Abstract
Cashew nut shell liquid (CNSL) is a byproduct of cashew processing that has largely been overlooked as a biomass resource for biodiesel production. While some research has been conducted on CNSL in diesel engines, there remains a lack of studies on using processed CNSL with industrial waste catalysts for diesel engines. This study focuses on the performance and emissions of catalytically cracked CNSL (CC-CNSL) created with fly ash as a catalyst. Blends of 25%, 50%, 75%, and 100% CC-CNSL-diesel were used as a fuel in a single-cylinder diesel engine under different load conditions. The CC-CNSL25 blend, which contains 25% CC-CNSL, outperformed the others with a 2% increase in brake thermal efficiency. Additionally, it showed substantial reductions in emissions, i.e., 11.76% less carbon monoxide, 9.09% reduced smoke density, 8.57% lower hydrocarbon emissions, and 5.27% decreased specific fuel consumption compared to conventional diesel at full load. This research highlights fly ash-catalyzed CNSL processing as an effective method for converting agricultural waste into high-quality biodiesel. It offers a dual advantage as a sustainable fuel source while addressing waste management challenges.
1. Introduction
Emissions from fossil fuels produced by motor vehicles harm the environment and contribute significantly to air pollution. The use of fossil fuels is the root cause of several major environmental issues [1]. Crude oil plays a critical role in the transportation industry. One-fourth of the world’s current oil consumption comes from this sector, and roughly two-thirds of the world’s oil reserves are located in regions that heavily depend on it. The majority of this oil is imported, with the Persian Gulf serving as the primary source of crude oil from other regions. However, continued reliance on fossil fuels is unsustainable due to their decreasing availability and the unreliability of global supply chains. Oil is a finite resource that will eventually be depleted. While it takes millions of years for new oil reserves to form, the world’s current known reserves are expected to last only about 40 more years. Once these resources are exhausted, alternative fuels will be required to meet global energy demands [2]. Furthermore, most of the world’s fossil fuel reserves are located in regions with unstable political systems. This makes our energy supply heavily dependent on sources that are both politically and environmentally unsustainable. The solution lies in the use of non-conventional forms of fuel. Most alternative fuels are renewable, meaning that they are virtually limitless in supply and can be produced sustainably without the risk of depletion. Moreover, many of these fuels can be produced domestically, reducing the need to rely on foreign fuel sources.
Biodiesel is composed of a long chain of chemical compounds such as methyl, ethyl, and propyl esters [3]. Biodiesel represents a revolutionary step forward in helping many nations reduce their dependence on conventional diesel fuel. It is produced by chemically processing vegetable oil or animal fat with alcohols, resulting in a clean-burning fuel. One of biodiesel’s key advantages is its compatibility with most diesel engines, requiring little to no modification to existing engines or equipment. In essence, biodiesel is a domestically produced, environmentally friendly fuel. It can be made from used vegetable oils, animal fats, or reclaimed grease from buildings and restaurants. Biodiesel has a lower toxicity level and emits fewer harmful byproducts than traditional diesel [4]. It can be used in its pure form (B100) or blended with diesel. Because it is made from organic materials such as food crops rather than fossil fuels, biodiesel offers a more sustainable and regenerative alternative to petroleum-based fuels. This makes it a promising long-term substitute, especially if other viable alternatives are not yet developed.
The liquid extracted from cashew nut shells has shown strong potential as an alternative to fossil fuels, owing to its numerous advantages. However, a key challenge lies in the decreasing availability of agricultural land, driven by rapid urban development. This issue is compounded by the fact that over 82% of farmers are classified as “small and marginal,” owning less than 2 hectares of land, which makes large-scale crop production increasingly difficult. Research on cashew nut shell liquid (CNSL) biodiesel shown in Table 1 highlights its promise as a sustainable alternative to conventional diesel, although further improvements in efficiency and emissions are still needed. Dual-fuel operation using CNSL biodiesel and hydrogen has demonstrated increased brake thermal efficiency (BTE), along with reductions in carbon monoxide (CO), hydrocarbons (HCs), and carbon dioxide (CO2), despite a slight increase in nitrogen oxide (NOx) emissions. Blending CNSL biodiesel with butanol has been found to enhance atomization and combustion quality, leading to improved BTE and significant reductions in CO and HC emissions. Similarly, the use of hexanol as an additive further reduced CO and HC emissions, decreased fuel consumption, and slightly improved engine efficiency, enabling its use in unmodified diesel engines. The combination of a B20 blend with ethanol and hydrogen yielded the most notable performance improvement, with BTE reaching nearly 38% compared to standard diesel, while also reducing CO and HC emissions though again with a rise in NOx levels. Regarding fuel properties, low-percentage CNSL blends such as B10 were able to meet diesel standards without preheating. However, higher blends like B20 and B30 required either preheating or stabilization using an acetone–butanol–ethanol mixture [5]. These findings suggest that, under appropriate conditions and with the use of additives or blended fuels, CNSL biodiesel can enhance engine performance while reducing emissions, making it a promising pathway for sustainable transportation fuel.

Table 1.
Studies on cashew nut shell liquid biodiesel.
This study specifically focuses on the bioenergy potential of cashew nut shells as a transportation fuel through catalytic cracking of CNSL, using fly ash as a catalyst. The goal is to evaluate the emission and performance characteristics of this biofuel when used in a single-cylinder diesel engine. Notably, the application of catalytically cracked CNSL with fly ash has not previously been explored in diesel engine settings. In this investigation, CNSL was blended with diesel fuel at varying proportions of 25%, 50%, 75%, and 100%, aiming to identify optimal performance levels in diesel engines. Therefore, this research assesses both the emission characteristics and engine performance when fueled with catalytically cracked cashew nut shell liquid biodiesel, offering insight into its viability as a sustainable fuel alternative.
2. Test Fuel
Native to India, which also has the world’s largest cashew production and processing capability, the cashew tree is scientifically known as Anacardium occidentale. India uses around 868,000 ha for cashew production, and the nation produces 665,000 tons of cashews per year, with an average output of 860 kg per hectare. About 1,138,000 metric tons of raw cashew nut seeds were processed at 3650 cashew processing mills located throughout the different states of India. Cashews in India were processed using a variety of unit operations and approaches based on the kind of raw material being used, the mill’s location, the amount of technological mechanization, and the reliability of the energy supply [5].
Drying raw seed in the open sun, steaming raw nuts, and drying kernels with electrical energy are the unit operations that need the most time and energy in the processing of cashews. In small-scale cashew nut processing mills, steaming the raw cashew nut seed is a common step before the cutting and shelling processes. One of the most typical processing steps is this one. When cashew nuts are processed via a mill, the shells account for around 67% of the raw cashew nut seeds’ total weight because of the cutting and shelling steps involved. In the processing of cashews, it was noticed that the amount of energy required to create the same number of goods of comparable quality varied greatly. These cashew mills have large differences in the amount of energy that they use, which reveals that there is room for energy saving in the range of 30 to 48% [9]. During the extraction process, the anacordic acid that is found in the CNSL is subjected to the decarboxylation procedure so that it may be converted into cardanol. In addition to cardanol, it also contains cardol and other polymeric material, both of which may be eliminated by distillation of CNSL or pyrolysis, respectively. Remember that the CNSL, after being handled in a number of various ways, will display a wide range of measurable physical and thermal properties. This is because every strategy makes its own special promise.
3. Methodology
When pulverized coal is burned in a coal-fired boiler, it produces a fine, powdery particulate substance known as fly ash, which is released into the air. This material is carried away by the flue gas and is typically removed using electrostatic precipitators, large collection chambers, or mechanical devices such as cyclones. Most fly ash particles are spherical and range in size from 0.5 to 100 μm. These particles solidify while still in motion within the exhaust gases. Fly ash consists largely of glassy particles that contain a variety of crystalline phases, including quartz, mullite, and various iron oxides. The primary component of fly ash is silicon dioxide (SiO2), which exists in two forms: an amorphous form that is soft and pliable and a crystalline form that is hard and potentially hazardous. Other key components include aluminum oxide (Al2O3) and iron oxide (Fe2O3). In electric utility power generation, coal-fired boiler furnaces are typically classified into three types: dry-bottom boilers, wet-bottom boilers, and cyclone furnaces. The most common of these is the dry-bottom boiler. When pulverized coal is burned in this type of boiler, around 80% of the total ash is released as fly ash and carried away with the flue gas. In contrast, when pulverized coal is burned in a wet-bottom furnace also known as a slag-tap furnace up to 50% of the ash remains in the furnace as molten slag, while the rest is removed with the flue gas as fly ash. In cyclone furnaces, where crushed coal is used instead of pulverized coal, much of the ash is fused into boiler slag, and only 20–30% is emitted as dry fly ash in the flue gas [10].
Role of Fly Ash as a Catalyst
The catalytic activity of supported catalysts is governed by the active component of the catalyst and the interaction between the support and the active component. Supported catalysts are widely used in heterogeneous catalysis [11]. Metal oxides such as Al2O3, SiO2, TiO2, and MgO are commonly used as catalyst supports. Al2O3 and SiO2 make up the bulk of fly ash’s chemical composition. The high combustion temperatures these compounds undergo have enhanced their thermal stability, making them ideal for use as catalyst supports. Over the last few decades, researchers have found that fly ash-supported catalysts are more active in a wide variety of processes, including CO2 reforming of methane, ammonia decomposition, catalytic reduction of NOx and SOx, methane oxidation, dye decomposition, and petroleum hydrocracking [12].
The fly ash, in its original form, was passed through a sieve with a mesh size of 100 to remove any larger or foreign particles before being mechanically crushed into a fine powder in a ball mill. It was found that the particle size distribution ranged from 40 to 90 µm. To activate the fly ash, it was placed in a silica crucible and heated to a temperature between 900 °C and 1000 °C for one hour before being used in the study. The fly ash produced after the removal of carbon, sulfur, and other impurities through the process of thermal activation is referred to as activated fly ash. The activated fly ash was then mixed with a 40% sodium hydroxide solution. After the slurry was prepared, it was placed in a ball mill and held in a furnace at a temperature of 500 °C for four hours. Table 2 displays the percentage of elements present in raw fly ash.

Table 2.
Elements present in raw fly ash.
4. Experimental Details
4.1. Catalytic Cracking of CNSL
A catalytic reaction is the process by which one organic component is transformed into another by the application of heat in the presence of a catalyst. In sharp contrast to the heat-only thermal cracking method. When the cashew nut is cracked open, the liquid within undergoes a thermal breakdown, producing heavy oxygen-containing compounds, the bulk of which are monobasic fatty acids. When these materials are subjected to decarboxylation, CO, CO2, water (H2O), and a hydrocarbon residue are formed. These compounds, which were first produced by the thermal breakdown of triglycerides, are then further transformed by the influence of the catalyst.
The liquid that is extracted from the cashew nut shells is added to the fly ash, and the mixture is stirred quickly until a slurry is formed. The reactor then receives this slurry. The mixture was then heated to a temperature of 450 to 500 °C with the aid of an electrical coil. Using an electrical controller might make it simpler to keep the room at the ideal temperature. When the temperature hits this degree, the process starts, and the fly ash serves as a catalyst to get things going. Finally, the oil vapors leave the reactor and are condensed in a water-cooled condenser. The process of condensing gases into liquids requires a special appliance known as a condenser. The transformation from gas to liquid marks the beginning of the change in phase. After the hydrocarbon fuel has been created, it is finally cooled in a fuel tank that is kept separate.
For the experimental investigation, four blends of catalytically cracked cashew nut shell liquid (CC-CNSL) with diesel were prepared: CC-CNSL25 (25% CC-CNSL + 75% diesel), CC-CNSL50 (50% CC-CNSL + 50% diesel), CC-CNSL75 (75% CC-CNSL + 25% diesel), and CC-CNSL100 (100% CC-CNSL). The required volumes of CC-CNSL and diesel were measured in graduated mixing vessels according to the respective volumetric ratios, and each blend was homogenized using ultrasonic treatment to ensure uniform mixing and stability. To achieve uniform mixing and long-term stability of CC-CNSL-diesel blends, an ultrasonic bath set to 40 kHz was used for 30 min, at room temperature, to create cavitation bubbles that facilitate very powerful localized mixing and disrupt any potential phase separation that can occur between the biodiesel and the conventional diesel. During the sonication time, the cavitation reduces the surface tension separating the two fuel components by creating micro-emulsions and enabling molecular level mixing which entirely eliminates stratification and ensures consistent fuel properties throughout the blend. The cavitation effects produced by sonication aid in achieving better dispersion of the biodiesel’s oxygenated molecules among the diesel network and, therefore, alter the fuel’s atomization characteristics and overall combustion behavior during operation of the engine. The blended fuels were put in airtight containers after ultrasonication and were subjected to stability testing for phase separation after every twenty-four hours for a week, with no visible separation appearing in any of the CC-CNSL blends up to 50% concentration and high volume of the CC-CNSL fuels. The ultrasonic mixing process was also used to help unify any density and viscosity variation between CC-CNSL and diesel and assure stable fuel injection characteristics (i.e., viscosity), without injector clogging issues in regard to fuel property variation under long periods of engine operation.
4.2. Engine Specifications
The test engine is a stationary diesel engine with a single cylinder. This type of engine has a variety of uses, including power generation in agricultural and industrial settings. An eddy current dynamometer is employed to provide varying loads to the engine by adjusting the current flowing through the dynamometer. To maintain a constant 1500 rpm, the position of the fuel pump rack must be adjusted. A mechanical injector with three holes and a pump-nozzle injection system, featuring a 300 µm nozzle hole size, has been used for the experiment. The fuel has a pressure tolerance of 200–300 bar. The combustion chamber is a 2.5 cm deep hemispherical bowl that utilizes a piston-style combustion chamber. Figure 1 shows the engine layout.
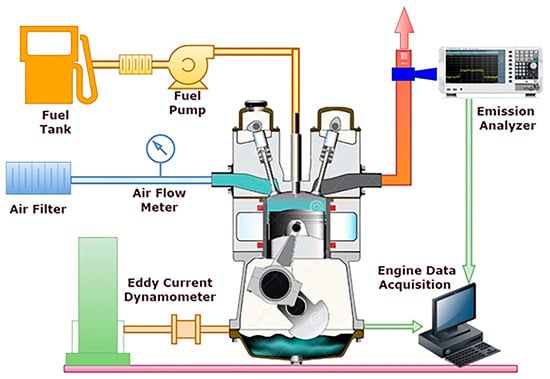
Figure 1.
Test Engine layout.
The fuel flow rate was monitored manually using a burette and a stopwatch, while the intake air flow rate was measured manually with an orifice meter. These two gauges are integrated into the system for use. An AVL pressure transducer was installed to measure and record the pressure within the combustion chamber. The rate at which heat is lost is calculated by analyzing the recorded pressure signals. An AVL 444 Digas analyzer was used to estimate the exhaust emissions of HC, CO, NOₓ and O2 (oxygen). Table 3 shows the experiments instruments details and uncertainty.

Table 3.
Experiments instruments details and uncertainty.
4.3. Engine Test Procedure
First, the engine is run for 30 min on diesel to bring it to a stable state. Afterward, different blends CC-CNSL are tested. This is performed before the engine undergoes testing. During this phase, the engine warms up to its operating temperature with the help of the coolant and lubricating oil temperatures. Once the engine is operational, all of the test fuels are used, and the tests are carried out under the appropriate environmental conditions. After each experiment with a blend, the entire quantity of fuel in the fuel lines is completely drained. Following this, the next blend is prepared for use in the experiment. To increase the reliability of the results, each measurement was conducted five times at regular intervals, and the average of these readings was used in the subsequent computations and analyses. The reproducibility of the measurements was sound, and the results fell within the acceptable range. The required injection pressure was then set by adjusting a screw located on top of the injector assembly. This was performed after verifying the desired pressure using a mechanical pressure gauge system. The injection pressure was set to 235 bar during the investigation to enhance the atomization process of the blends. Similar procedures were followed to monitor engine performance and emissions, and the data was analyzed.
5. Results and Discussion
The analysis of BTE with respect to the brake power (BP) for different blends is shown in Figure 2. The BTEs are very close to those of diesel fuel because the calorific values of all blends are comparable to those of diesel fuel. Additionally, the presence of a higher quantity of oxygen, particularly in CNSL25, resulting in more complete combustion and lower heat losses during the expansion stroke and has led to an 2% improvement in combustion compared to pure diesel. Catalytic cracking breaks down long-chain fatty acids into shorter hydrocarbons with improved volatility, leading to better fuel-air mixing and higher flame propagation rates [13]. As noted previously, the presence of oxygenates in CC-CNSL enables complete oxidation of carbon atoms, providing greater energy release per unit of fuel. The improved cetane number reduces the ignition delay of CC-CNSL25, allowing for more control over combustion timing and sufficiently high pressure rise rates.
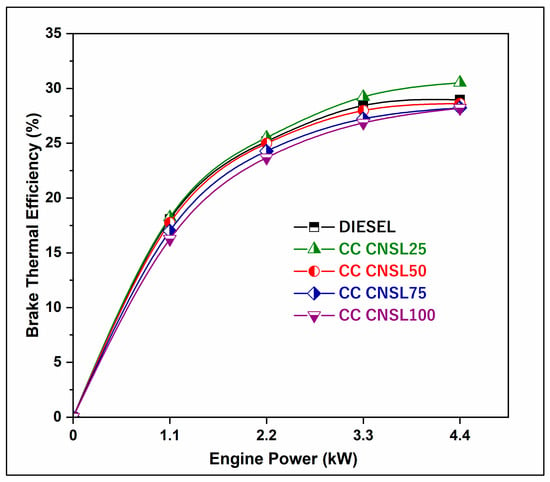
Figure 2.
BP vs. BTE.
Figure 3 shows the variation in brake specific energy consumption (SEC) between different blends. The relationship can be interpreted in terms of load. It was found that as the percentage of biodiesel in the blends increases, the variance in specific energy consumption with load decreases considerably for all fuels. Since a smaller fraction of heat is lost under higher loads, the percentage increase in fuel required to drive the engine is less than the percentage increase in braking power [14]. The reduction in SEC of 5.27% for CC-CNSL25 is mainly due to an improvement in combustion efficiency from the natural oxygen content in biodiesel molecules. This results in a lower stoichiometric air-fuel ratio. Additionally, catalytic cracking makes the fuel more volatile and improves fuel atomization, leading to better fuel droplet distribution and minimizing wall wetting effects in the combustion chamber. The hydrocarbon chains in CC-CNSL25 are shorter and evaporate faster compared to FC-CNSL25, mixing better with air, which enhances fuel utilization and produces more power output.
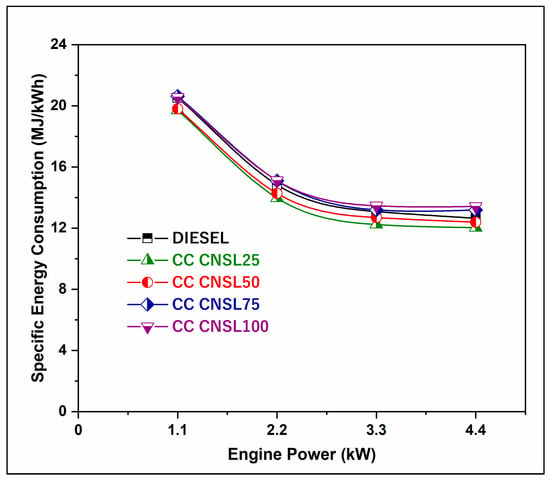
Figure 3.
BP vs. BSEC.
As combustion completeness improves with CC-CNSL25, energy losses from unburned fuel particles are reduced, improving the overall fuel economy of the engine. The variation in CO emissions among fuel blends as a function of engine load is shown in Figure 4. The volume of CO released was between 0.02% and 0.05% that of diesel. The higher degree of complete oxidation experienced by biodiesel blends, compared to diesel, may account for their lower CO emissions. There may have been less CO produced because part of the CO generated during biodiesel combustion was converted to CO2 by combining with an additional oxygen molecule already present in the biodiesel chain [15]. From the figure, it can be deduced that CO initially increased with load, but then began to decrease again until it reached full load, at which point it started to dramatically increase again. The 11.76% decrease in CO emissions for CC-CNSL25 can be explained by the increased availability of oxygen in the biodiesel molecular structure, which allows for the oxidation of CO to CO2 during combustion.
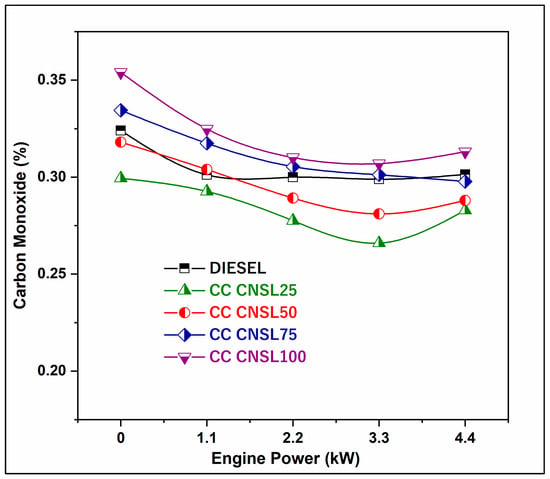
Figure 4.
BP vs. CO emission.
This is further aided by the improved fuel atomization and evaporation characteristics of the catalytically cracked CNSL fuel, which enables better mixing of the fuel and air, preventing fuel-rich regions where incomplete combustion may occur. Oxygen atoms are also chemically bonded to the hydrocarbon chains, making oxygen available to regions nearly devoid of air and preventing CO generation due to incomplete oxidation. Additionally, the availability of extra oxygen increases the flame temperature when using oxygenated fuels, which results in the conversion of any initial CO to CO2 through secondary oxidation reactions [16].
The amount of smoke produced by an engine running on different blends and diesel is shown in Figure 5 as a function of engine load. It is clear from this graph that the B25 blend generates far less smoke than diesel alone. Under full load conditions, the smoke density produced by the B20 biodiesel mix is 71 HSU. The smoke is a result of incomplete combustion. In general, biodiesel produces less smoke than conventional diesel. This may be because the biodiesel chain contains an oxygen molecule, which promotes more thorough combustion than conventional diesel. The 9.09% reduction in smoke density for CC-CNSL25 is primarily due to the improved combustion completeness associated with the inherent oxygen content in biodiesel molecules, which helps combust and oxidize soot while forming carbon particulates. Catalytic cracking produces lighter hydrocarbon fractions with improved volatility, minimizing fuel pyrolysis and maximizing the amount of fuel combusted at elevated temperatures by reducing the formation and aggregation of carbon particles [17]. The oxygen atoms in the biodiesel molecular structure provide local oxidizing species that convert nascent soot particles to CO and CO2 before they can agglomerate into larger particulates.
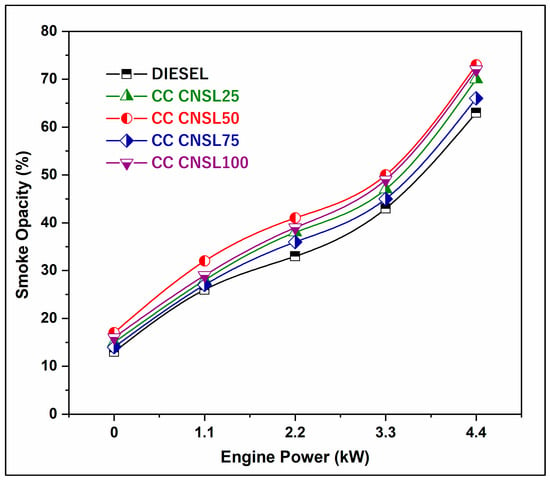
Figure 5.
BP vs. Smoke Density.
The superior mixing and atomizing properties of CC-CNSL are also expected to provide more homogeneous combustion, thus preventing fuel-rich zones where soot formation typically occurs due to limited oxygen [18].
The graph presents a comparison of the changes in hydrocarbon (HC) emissions that occur with varying engine load for different fuel blends. Increasing the load results in a commensurate increase in HC emissions, as shown in Figure 6. At 75% of the engine’s rated capacity, the HC emissions for both the B25 and B50 blends are within acceptable ranges. The higher the mix fraction, the larger the increase in HC emissions at high engine loads compared to operation with diesel fuel. Because biodiesel has poor atomization and is less volatile than conventional diesel, some of the fuel droplets may not burn completely, and the fuel does not have enough time to undergo full combustion. As a result, there is an increase in HC emissions when the load is increased. For CC-CNSL25, the 8.57% reduction in HC emissions is attributed to the improved volatility and evaporation characteristics resulting from catalytic cracking, which reduces fuel film formation on cylinder walls and in crevice volumes. Biodiesel’s higher oxygen content allows for more complete combustion of hydrocarbon chains, reducing the amount of unburned fuel molecules escaping through the exhaust during the exhaust stroke [19]. Catalytic cracking produces lighter hydrocarbon fractions with lower boiling points, which, once vaporized, increase the likelihood of preventing incomplete combustion in fuel-rich regions. The small rise in HC emissions with higher percentage blends suggests that excessive biodiesel may create over-rich mixtures and quenching effects due to biodiesel’s higher viscosity and different combustion chemistry.
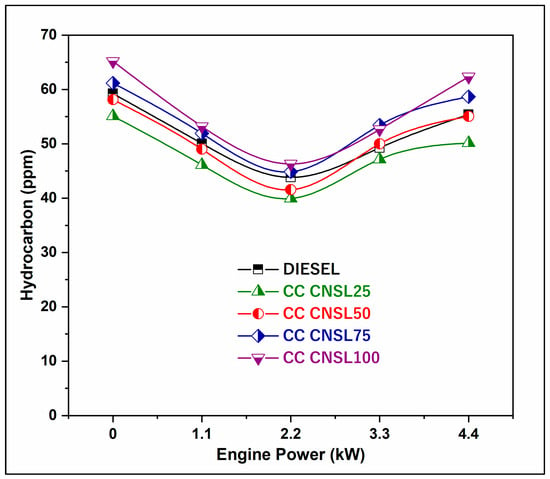
Figure 6.
BP vs. HC Emission.
The values of NOx in the exhaust emissions from different CNSL blends with diesel and pure diesel are displayed in Figure 7. This data clearly shows that the NOx emissions from the blends with a smaller amount of biodiesel were much higher than those from pure diesel. The higher exhaust gas temperatures observed support this finding. The main reason for this is that as the load increases, both power production and fuel consumption rise. This increase is due to biodiesel blends containing higher levels of oxygen, which leads to increased oxidation when exposed to high temperatures. The higher NOx emissions from higher CC-CNSL blend ratios occur because higher heat release rates, or combustion temperatures, arise from the oxygen-rich fuel and improved combustion efficiencies. The increased oxygen available in the fatty compounds intensifies the temperature and the oxidation of atmospheric N2 above 1500–1800 K, predominantly during the peak combustion period. The increase in heat release rates and combustion efficiency inherent in using oxygenated fuels also creates thermodynamic conditions that favor thermal NOx formation through the Zeldovich mechanism. The prolonged residence time of hot combustion products with higher nitrogen concentrations in an oxygen-rich environment encourages both thermal and prompt NOx formation, which aligns with the substantial increases in NOx emissions observed as CC-CNSL blends increased.
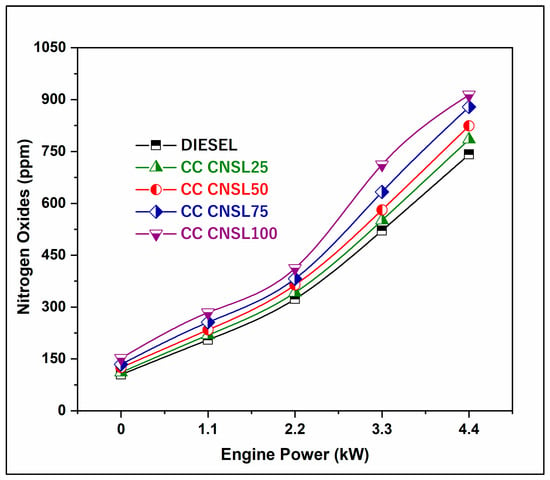
Figure 7.
Brake Power vs. NOx.
6. Conclusions
The experimental findings indicate that cashew nut shell liquid (CNSL) produced through catalytic cracking (CC-CNSL) may be a suitable alternative to diesel fuel. Among the blends tested, CC-CNSL25 yielded the most favorable performance. At full load, the BTE of CC-CNSL25 was the same as that of diesel fuel, with a 2% improvement, while the specific fuel consumption (SFC) was reduced by 5.27%. The emission characteristics were also promising: smoke density decreased by 9.09%, CO emissions were reduced by 11.76%, and HC emissions were reduced by 8.57%, relative to pure diesel. While higher blends such as CC-CNSL100 produced more oxygen and, in turn, increased NOx formation, CC-CNSL25 consistently demonstrated favorable combustion behavior. The implementation of CC-CNSL offers a cleaner energy alternative by converting waste industrial by-products into biofuels, addressing both the utilization of industrial waste and the dependence on fossil fuels. This contributes directly to SDG 7 and SDG 12 and indirectly to SDG 13 by providing a low-emission, sustainable biofuel. By creating new pathways for local biofuel processing, it advances SDG 8 and also indirectly supports SDG 9. Overall, the results of this study suggest that, based on both performance and environmental attributes, CC-CNSL at a 25% blend should be a viable candidate for emerging sustainable diesel engine applications. Further research should focus on catalyst formulations to reduce NOx emissions further and explore engine durability and fuel stability with prolonged use of CC-CNSL blends.
Author Contributions
Conceptualization, S.J.; Methodology, M.M.; Software, S.C.V.R.M.N.; Validation, C.S.R.; Formal analysis, N.P.; Investigation, S.P.; Resources, N.K.; Data curation, A.S.Y.; Supervision, A.S. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The original contributions presented in this study are included in the article. Further inquiries can be directed to the corresponding authors.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Sharma, A.; Murugan, S. Potential for using a tyre pyrolysis oil-biodiesel blend in a diesel engine at different compression ratios. Energy Convers. Manag. 2015, 93, 289–297. [Google Scholar] [CrossRef]
- Sharma, A.; Murugan, S. Investigation on the behaviour of a DI diesel engine fueled with Jatropha Methyl Ester (JME) and Tyre Pyrolysis Oil (TPO) blends. Fuel 2013, 108, 699–708. [Google Scholar] [CrossRef]
- Xue, J.; Grift, T.E.; Hansen, A.C. Effect of biodiesel on engine performances and emissions. Renew. Sustain. Energy Rev. 2011, 15, 1098–1116. [Google Scholar] [CrossRef]
- Sharma, A.; Murugan, S. Effect of blending waste tyre derived fuel on oxidation stability of biodiesel and performance and emission studies of a diesel engine. Appl. Therm. Eng. 2017, 118, 365–374. [Google Scholar] [CrossRef]
- Thanigaivelan, V.; Loganathan, M.; Vikneswaran, M.; Venkatramanan, S.; Manickam, M. Effect of hydrogen and ethanol addition in cashew nut shell liquid biodiesel operated direct injection (DI) diesel engine. Int. J. Hydrogen Energy 2022, 47, 5111–5129. [Google Scholar] [CrossRef]
- Lionus Leo, G.M.; Jayabal, R.; Kathapillai, A.; Sekar, S. Performance and emissions optimization of a dual-fuel diesel engine powered by cashew nut shell oil biodiesel/hydrogen gas using response surface methodology. Fuel 2025, 384, 133960. [Google Scholar] [CrossRef]
- Duraisamy, S.; Giri, J.; Rao, T.S.S.B.; Kanan, M.; Manimaran, R. Optimization of cashew nut shell biodiesel production with industrial waste catalysts and butanol additives for ecofriendly CRDI engine applications. Sci. Rep. 2025, 15, 9573. [Google Scholar] [CrossRef] [PubMed]
- Pandian, A.K.; Munuswamy, D.B.; Radhakrishanan, S.; Devarajan, Y.; Ramakrishnan, R.B.B.; Nagappan, B. Emission and performance analysis of a diesel engine burning cashew nut shell oil bio diesel mixed with hexanol. Pet Sci. 2018, 15, 176–184. [Google Scholar] [CrossRef]
- Adekanbi, M.L.; Olugasa, T.T. Utilizing cashew nut shell liquid for the sustainable production of biodiesel: A comprehensive review. Clean. Chem. Eng. 2022, 4, 100085. [Google Scholar] [CrossRef]
- Biswal, T.; Shadangi, K.P.; Sarangi, P.K. Fly ash derived catalyst for biodiesel production. In Biorefinery Production of Fuels and Platform Chemicals; Wiley: Hoboken, NJ, USA, 2023; pp. 203–232. [Google Scholar]
- Navia, R.; Pecchi, G.; Azócar, L. Fly ash as a new versatile acid-base catalyst for biodiesel production. Renew. Energy 1931, 162, 2020. [Google Scholar]
- Malonda Shabani, J.; Babajide, O.; Oyekola, O.; Petrik, L. Synthesis of hydroxy sodalite from coal fly ash for biodiesel production from waste-derived maggot oil. Catalysts 2019, 9, 1052. [Google Scholar] [CrossRef]
- Gwoda, S.; Valette, J.; dit Sidibé, S.S.; Piriou, B.; Blin, J.; Ouédraogo, I.W.K. Use of cashew nut shell liquid as biofuel blended in diesel: Optimisation of blends using additive acetone–butanol–ethanol (ABE (361)). Clean. Chem. Eng. 2024, 9, 100117. [Google Scholar] [CrossRef]
- Kumar, S.S.; Rajan, K.; Mohanavel, V.; Ravichandran, M.; Rajendran, P.; Rashedi, A.; Sharma, A.; Khan, S.A.; Afzal, A. Combustion, Performance, and Emission Behaviors of Biodiesel Fueled Diesel Engine with the Impact of Alumina Nanoparticle as an Additive. Sustainability 2021, 13, 12103. [Google Scholar] [CrossRef]
- Sharma, A.; Murugan, S. Effect of nozzle opening pressure on the behaviour of a diesel engine running with non-petroleum fuel. Energy 2017, 127, 236–246. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhong, Y.; Lu, S.; Zhang, Z.; Tan, D. A comprehensive review of the properties, performance, combustion, and emissions of the diesel engine fueled with different generations of biodiesel. Processes 2022, 10, 1178. [Google Scholar] [CrossRef]
- Sharma, A.; Dhakal, B. Performance and emission studies of a diesel engine using biodiesel tyre pyrolysis oil blends. SAE Tech. Pap. 2013, 2, 1–7. [Google Scholar] [CrossRef]
- Karthick, M.; Logesh, K.; Baskar, S.; Sharma, A. Performance and Emission Characteristics of Single-Cylinder Diesel Engine Fueled with Biodiesel Derived from Cashew Nut Shell. In Advancement in Materials, Manufacturing and Energy Engineering, Volume II; Springer: Berlin/Heidelberg, Germany, 2022; pp. 521–529. [Google Scholar]
- Logesh, K.; Karthick, M.; Baskar, S.; Sharma, A. Performance and Emissions Characteristics of Diesel Engine Run on Citrullus Colocynthis Biodiesel with Zinc Oxide Additive. In Advancement in Materials, Manufacturing and Energy Engineering, Volume II; Springer: Berlin/Heidelberg, Germany, 2022; pp. 513–520. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).