Solution-Processed Organic Light-Emitting Electrochemical Cells (OLECs) with Blue Colour Emission via Silver-Nanowires (AgNWs) as Cathode †
Abstract
:1. Introduction
2. Materials and Device Fabrication
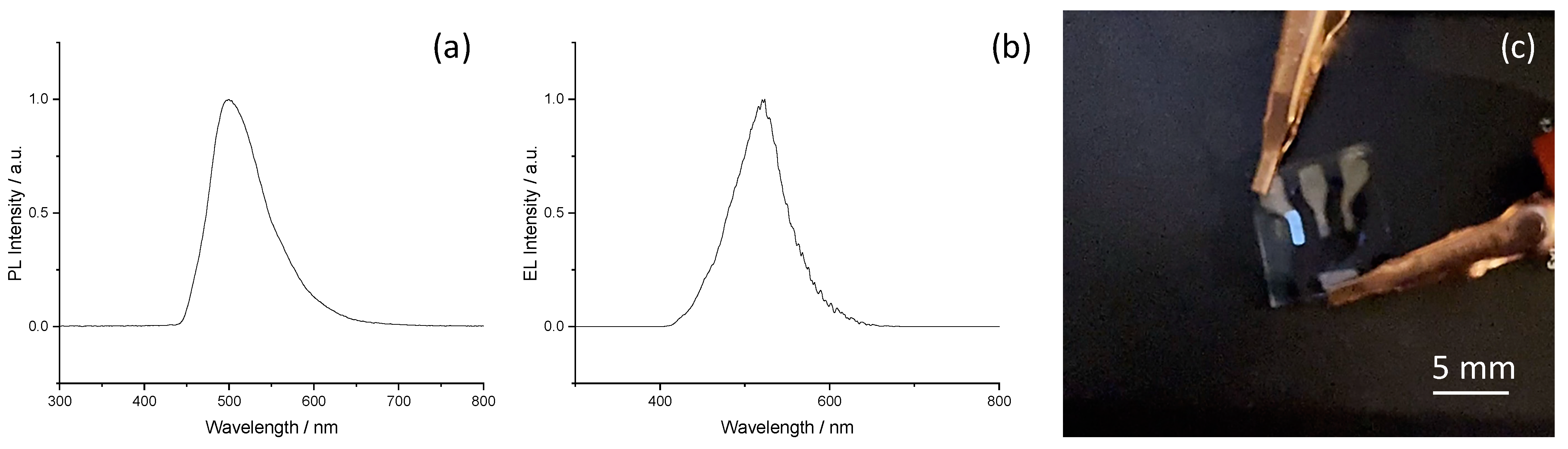
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Komolafe, A.; Zaghari, B.; Torah, R.; Weddell, A.S.; Khanbareh, H.; Tsikriteas, Z.M.; Vousden, M.; Wagih, M.; Jurado, U.T.; Shi, J.; et al. E-Textile Technology Review—From Materials to Application. IEEE Access 2021, 9, 97152–97179. [Google Scholar] [CrossRef]
- Cinquino, M.; Prontera, C.T.; Pugliese, M.; Giannuzzi, R.; Taurino, D.; Gigli, G.; Maiorano, V. Light-Emitting Textiles: Device Architectures, Working Principles, and Applications. Micromachines 2021, 12, 652. [Google Scholar] [CrossRef] [PubMed]
- Komolafe, A.; Torah, R.; Wei, Y.; Nunes-Matos, H.; Li, M.; Hardy, D.; Dias, T.; Tudor, M.; Beeby, S. Integrating Flexible Filament Circuits for E-Textile Applications. Adv. Mater. Technol. 2019, 4, 1900176. [Google Scholar] [CrossRef] [Green Version]
- Hardy, D.A.; Moneta, A.; Sakalyte, V.; Connolly, L.; Shahidi, A.; Hughes-Riley, T. Engineering a Costume for Performance Using Illuminated LED-Yarns. Fibers 2018, 6, 35. [Google Scholar] [CrossRef] [Green Version]
- Arumugam, S.; Li, Y.; Pearce, J.; Charlton, M.D.B.; Tudor, J.; Harrowven, D.; Beeby, S. Spray-Coated Organic Light-Emitting Electrochemical Cells Realized on a Standard Woven Polyester Cotton Textile. IEEE Trans. Electron. Devices 2021, 68, 1717–1722. [Google Scholar] [CrossRef]
- Pei, Q.; Yang, Y.; Yu, G.; Zhang, C.; Heeger, A.J. Polymer Light-Emitting Electrochemical Cells: In Situ Formation of a Light-Emitting p–n Junction. J. Am. Chem. Soc. 1996, 118, 3922–3929. [Google Scholar] [CrossRef] [PubMed]
- Tang, S.; Sandström, A.; Fang, J.; Edman, L. A Solution-Processed Trilayer Electrochemical Device: Localizing the Light Emission for Optimized Performance. J. Am. Chem. Soc. 2012, 134, 14050–14055. [Google Scholar] [CrossRef] [PubMed]
- Arumugam, S.; Li, Y.; Court, K.; Piana, G.; Charlton, M.; Tudor, J.; Harrowven, D.; Beeby, S. Solution processed blue light emitting electrochemical cells fabricated and encapsulated fully in ambient environment. In Proceedings of the 2021 IEEE International Conference on Flexible and Printable Sensors and Systems (FLEPS), Virtual, 20–23 June 2021; pp. 1–4. [Google Scholar]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Court, K.; Arumugam, S.; Li, Y.; Charlton, M.D.B.; Tudor, J.; Harrowven, D.; Beeby, S. Solution-Processed Organic Light-Emitting Electrochemical Cells (OLECs) with Blue Colour Emission via Silver-Nanowires (AgNWs) as Cathode. Eng. Proc. 2022, 15, 21. https://doi.org/10.3390/engproc2022015021
Court K, Arumugam S, Li Y, Charlton MDB, Tudor J, Harrowven D, Beeby S. Solution-Processed Organic Light-Emitting Electrochemical Cells (OLECs) with Blue Colour Emission via Silver-Nanowires (AgNWs) as Cathode. Engineering Proceedings. 2022; 15(1):21. https://doi.org/10.3390/engproc2022015021
Chicago/Turabian StyleCourt, Katie, Sasikumar Arumugam, Yi Li, Martin D. B. Charlton, John Tudor, David Harrowven, and Steve Beeby. 2022. "Solution-Processed Organic Light-Emitting Electrochemical Cells (OLECs) with Blue Colour Emission via Silver-Nanowires (AgNWs) as Cathode" Engineering Proceedings 15, no. 1: 21. https://doi.org/10.3390/engproc2022015021
APA StyleCourt, K., Arumugam, S., Li, Y., Charlton, M. D. B., Tudor, J., Harrowven, D., & Beeby, S. (2022). Solution-Processed Organic Light-Emitting Electrochemical Cells (OLECs) with Blue Colour Emission via Silver-Nanowires (AgNWs) as Cathode. Engineering Proceedings, 15(1), 21. https://doi.org/10.3390/engproc2022015021








