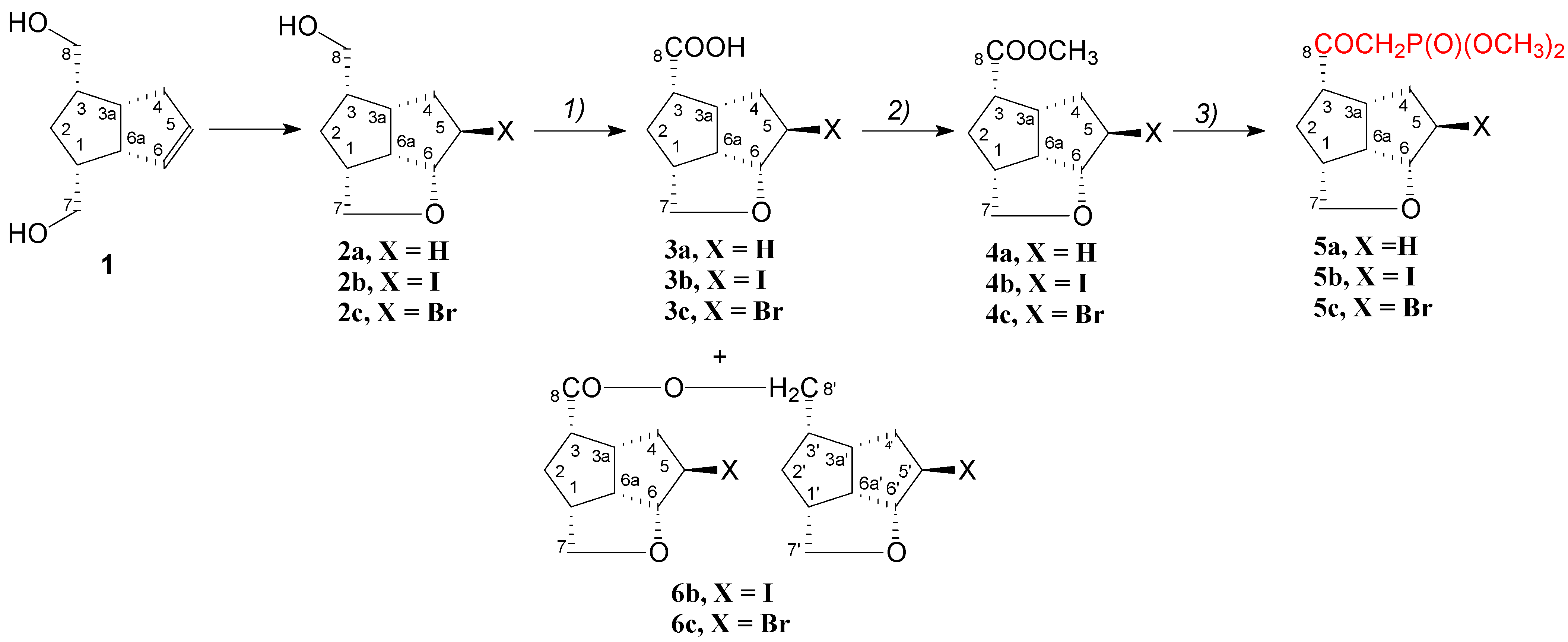1. Introduction
The inactivation of prostaglandin (PG) and prostaglandin analogs (PGs) is realized with enzyme oxidation of the 15α-OH to the 15-keto group via the 15-PGDH pathway. To slow down this oxidation, some structural modifications were made: the introduction of a 15-methyl group, a 16-OH,16-methyl group, two methyl groups at C16, cyclopentyl and cyclohexyl scaffolds, etc [1]. In this direction, we previously introduced bicyclo[3.3.0]octene or bicylo[3.3.0]octane fragments in β-ketophosphonates [2,3] to obtain the PG analogs I and II (Figure 1), knowing that these fragments are encountered in natural products, some of them with anticancer activity.
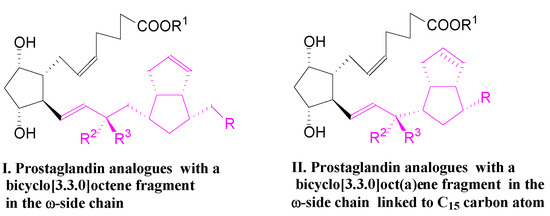
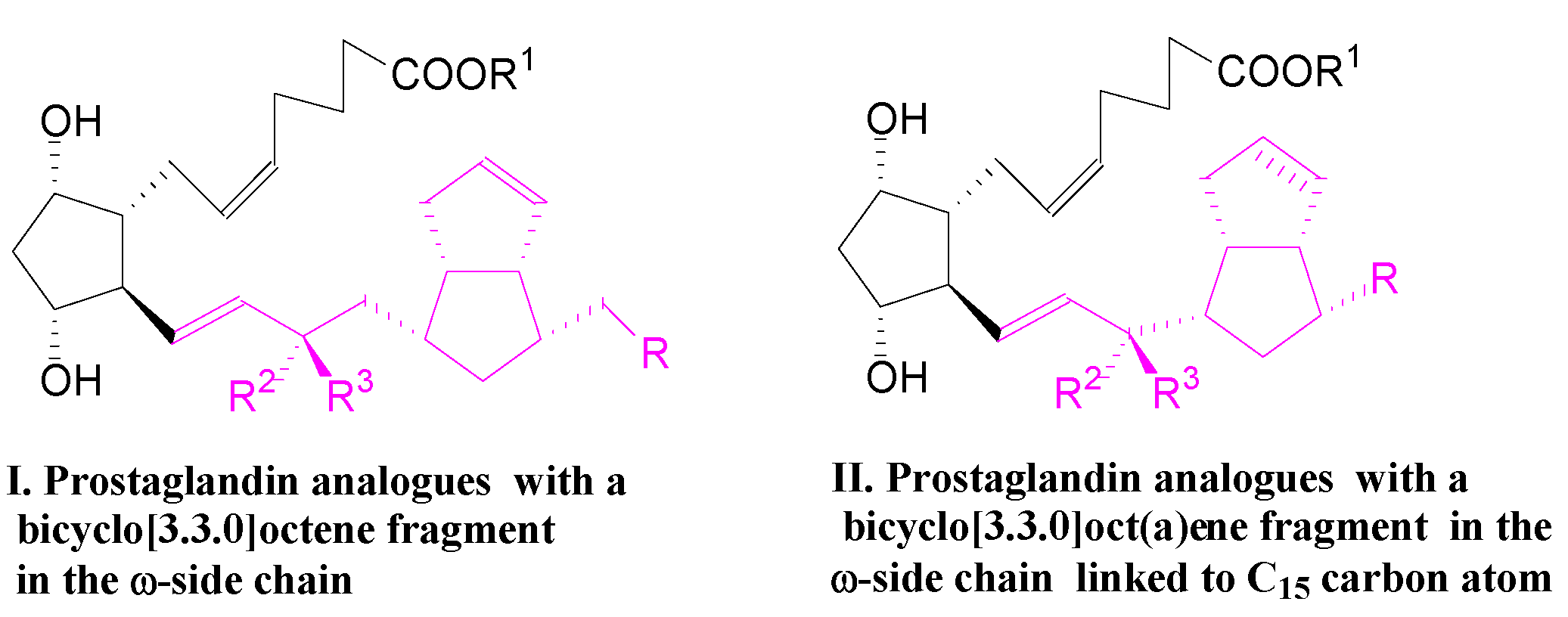
Figure 1.
Prostaglandin analogues with a bicyclo[3.3.0]octene and bicyclo[3.3.0]octane fragments in the ω-side chain, of types I and II.
In the first compound, I, the bicyclo[3.3.0]octene fragment is linked to the C16 carbon atom, which is a small but significant hindrance of the 15-PGDH enzyme to inactivate the PG analogue via the oxidation of 15α-OH to the 15-keto group [2].
In the second compound, II, the bicyclo[3.3.0]octene and bicyclo[3.3.0]octane fragments linked to the C15 carbon atom are expected to slow down the inactivation of the PG analog [3].
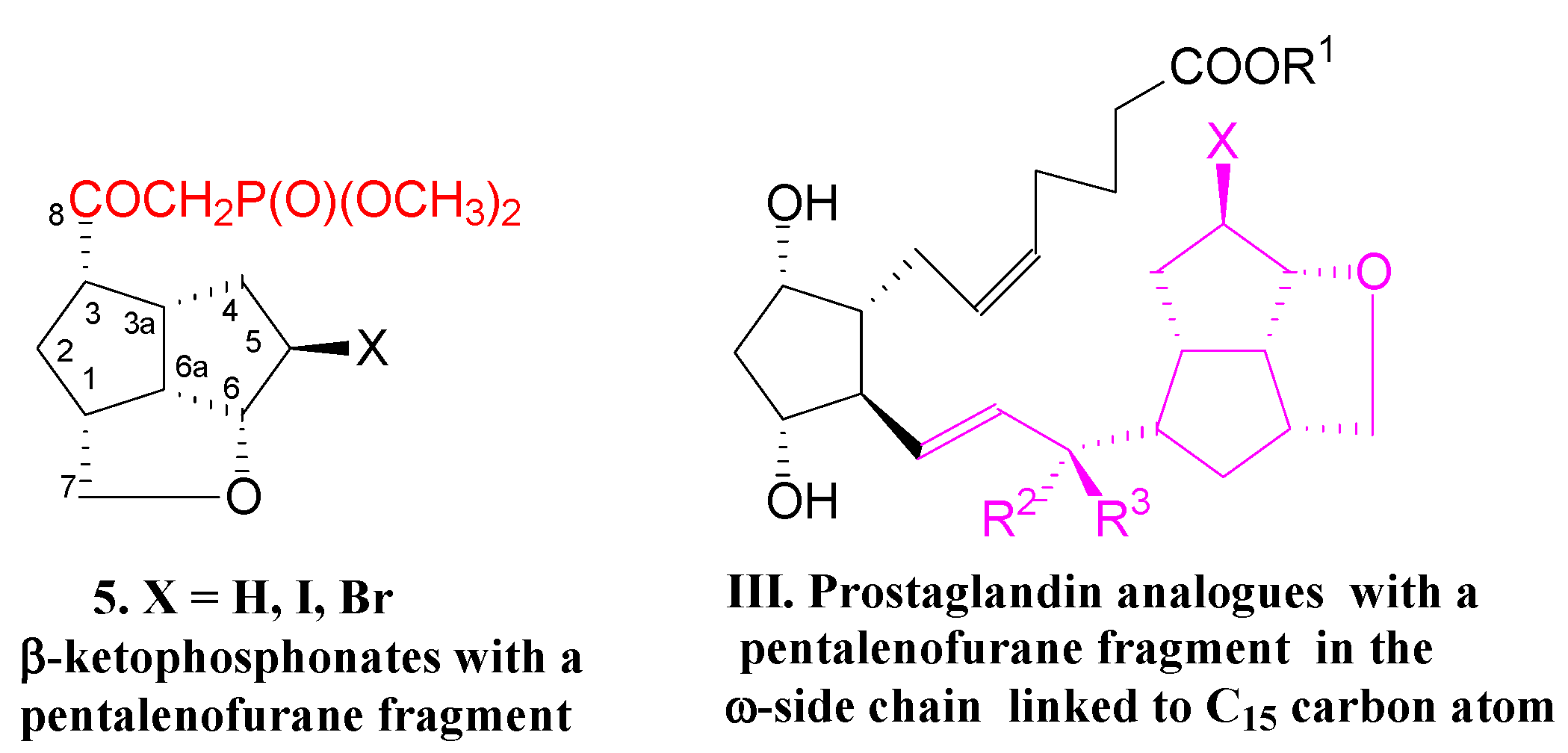
Now, we present the synthesis of new key β-ketophosphonates 5, with a more bulky pentalenofurane scaffold linked to the keto group to build type III PG analogues (Figure 2):
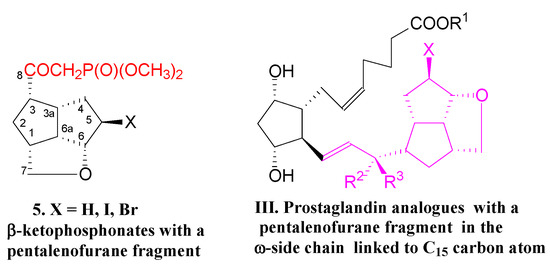
Figure 2.
β-Ketophosphonates 5 with a pentalenofurane fragment in the molecule to obtain new type III prostaglandin analogues.
2. Materials and Methods
Syntheses of the compounds were realized in three high-yield reactions, starting from the pentalenofurane alcohols 2. The alcohols were oxidated with Johns reagent to the acids 3, which were esterified to the methyl esters 4. In the last step, the esters 4 were reacted with lithium salt of dimethyl methanephosphonate at a low temperature to give the β-ketophosphonates 5 (Scheme 1). The secondary compounds 6b and 6c were formed in small amounts in the Johns oxidation of 2b and 2c, and the NMR spectroscopy showed that their structure is that of an ester of the acid with the starting alcohol.
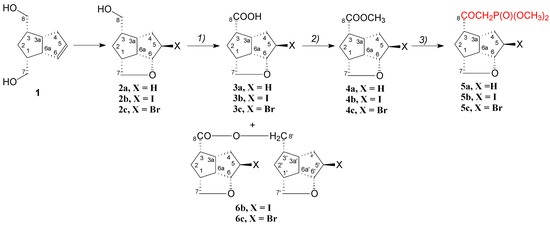
Scheme 1.
Synthesis of pentalenofurane β-ketophosphonates 5a–5c. (1) Jones reagent (2.4 M), acetone, −15 to 0 °C, 2a, 81.8% 3a; with 2b, 85.15% 3b; with 2c, 73.7% 3c, (2) MeOH, TsOH, rt, overnight, 86.4% 4a; 92.4% 4b; 81.0% 4c, (3) dimethyl methanephosphonate, n-BuLi, −75 °C to −65 °C, 88.0% 5a; 78.6% 5b; 83.3 % 5c.
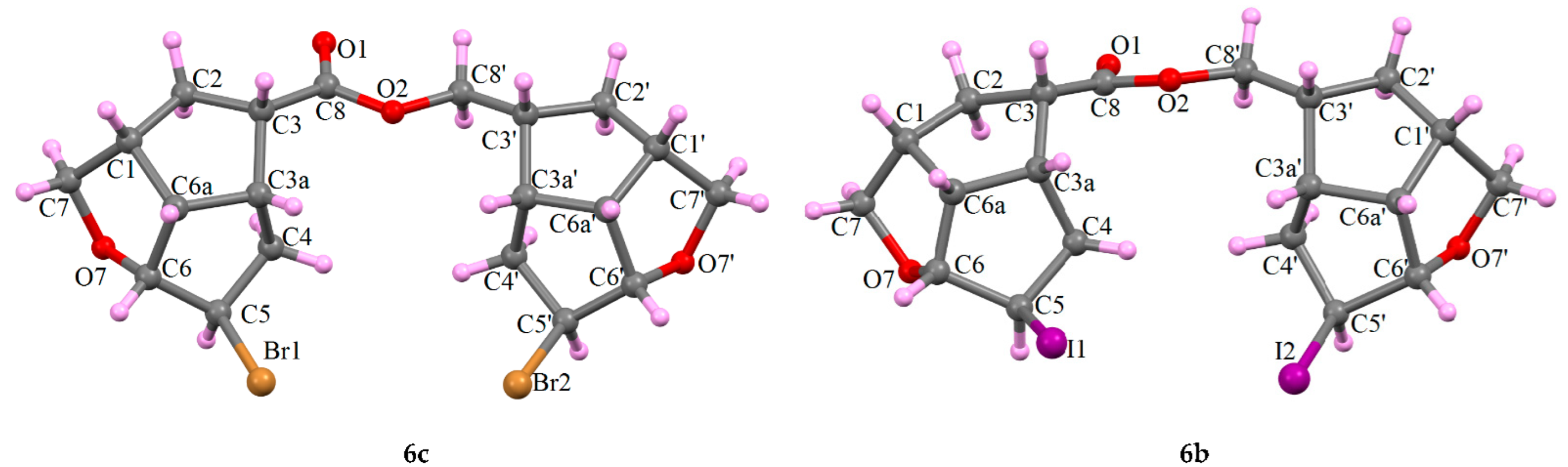
Their molecular structures were confirmed using the single crystal X-ray determination method for 6c and the XRPD powder method for 6b (Figure 3):
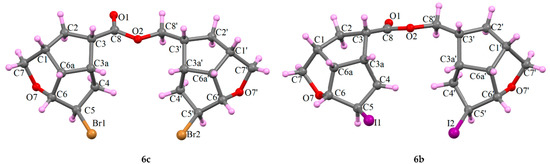
Figure 3.
X-ray molecular configuration of the asymmetric unit of the secondary compounds 6c and 6b.
3. Results
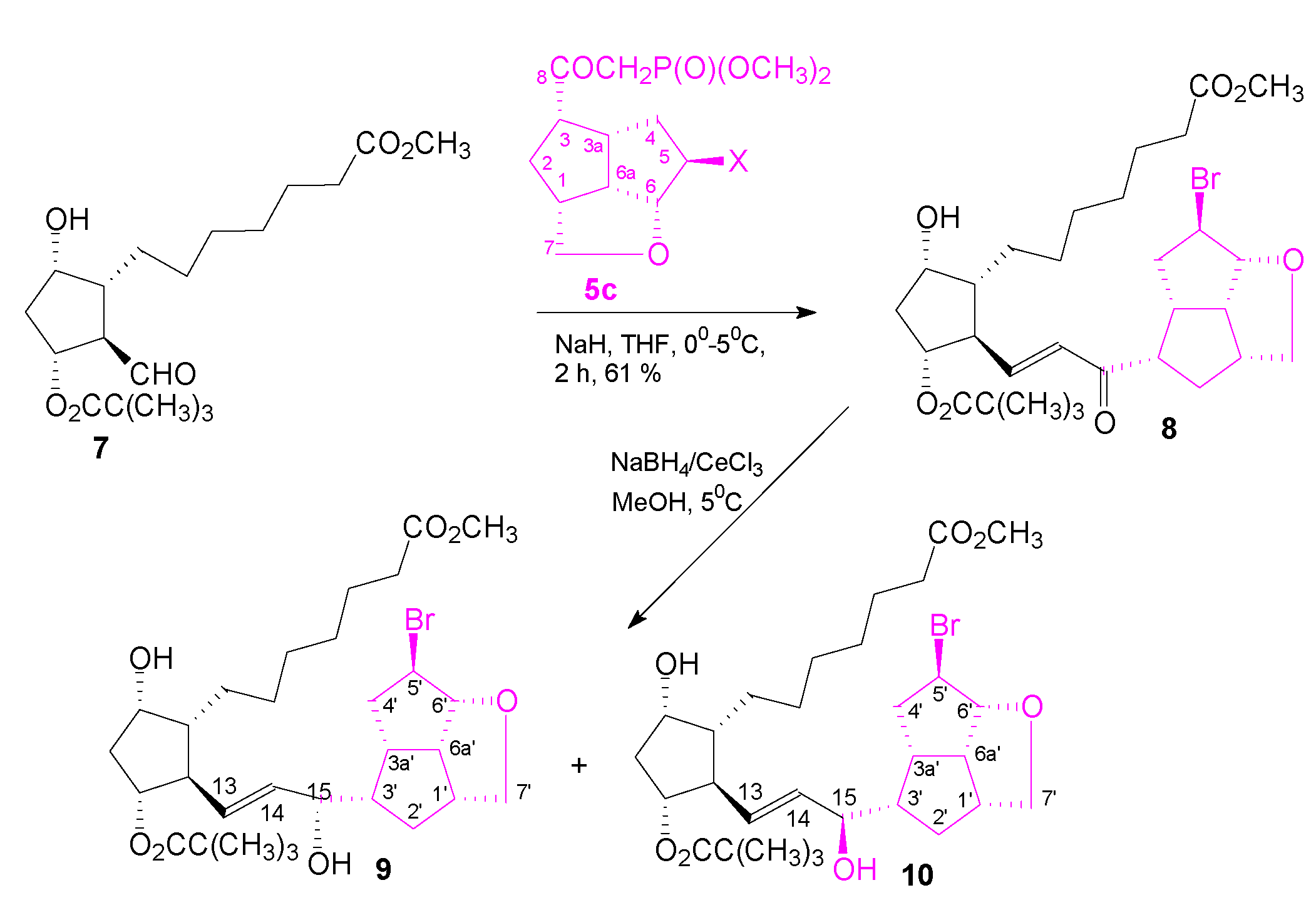
Three key intermediate β-ketophosphonates 5 were synthesized in a high-yield, short-sequence synthesis, as presented in Scheme 1, and fully characterized. β-Ketophosphonate 5 was used to obtain type III PG analogs in the E-HEW selective olefination of the aldehyde 7, with the hydrogenated α-side chain, to the ketoprostaglandin analog 8 (Scheme 2):
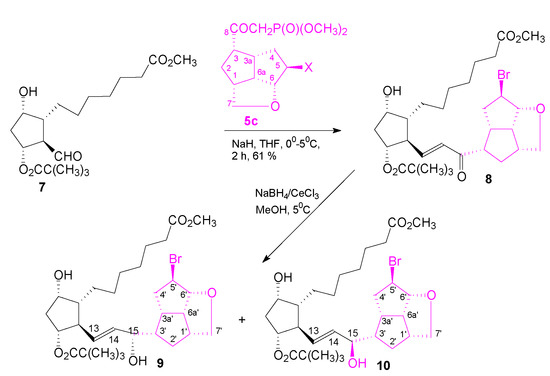
Scheme 2.
Synthesis of F1 PG analogs 8, 9 and 10 with a pentalenofurane fragment in the ω-side chain.
The reduction of the enone group to the desired allylic alcohol 9 with the selective but bulky reducing reagent aluminum diisobornyloxyisopropoxide, usually used in the PG field, did not proceeded as in the case of the PG analog II (R1,R2 = O) (Figure 1), as expected. The Luche reduction of enone 8 with NaBH4 and CeCl3 gave the allylic alcohol 9 together with its 15-epimer, 10, in a ratio of 1:1. As in the reduction, the bulky, constrained pentalenofurane scaffold in the ω-side chain was used to slow down the inactivation of the PGs analogs via the enzyme 15-PGDH pathway.
4. Conclusions
The synthesis of key β-ketophosphonates 5a–5c with a pentalenofurane scaffold linked to the keto group was realized in a sequence of three high-yield reactions. Two by-products formed in the oxidation of alcohols 2 were characterized using NMR and confirmed using single crystal X-ray crystallography for 6c and the XRPD powder method for 6b. For the first time, the key intermediates 5 were used to obtain the PGF1 analogs 8–10 with a pentalenofurane scaffold in the ω-side chain.
Funding
The funds were provided by Orizont-2000, 45/1999/1.
Data Availability Statement
Not applicable.
Acknowledgments
We acknowledge the Ministry of Research, Innovation and Digitalization for the grant Orizont-2000, 45/1999/1, and A.H. gratefully acknowledges the University of Bucharest—UniRem project no. 244 and the contract CNFIS-FDI-2020-0355.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Tănase, C.; Drăghici, C.; Căproiu, M.T.; Hanganu, A.; Borodi, G.; Maganu, M.; Gal, E.; Pintilie, L. β-Ketophosphonates with Pentalenofuran Scaffolds Linked to the Ketone Group for the Synthesis of Prostaglandin Analogs. Int. J. Mol. Sci. 2021, 22, 6787. [Google Scholar] [CrossRef] [PubMed]
- Tănase, C.I.; Căproiu, M.T.; Drăghici, C. New β-ketophosphonates for the synthesis of prostaglandin analogues. 1. Phosphonates with a bicyclo[3.3.0]octene scaffold spaced by a methylene group from the β-ketone. Prostaglandins Leukot. Essent. Fat. Acids 2021, 173, 102325. [Google Scholar] [CrossRef] [PubMed]
- Tănase, C.I.; Drăghici, C.; Caproiu, M.T. New ß-ketophosphonates for the synthesis of prostaglandin analogues. 2. Phosphonates with a bicyclo[3.3.0]octene and bicyclo[3.3.0]octane scaffolds linked to the ß-ketone group. New J. Chem. 2020, 44, 20405–20410. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).