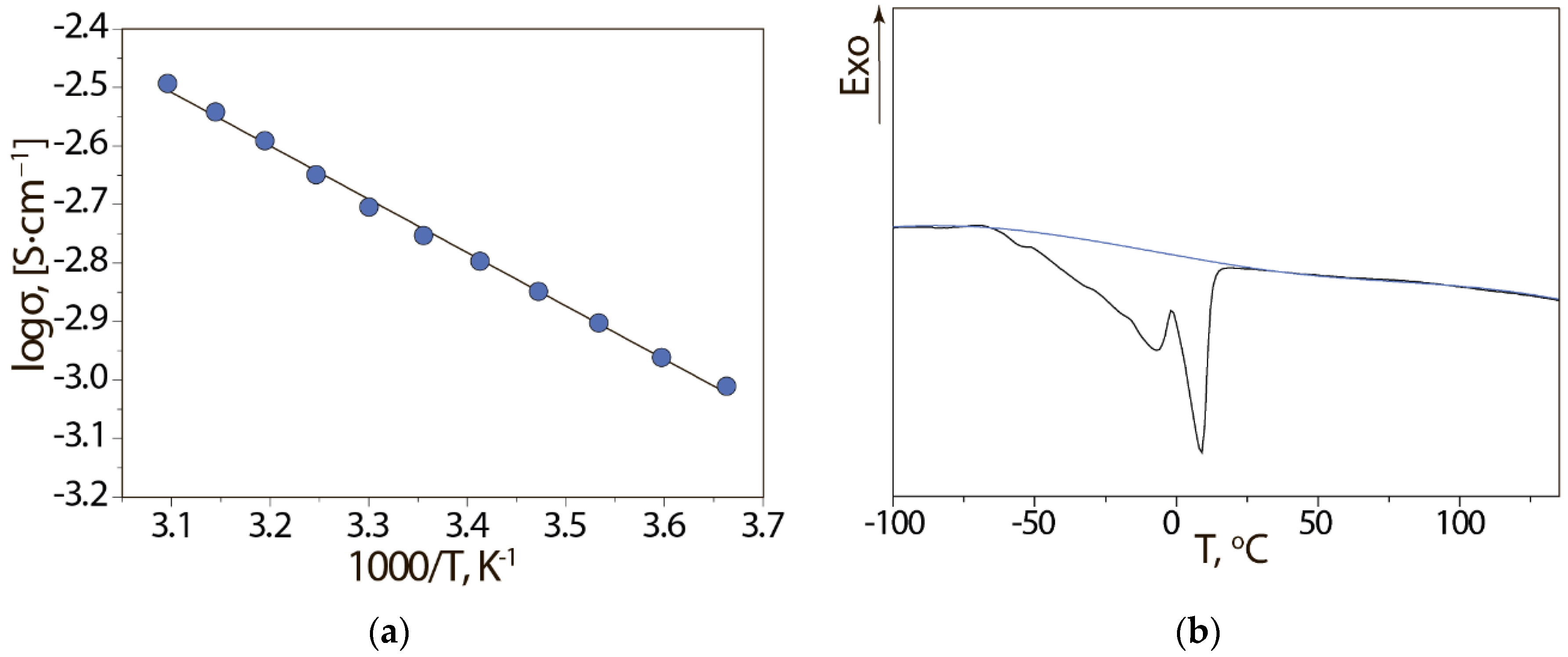
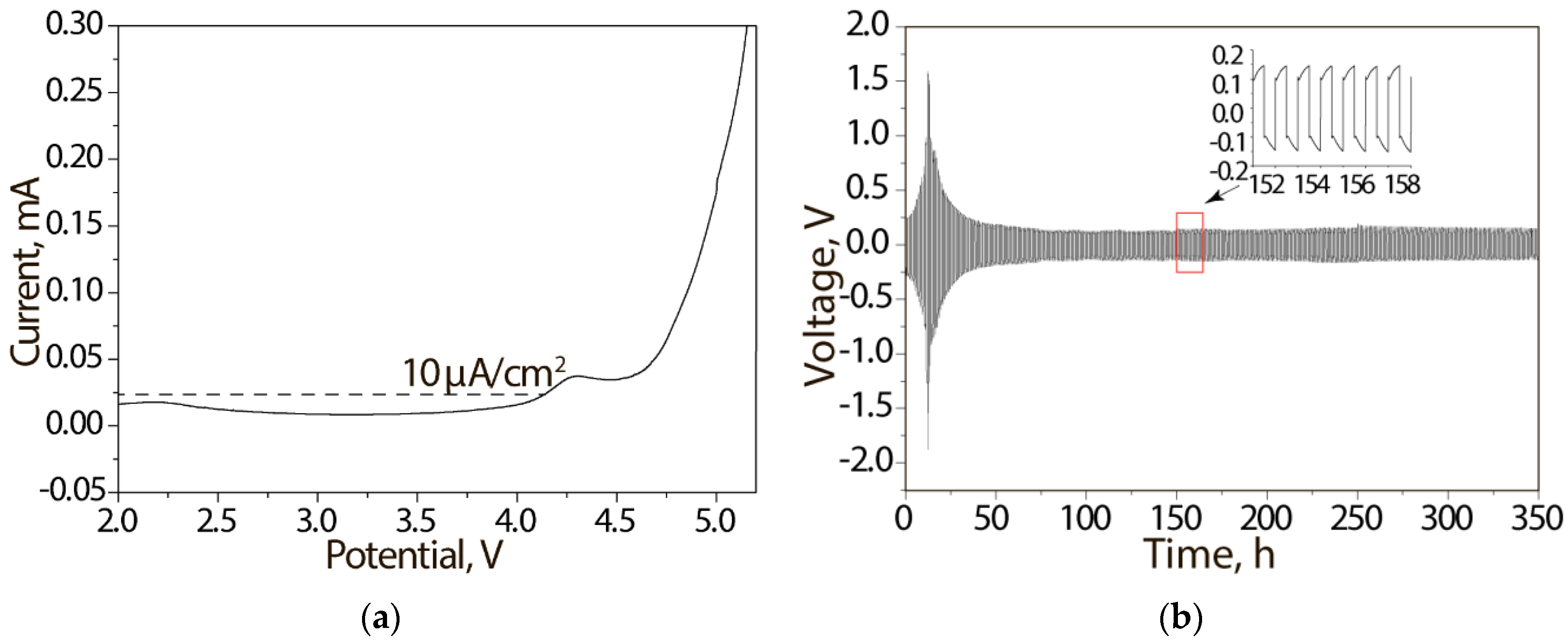
3. Results and Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Li, H.; Xu, Z.; Yang, J.; Wang, J.; Hirano, S. Polymer electrolytes for rechargeable lithium metal batteries. Sustain. Energy Fuels 2020, 4, 5469. [Google Scholar] [CrossRef]
- Rao, X.; Lou, Y.; Zhong, S.; Wang, L.; Li, B.; Xiao, Y.; Peng, W.; Zhong, X.; Huang, J. Strategies for Dendrite-Free lithium metal Anodes: A Mini-review. J. Electroanal. Chem. 2021, 897, 115499. [Google Scholar] [CrossRef]
- Tu, Z.; Choudhury, S.; Zachman, M.J.; Wei, S.; Zhang, K.; Kourkoutis, L.F.; Archer, L.A. Designing Artificial Solid-Electrolyte Interphases for Single-Ion and High-Efficiency Transport in Batteries. Joule 2017, 1, 394. [Google Scholar] [CrossRef] [Green Version]
- Istomina, A.S.; Yaroslavtseva, T.V.; Reznitskikh, O.G.; Kayumov, R.R.; Shmygleva, L.V.; Sanginov, E.A.; Dobrovolsky, Y.A.; Bushkova, O.V. Li-Nafion Membrane Plasticised with Ethylene Carbonate/Sulfolane: Influence of Mixing Temperature on the Physicochemical Properties. Polymers 2021, 13, 1150. [Google Scholar] [CrossRef] [PubMed]
- Doyle, M.; Lewittes, M.E.; Roelofs, M.G.; Perusich, S.A.; Lowrey, R.E. Relationship between ionic conductivity of perfluorinated ionomeric membranes and nonaqueous solvent properties. J. Memb. Sci. 2001, 184, 257. [Google Scholar] [CrossRef]
- Kulova, T.; Skundin, A.; Chekannikov, A.; Novikova, S.; Voropaeva, D.; Yaroslavtsev, A. Sodium rechargeable batteries with electrolytes based on Nafion membranes intercalated by mixtures of organic solvents. Batteries 2018, 4, 61. [Google Scholar] [CrossRef] [Green Version]
- Walker, W.; Giordani, V.; Uddin, J.; Bryantsev, V.S.; Chase, G.V.; Addison, D. A Rechargeable Li–O2 Battery Using a Lithium Nitrate/N,N-Dimethylacetamide Electrolyte. J. Am. Chem. Soc. 2013, 135, 2076. [Google Scholar] [CrossRef] [PubMed]
- Voropaeva, D.Y.; Novikova, S.A.; Yaroslavtsev, A.B. Polymer electrolytes for metal-ion batteries. Russ. Chem. Rev. 2020, 89, 1132. [Google Scholar] [CrossRef]
- Li, H.; Shen, X.; Hua, H.; Gao, J.; Wen, Z.; Wang, X.; Peng, L.; Wu, D.; Zhang, P.; Zhao, J. A novel single-ion conductor gel polymer electrolyte prepared by co-irradiation grafting and electrospinning process. Solid State Ion. 2020, 347, 115246. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Voropaeva, D.; Yaroslavtsev, A. Nafion Solvated by Ethylene Carbonate, Dimethyl Carbonate and Dimethylacetamide as Electrolyte for Lithium Metal Batteries. Eng. Proc. 2022, 19, 29. https://doi.org/10.3390/ECP2022-12667
Voropaeva D, Yaroslavtsev A. Nafion Solvated by Ethylene Carbonate, Dimethyl Carbonate and Dimethylacetamide as Electrolyte for Lithium Metal Batteries. Engineering Proceedings. 2022; 19(1):29. https://doi.org/10.3390/ECP2022-12667
Chicago/Turabian StyleVoropaeva, Daria, and Andrey Yaroslavtsev. 2022. "Nafion Solvated by Ethylene Carbonate, Dimethyl Carbonate and Dimethylacetamide as Electrolyte for Lithium Metal Batteries" Engineering Proceedings 19, no. 1: 29. https://doi.org/10.3390/ECP2022-12667
APA StyleVoropaeva, D., & Yaroslavtsev, A. (2022). Nafion Solvated by Ethylene Carbonate, Dimethyl Carbonate and Dimethylacetamide as Electrolyte for Lithium Metal Batteries. Engineering Proceedings, 19(1), 29. https://doi.org/10.3390/ECP2022-12667






