Abstract
In this study, an efficient and regioselective synthetic method was developed for the preparation of 3-hydroxy-3-((2-(naphthalen-2-yloxy)-2-oxoethoxy)carbonyl)pentanedioic acid, a multifunctional ether–ester compound of potential interest for pharmaceutical and material science applications. The target compound was synthesized via the nucleophilic substitution (SN2) and esterification reactions of 2-naphthyl chloroacetate with the monosodium salt of citric acid. Optimization of the reaction conditions was carried out by varying the molar ratio of the reagents, reaction temperature, and duration. The highest yield of 83% was achieved under the conditions of a 2:1 molar ratio of chloroacetate to citrate, a temperature of 70–80 °C, and a reaction time of 6 h. The enhanced product yield observed under these conditions is attributed to the dual reactivity of the citric acid monosodium salt, which contains a free hydroxyl group capable of undergoing SN2 etherification, and free carboxylic acid groups that participate in esterification with the electrophilic 2-naphthyl chloroacetate. The stoichiometric 2:1 ratio ensures that both reactive centers on the citrate anion are fully utilized, leading to efficient and selective transformation into the desired product. Mechanistically, the ether bond formation proceeds through the classical Williamson ether synthesis pathway, where the alkoxide formed from the hydroxyl group attacks the electrophilic carbon of the chloroacetate, displacing the chloride ion. Concurrently, esterification enhances molecular complexity and stability. The results underline the synthetic utility of citric acid derivatives in forming complex organic architectures via environmentally benign routes. This study not only contributes a practical approach to multifunctional molecule synthesis but also reinforces the applicability of green chemistry principles in ester–ether coupling strategies.
1. Introduction
Naphthol derivatives and their corresponding ethers, as well as carboxylic acid-based compounds, have attracted considerable attention in pharmaceutical research due to their notable antibacterial and analgesic activities. Moreover, molecules bearing reactive functional groups such as halogen, hydroxyl, and methylamino are actively explored as fungicidal agents in agriculture. Aromatic amines, on the other hand, find broad utility in industrial applications including dye manufacture, coatings, and petrochemical processes [1,2]. Chloroacetylation reactions of aromatic compounds play a critical role in the synthesis of this class of bioactive agents. Targeted studies are being actively conducted on the chloroacetylation of phenols, aromatic hydrocarbons, and heterocyclic compounds using chloroacetyl chloride. In particular, the development of effective synthetic mechanisms is essential for obtaining reagents, substrates, and catalysts for asymmetric synthesis, as well as for designing immunostimulatory agents and drug candidates with anti-diabetic, anticancer, and antibacterial properties. Broad research efforts are underway to synthesize highly effective phenol-derived compounds for applications in agriculture and medicine. These efforts are yielding competitive products based on natural raw materials and synthetic organic chemistry. In this context, chloroacetylation of aminophenol isomers, followed by nucleophilic substitution reactions of the resulting intermediates, and investigation of their structural features are of particular scientific interest, especially in the development of novel biologically active molecules.
Chloroacetylation of naphthols has been extensively studied in the field of organic chemistry. Notably, Knut Sommer investigated novel catalysts for chloroacetylation reactions [3], while Burkhard Matthes focused on the development of insecticidal agents based on the chloroacetylation of arene derivatives using chloroacetyl chloride [4]. Research conducted by Zainab Ramli and Firas Zayed has emphasized acyl compounds containing electron-donating groups in aromatic and heterocyclic rings [5,6]. Similarly, Ullastiina Hakala and V.F. Traven explored the promotion of organic synthesis reactions using ionic liquids and microwave-assisted techniques [7,8]. Michael Kraus investigated the synthesis of anthocyanidins via the chloroacetylation of phenols [9]. In Uzbekistan, several leading scientists have contributed to the field, including I.P. Tsukervanik and A.K. Abdushukurov, who examined the reactions of aromatic hydrocarbons and cresols with chloroacetyl chloride, ultimately obtaining novel compounds with fungicidal and herbicidal activities [10,11,12].
Our previous studies have also explored the chloroacetylation of various phenols with chloroacetyl chloride, followed by the structural modification of the resulting products [13,14,15,16,17,18,19]. These investigations have mainly focused on the regioselective O-chloroacetylation of phenolic compounds, providing a platform for further functionalization and biological evaluation.
The aim of this study was to investigate the nucleophilic substitution reaction of the product obtained from the chloroacetylation of 2-naphthol with citric acid, and to elucidate the structure of the resulting compound. The final product, identified as 2-naphthylcarboxymethylene citrate (3-hydroxy-3-((2-naphthalen-2-yloxy)-2-oxoethoxy)pentanedioic acid), was successfully applied as an analytical reagent for the determination of Fe(III) ions. The analytical application of this compound has been previously published [16]. In the present work, we focused on the synthetic route and characterization of 3-hydroxy-3-((2-naphthalen-2-yloxy)-2-oxoethoxy)pentanedioic acid in detail.
2. Experimental
The purity and identity of the synthesized compounds were evaluated by thin-layer chromatography (TLC) on Silufol-254 plates using a iso-octane:ethyl acetate (9:1) solvent system, with ultraviolet (UV) light as the visualization method. For the TLC stationary phase, silica gel-coated aluminum plates (silica gel 60 F254) bought from MERCK, India were utilized. Fourier transform infrared (FTIR) spectra were recorded in the 400–4000 cm−1 range using the KBr pellet method on a Specord IR-71 spectrophotometer (Carl Sies, Germany). The nuclear magnetic resonance (1H and 13C NMR) spectra were obtained on a Bruker 400 MHz NMR spectrometer (Germany), using tetramethylsilane (TMS) as an internal standard. Chemical shift values were reported in parts per million (ppm). The uncorrected melting points of the synthesized compounds were determined using the open capillary method on an Mvtec melting point apparatus.
Chloroacetylation of 2-Naphthol. In a round-bottom flask equipped with a reflux condenser and a gas outlet tube for hydrogen chloride, 0.1 mol of 2-naphthol was dissolved in 50 mL of dichloroethane. Then, 0.11 mol of chloroacetyl chloride was added, and the mixture was refluxed for 20 h. After the evolution of hydrogen chloride ceased, the solvent was removed under ambient conditions, and the residue was cooled to room temperature. The product yield was 95%. It is a dissoluble solid compound with a melting point of 39–41 °C. Rf = 0.64 (iso-octane:ethyl acetate = 9:1). IR spectrum (KBr, ν, cm−1): 3506 (Ar C–H), 3058–2944 (C=C–H), 2341 (CH2), 1741 (>C=O), 1456–1602 (C=C), 1272 (C–O–), 1224 (C–O–C), 734 (Cl–CH2), 840–758 (C–H), 1506 (Ar), 1061 (C–Cl). 1H NMR spectrum (CDCl3, δ, ppm): 3.04 s (–OCH2Cl), 4.33 s (2H, –COOCH2–), 7.21–7.85 m (7H, d, J = 1.04–3.04 Hz, Ar–H). 13C NMR spectrum (CDCl3, δ, ppm): 41.07, 77.16, 118.41, 120.47, 126.15, 126.90, 127.80, 129.78, 131.72, 133.69, 147.97, 166.22.
Reaction of Monosodium Citrate with O-Chloroacetylated Naphthol in the Presence of Catalytic Amounts of Dimethylformamide. In a 100 mL flat-bottom flask equipped with a magnetic stirrer, 0.015 mol of monosodium salt of citric acid and 0.09 mol of O-chloroacetylated naphthol were added. Then, 2.4 mL (0.03 mol) of dimethylformamide (DMF) was introduced. The mixture was stirred magnetically at 70–80 °C for 6 h. The molar ratio of the reactants was 1:2:6 (citric acid salt:naphthol derivative:DMF). During this time, the monosodium salt of citric acid completely dissolved. After completion of the reaction, the mixture was washed with benzene and filtered. The benzene filtrate was dried over sodium sulfate and then concentrated using a flask with a reflux condenser by distilling off the benzene at 78 °C. The final product was obtained with a 75% yield. The crude product was recrystallized from acetic acid (AcOH), yielding a purified compound with a melting point of 33–35 °C. Rf = 0.53 (iso-octane:ethyl acetate = 9:1). IR spectrum (ν, cm−1): 3355.83 (O–H), 947.33, 860.17, 808.26, 740.31 (C=C–H), 2960.37 and 2873.18 (CH2), 1743.59 (>C=O), 1585.97–1420.05 (C=C), 1290.91, 1230.74, 1190.78, 1154.62 (C–O), 1290–1060 (C–O–C). 1H NMR spectrum (δ, ppm): 2.852, 2.854, 2.856, 7.149, 7.183, 7.268, 7.367, 7.610, 7.679, 7.708, 7.971. 13C NMR spectrum (δ, ppm): 31.906, 36.968, 59.775, 77.160, 109.519, 118.250, 123.285, 126.341, 127.752, 128.693, 129.652, 134.802, 154.244, 163.535.
3. Reaction Results and Discussion of Results
Chloroacetyl chloride (ClCH2COCl) is a highly electrophilic and reactive compound widely employed as an efficient alkylating—more precisely, acylating—agent toward O-, N-, and S-nucleophiles. Its pronounced reactivity arises from the presence of two functional moieties: the carbonyl chloride group (–COCl), which strongly polarizes the carbonyl carbon and enhances electrophilicity, and the –CH2Cl fragment, which can participate in secondary SN2-type transformations. Together, these features render the reagent remarkably active in nucleophilic substitution and acylation processes.
Chloroacetyl chloride readily reacts with phenols, alcohols, amines, and thiols, forming the corresponding O-, N-, and S-chloroacetylated derivatives. One of its most characteristic transformations is the O-chloroacetylation of phenolic substrates, which typically proceeds rapidly and efficiently, often without the need for catalysts. In reactions with amines, it affords N-chloroacetylated amides, whereas thiols yield their S-chloroacetylated analogues. Due to the reactivity of the –CH2Cl group, additional alkylation pathways may also occur under suitable conditions.
The choice of solvent influences the reagent’s behavior: polar aprotic solvents such as dichloromethane or DMF tend to accelerate the reaction, while aqueous or alcoholic media promote rapid hydrolysis, reflecting the intrinsic electrophilicity of the molecule. Overall, chloroacetyl chloride is regarded as a potent bifunctional alkylating/acylating reagent and is frequently utilized in organic synthesis, particularly in the chloroacetylation of phenolic compounds under catalyst-free conditions.
The influence of solvents on the course of the O-chloroacetylation reaction of 2-naphthol was investigated. The reagents were used in equimolar amounts, and the reactions were carried out at the boiling points of the respective solvents [19,20]. The reactions proceed according to the following equation:
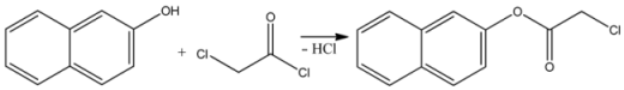
The mechanism of this reaction was proposed as follows: during the reaction between the –OH group of the 2-naphthol molecule and chloroacetyl chloride, the electron density in the chloroacetyl chloride molecule shifts toward the more electronegative oxygen atom, making the oxygen partially negatively charged. Due to the electronegative effects of both the chlorine and oxygen atoms, the carbon atom becomes partially positively charged and interacts with the lone electron pair of the hydroxyl group in the 2-naphthol molecule to form an intermediate complex. As the reaction proceeds, a covalent bond forms between the oxygen and carbon atoms, resulting in the formation of a new complex, from which the reaction product is released along with hydrogen chloride [21,22]. The resulting reaction product was identified as naphthalen-2-yl 2-chloroacetate and was analyzed using IR and NMR spectroscopy. The IR spectrum confirms that the synthesized compound is indeed naphthalen-2-yl 2-chloroacetate. The positions and intensities of the observed functional groups corresponded well with the proposed structure of the compound (Table 1).

Table 1.
FTIR Spectral Analysis of Naphthalen-2-yl 2-chloroacetate.
1H NMR (400 MHz, CDCl3) spectrum revealed the following chemical shifts and integrations: multiple signals in the range of δ 7.85–7.21 ppm correspond to seven aromatic protons, indicating the presence of a naphthalene ring; a singlet at δ 4.32 ppm is attributed to the methylene protons (–CH2–O–Ar) bonded to oxygen; another singlet at δ 3.04 ppm corresponds to the methylene protons adjacent to chlorine (–CH2–Cl); and a residual solvent signal at δ 7.26 ppm was assigned to CDCl3. The chemical shifts, signal intensities, and splitting patterns are in complete agreement with the proposed structure of the compound.
13C NMR (100 MHz, CDCl3) spectrum exhibited the following carbon signals: δ 166.22 ppm is assigned to the ester carbonyl carbon (C=O); δ 147.97 ppm corresponds to an aromatic carbon adjacent to the phenolic oxygen, appearing downfield due to deshielding effects; multiple signals in the range of δ 118.41–133.69 ppm are attributed to the aromatic carbons of the naphthalene core; δ 77.16 ppm corresponds to the methylene carbon bonded to oxygen (–CH2–O–Ar); and δ 41.07 ppm is attributed to the methylene carbon bonded to chlorine (–CH2–Cl). These spectroscopic data are fully consistent with the proposed molecular structure and confirm the successful synthesis of naphthalen-2-yl 2-chloroacetate.
O-chloroacetyl derivatives were synthesized via the reaction of 2-naphthol with chloroacetyl chloride, and in order to investigate their potential biological activity, nucleophilic substitution reactions were carried out between the O-chloroacetyl products and the mono-substituted sodium salt of citric acid.
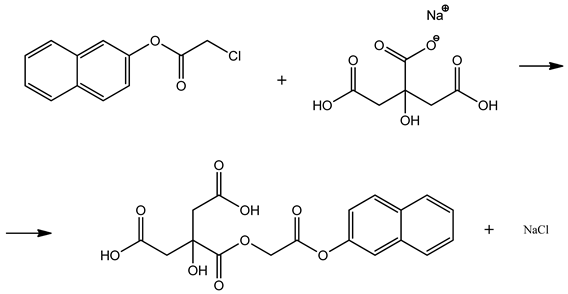
The most suitable method for performing this reaction was identified. The nucleophilic substitution reactions were carried out in various solvents, including ethanol, DMF, and acetone. In order to improve the product yield and determine optimal reaction conditions, the reaction of naphthalen-2-yl 2-chloroacetate with sodium citrate was specifically conducted in dimethylformamide (DMF) as a solvent. To evaluate the effects of reaction time and temperature on the product yield, the reactions were performed at different temperatures for 1 and 2 h, respectively. The obtained results are summarized in Table 2.

Table 2.
Effect of Temperature on the Yield of 3-hydroxy-3-((2-(naphthalen-2-yloxy)-2-oxoethoxy)carbonyl)pentanedioic acid.
To further improve the yield of the reaction product, a series of experiments were conducted by varying the molar ratios of monochloroacetic acid 2-naphthyl ester and sodium citrate. These reactions were carried out at a constant temperature of 70–80 °C in a water bath for 2 h under different reagent ratios. The obtained results are summarized in Table 3.

Table 3.
Effect of the molar ratios of reagents on the yield of 3-hydroxy-3-((2-(naphthalen-2-yloxy)-2-oxoethoxy)carbonyl)pentanedioic acid.
When the reaction was carried out at a reagent molar ratio of 2:1 and a temperature of 70–80 °C, the product yield reached 83%. As can be seen from the table data, increasing the molar ratio of the starting materials and elevating the temperature had a positive effect on the reaction yield. This trend is also confirmed by the results presented in Table 4.

Table 4.
Effect of Reaction Time on the Yield of 3-hydroxy-3-((2-(naphthalen-2-yloxy)-2-oxoethoxy)carbonyl)pentanedioic acid.
In reactions conducted for 2, 3, 4, 5, and 6 h using a 2:1 molar ratio of monochloroacetic acid 2-naphthyl ester and sodium citrate, the product yields were 62%, 75%, 78%, 80%, and 83%, respectively.
In the synthesis of 3-hydroxy-3-((2-(naphthalen-2-yloxy)-2-oxoethoxy)carbonyl)pentanedioic acid, the 2:1 molar ratio between 2-naphthyl chloroacetate and the monosodium salt of citric acid proved to be optimal, with the highest yield of the target product observed under these conditions.
To rationalize this result theoretically, several key factors must be considered. Firstly, the monosodium salt of citric acid contains one carboxyl group neutralized by sodium, while the remaining two carboxylic acid groups and the single alcoholic (–OH) group remain unreacted. Among these, the –OH group acts as a strong nucleophile and undergoes an SN2 substitution reaction with 2-naphthyl chloroacetate, resulting in the formation of an ether bond. This process follows the classical Williamson ether synthesis mechanism [23,24,25,26].
In addition, one of the free carboxyl groups of citric acid can also react with the electrophilic carbon in 2-naphthyl chloroacetate via esterification. Therefore, a single citrate molecule possesses two distinct reactive sites: one alcoholic (–OH) group capable of ether formation and one carboxylic acid (–COOH) group capable of ester formation. Consequently, two molecules of 2-naphthyl chloroacetate are required per one molecule of citric acid salt, which is crucial for maximizing the reaction yield.
When the reaction was carried out at a 2:1 molar ratio, the reactivity of the carboxyl group coordinated with sodium was also enhanced. Moreover, the presence of excess electrophile (i.e., 2-naphthyl chloroacetate) facilitates the complete progression of the reaction, ensuring the full formation of the desired product while minimizing by-products. As is typical in SN2 mechanisms, the nucleophilic center (alkoxide ion) directly attacks the electrophilic center bearing a good leaving group (chloroacetate).
In conclusion, the 2:1 molar ratio between 2-naphthyl chloroacetate and the monosodium salt of citric acid is both stoichiometrically and mechanistically justified for achieving a high-yield reaction. The reaction proceeds via the Williamson ether synthesis mechanism, resulting in high selectivity and efficiency under the optimized conditions.
The IR spectrum (KBr, cm−1) displayed a broad absorption band at 3355.83 cm−1, characteristic of the O–H stretching vibration from the hydroxyl and carboxylic acid groups. The absorption peaks at 2960.37 and 2873.18 cm−1 correspond to aliphatic C–H stretching vibrations. A strong band at 1743.59 cm−1 indicates the presence of ester carbonyl (C=O) groups, while a second carbonyl stretch at 1660.71 cm−1 is attributable to the carboxylic acid function. Aromatic C=C stretching vibrations were observed in the 1585.97–1420.05 cm−1 range. The bands in the 1290–1060 cm−1 region support the presence of C–O–C (ether and ester) linkages. Additional fingerprint peaks were observed below 1000 cm−1, confirming the aromatic substitution pattern.
The 1H NMR spectrum revealed multiple signals in both the aliphatic and aromatic regions. The triplet signals at 2.852, 2.854, and 2.856 ppm corresponded to the methylene protons (–CH2–) adjacent to carbonyl and hydroxyl-bearing centers. The aromatic region displayed multiplets in the range of 7.149–7.971 ppm, which are characteristic of a naphthalene ring system, consistent with the substitution at the 2-position by an ether-linked ester moiety. The multiplicity and chemical shift values are in agreement with a disubstituted aromatic system containing an electron-donating –O– linker and electron-withdrawing ester groups.
The 13C NMR spectrum further corroborated the proposed structure, showing distinct signals for aliphatic, aromatic, and carbonyl carbons. Aliphatic sp3 carbons appeared at 31.906, 36.968, 59.775, and 77.160 ppm, attributed to CH2–COOH, CH2–C(OH), CH2–O–C=O, and the quaternary C(OH) center, respectively. Aromatic carbons were distributed over 109.519 to 134.802 ppm, in agreement with a substituted naphthalene moiety. Downfield signals at 154.244 and 163.535 ppm corresponded to aromatic carbon bonded to oxygen (Ar–O) and ester/carboxylic acid carbonyl carbons (C=O), respectively.
4. Conclusions
In this study, the synthesis of 3-hydroxy-3-((2-(naphthalen-2-yloxy)-2-oxoethoxy)carbonyl)pentanedioic acid was successfully achieved through the reaction of 2-naphthyl chloroacetate with the monosodium salt of citric acid under mild conditions. The influence of various reaction parameters, including molar ratios, temperature, and reaction time, was systematically investigated. It was found that a 2:1 molar ratio of 2-naphthyl chloroacetate to sodium citrate, combined with a reaction temperature of 70–80 °C and a duration of 6 h, afforded the highest yield of 83%.
Mechanistic considerations revealed that the monosodium citrate serves as a bifunctional nucleophile, possessing both a free hydroxyl and a carboxyl group capable of undergoing SN2 and esterification reactions, respectively. This dual reactivity is best accommodated by two equivalents of the electrophilic 2-naphthyl chloroacetate, enabling efficient formation of both ether and ester bonds in a single synthetic step. The reaction follows the classical Williamson ether synthesis mechanism, demonstrating high chemoselectivity and efficiency.
Overall, this approach offers a simple, selective, and high-yielding method for the construction of multifunctional ether–ester derivatives. The optimized conditions and mechanistic insights provided herein can serve as a foundation for the further development of green and atom-economical synthetic strategies involving polyfunctional acids and active esters. These findings contribute valuable knowledge to the field of organic synthesis and could facilitate the design of novel bioactive compounds or advanced intermediates for pharmaceutical and materials science applications.
Author Contributions
Conceptualization, A.C., I.N. and R.J.; writing—original draft preparation, R.J. and A.A.; visualization, R.J. and A.A.; writing—review and editing, R.J. and A.A.; supervision, A.C. and A.A. contributed equally to this paper. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available on request from the corresponding author.
Acknowledgments
The authors wish to acknowledge Karshi State Technical University and Karshi State University, Karshi, Uzbekistan, the Ministry of Higher and Secondary Specialized Education of the Republic of Uzbekistan, and the Ministry of Innovative Development of the Republic of Uzbekistan, Tashkent, Uzbekistan.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Berdimurodov, E.; Berdimuradov, K.; Eliboev, I.; Yusufov, M.; Yodgorov, C. Polyphenols for Dietary Polyphenols Application. In Science and Engineering of Polyphenols Fundamentals and Industrial Scale Applications; Wiley: Hoboken, NJ, USA, 2024; Chapter 14; pp. 385–401. [Google Scholar] [CrossRef]
- Tyman, J.H.P. Synthetic and Natural Phenols; Elsevier: New York, NY, USA, 1996. [Google Scholar]
- Reetz, M.T.; Sommer, K. Gold-Catalyzed Hydroarylation of Alkynes. Eur. J. Org. Chem. 2003, 2003, 3485–3496. [Google Scholar] [CrossRef]
- Mark Gordons Quantum Theory Group, Ames Laboratory, Iowa State University. 2000. Available online: https://www.msg.ameslab.gov (accessed on 21 June 2025).
- Nur, H.; Prasetyoko, D.; Ramli, Z.; Endud, S. Sulfation: A simple method to enhance the catalytic activity of TS-1 in epoxidation of 1-octene with aqueous hydrogen peroxide. Catal. Commun. 2004, 5, 725–728. [Google Scholar] [CrossRef][Green Version]
- Zayed, F.; Greiner, L.; Schulz, P.S.; Lapkin, A.; Leitner, W. Continuous catalytic Friedel-Crafts acylation in the biphasic medium of an ionic liquid and supercritical carbon dioxide. Chem. Commun. 2008, 79–81. [Google Scholar] [CrossRef] [PubMed]
- Hakala, U.; Wähälä, K. Microwave-promoted synthesis of polyhydroxydeoxybenzoins in ionic liquids. Tetrahedron Lett. 2006, 47, 8375–8378. [Google Scholar] [CrossRef]
- Kravtchenko, D.V.; Chibisova, T.A.; Traven, V.F. Unusual Fries rearrangement of 7-acyloxyquinolin-2-ones A new way to linear and angular furoquinolin-2-ones. Zhurnal Org. Khimii 1999, 35, 924. [Google Scholar]
- Kahle, K.; Kraus, M.; Richling, E. Polyphenol profiles of apple juices. Mol. Nutr. Food Res. 2005, 49, 797–806. [Google Scholar] [CrossRef]
- Mamatkulov, N.N.; Abdushukurov, A.K.; Khidirov, S.; Rakhmonova, S. Synthesis and Rearrangement of p-Tolyl Chloroacetate. Russ. J. Org. Chem. 2001, 37, 1668–1669. [Google Scholar] [CrossRef]
- Abdullaev, M.G.; Klyev, M.V. Kinetics of the Production of p-Acetaminophenol and p-Hydroxyphenylsalicylamide by Reductive Acylation of p-Nitrophenol on Palladium-Containing Anionites. Pharm. Chem. J. 2014, 47, 610–611. [Google Scholar] [CrossRef]
- Nurmukhammadov, Z.S.; Smanova, Z.A.; Tadzhimukhamedov, K.S.; Inatova, M.S. Synthesis and properties of a new analytical reagent, 2-hydroxy-3- nitrosonaphthalene-1-carbaldehyde. Russ. J. Org. Chem. 2014, 50, 895–897. [Google Scholar] [CrossRef]
- Sattorovich, R.J.; Uralivich, A.C.; Ravshan ugli, B.E. Synthesis of Hexamethylenetetramine Mono- and Di(P-Methoxyphenylacetochloride). Eng. Proc. 2024, 67, 53. [Google Scholar] [CrossRef]
- Choriev, A.U.; Jurayev, R.S.; Abdushukurov, A.K.; Abdullayev, M.G. Synthesis of 2-Izopropyl-5-methylphenylcarboxymethylen Tartrate. Eng. Proc. 2023, 37, 57. [Google Scholar]
- Jurayev, R.S.; Choriev, A.U.; Qaxxorov, N.T. Effect and Spectroscopic Analysis of Solutions in Trychloratsetylpyrogallol Synthesis. Chem. Proc. 2023, 14, 80. [Google Scholar] [CrossRef]
- Jurayev, R.S.; Choriev, A.U.; Qaxxorov, N.T. The Photometric Determination of Iron(III) with 2-Napthylcarboxymethylene Citrate. Eng. Proc. 2023, 48, 49. [Google Scholar] [CrossRef]
- Jurayev, R.S. Synthesis of 4,4′,4″-(((Benzene-1,2,3-triyltris(oxy))tris(2-oxoethane-2,1-diyl))tris(oxy)) tris(2,3-dihydroxy-4-oxobutanoic Acid) and 4,4′,4″-(((Benzene-1,2,4-triyltris(oxy))tris(2-oxoethane-2,1-diyl))tris(oxy))tris(2,3-dihydroxy-4-oxobutanoic Acid). Eng. Proc. 2024, 67, 75. [Google Scholar] [CrossRef]
- Sadikova, S.; Abdushukurov, A.; Choriyev, A.; Takhirov, Y. Nucleophilic substitution reaction of dichloroacetyl hydroquinone with sodium salts of oxyacids. Int. J. Pharm. Res. 2020, 12, 648–653. [Google Scholar] [CrossRef]
- Pasha, M.A.; Reddy, M.B.M.; Manjula, K. Zinc Dust: An Extremely Active and Reusable Catalyst in Acy- lation of Phenols, Thiophenol, Amines and Alcohols in a Solvent-Free System. Eur. J. Chem. 2010, 1, 385–387. [Google Scholar] [CrossRef]
- Gupta, R.; Kumar, V.; Gupta, M.; Paul, S.; Gupta, R. Silica Supported Zinc Chloride Acetylation of Amines, Alcohols and Phenols. Indian J. Chem. 2008, 47B, 1739–1743. [Google Scholar]
- Li, J.J. A Collection of Detailed Mechanisms and Synthetic Applications; Springer: Berlin/Heidelberg, Germany, 2009; 704p. [Google Scholar]
- Smith, M.B.; March, J. Advanced Organic Chemistry: Reactions, Mechanisms and Structure; Wiley: New York, NY, USA, 2013; 1200p. [Google Scholar]
- Ouellette, R.J. Organic Chemistry||Carboxylic Acids; Academic Press: Cambridge, MA, USA, 2018; pp. 625–663. [Google Scholar] [CrossRef]
- Ouellette, R.J. Organic Chemistry Study Guide||Ethers and Epoxides; Academic Press: Cambridge, MA, USA, 2015; pp. 277–297. [Google Scholar] [CrossRef]
- Ouellette, R.J. Organic Chemistry||Ethers and Epoxides; Academic Press: Cambridge, MA, USA, 2018; pp. 507–536. [Google Scholar] [CrossRef]
- Kunz, H. Comprehensive Organic Synthesis||Protecting Groups; Pergamon Press: Oxford, UK, 1991; pp. 631–701. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
