Abstract
Reactions of dinitrobenzoannulated heterocycles (furazan, thiadiazole, selenadiazole, pyridine) with anionic C-nucleophiles (mono- and diketones, nitroalkanes and related compounds) provided stable anionic adducts in high yields. Consecutive oxidation with ammonium cerium (IV) nitrate resulted in re-aromatization with the formation of the corresponding substitution products, formally representing C-H-functionalized benzoheterocycles.
1. Introduction
The functionalization of arenes provides their chemical diversity and opens a way to valuable substances that are widely used in medicine, pharmaceutics, agriculture and other areas. In recent years, the functionalization of the CH bond has become an important tool for the implementation of such processes. Nucleophilic substitution of hydrogen (SNH) in arenes has acquired intensive development as a more prospective way of functionalization than classical SNAr processes occurring through ipso-substitution of a nucleofuge. Two general SNH processes are oxidative (ONSH) and vicarious (VNSH) nucleophilic substitution of hydrogen proceeding through the generation of σн-adduct. In the case of highly electrophilic substrates, the intermediate sigma-adducts can be isolated and identified, and their chemical behavior can be studied.
It is well-known that highly electrophilic arenes and heteroarenes (superelectrophiles) readily form adducts with nucleophiles of various nature, including weak neutral nucleophiles such as π-excessive (het)arenes, enamines, etc. [1,2,3]. In the case of C-nucleophiles, these adducts can be isolated. Earlier we reported on the reactions of some azolo[b]pyridines with 1,3-dicarbonyl compounds [4,5,6]. In this work, we studied the reactions of dinitrobenzoannulated heterocycles (thiadiazole, selenadiazole, pyridine) with anionic C-nucleophiles (mono- and diketones, nitroalkanes and related compounds).
2. Results and Discussion
Among numerous highly electrophilic nitro (het)arenes, the following were selected for this study: 4,6-dinitrobenzothiadiazole 1a [7], 4,6-dinitrobenzoselenadiazole 1b [8], 5,7-dinitroquinoline 1c [9] and 5,7-dinitroquinoline-N-oxide 1d [9]. It was found that their reactions with mono- and diketones as well as 2-nitropropane in the presence of a base provided the previously unknown stable anionic adducts 2 in high yields (Figure 1, Table 1). These adducts were isolated in pure form and are characterized by (nuclear magnetic resonance spectroscopy (NMR) and high-resolution mass spectrometry (HRMS). Oxidation of compounds 2 with ammonium cerium (IV) nitrate was studied. In some cases (Table 1, entries 1, 2, 6, 8) the corresponding substitution products 3 were isolated formally representing C-H-functionalized benzoheterocycles. In case of adduct 2c, decomposition was observed, while in case of dinitroquinoline complex 2i, the starting compound 1c appeared to be the sole isolable product. In all other cases, 1NMR spectra showed a 1:1 mixture of the target substitution product and the corresponding starting material (Table 1, entries 4, 5, 7, 10, 11).
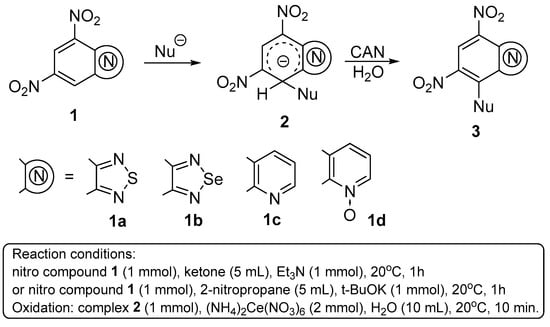
Figure 1.
Formation of anionic complexes with C-nucleophiles and their oxidation.

Table 1.
Yields of anionic σ-adducts 2 and oxidation products 3.
As it follows from the data presented in Table 1, the oxidation of adducts 2 generally proceed in two directions: formation of target substitution products 3 and decomposition to give starting compounds 1. Such behavior of anionic σ-complexes is not surprising since it was reported earlier for 1,3,5-trinitrobenzene (TNB) adducts with CH-acidic compounds [10]. The kinetic study of the decomposition of the TNB-acetophenone complex revealed a strong dependence on the pH of the reaction media. However, in a number of cases we were able to isolate polyfunctional derivatives of highly electrophilic benzoannulated heterocycles (Figure 2).
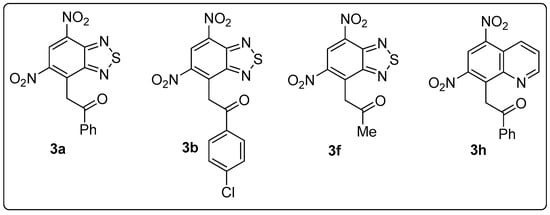
Figure 2.
Compounds synthesized by oxidation of anionic σ-complexes of dinitrobenzohetarenes and C-nucleophiles.
3. Experimental Procedures
Anionic σ-adducts 2a,b,d-f,h,j (general procedure). To a solution of dinitro compound 1 in an appropriate ketone (5 mL), Et3N (0.14 mL, 1 mmol) was added. The mixture was stirred for 1 h at 20 °C, poured in ether (50 mL), and the resulting precipitate was filtered off, washed with ether and dried to give the target adduct (see Table 1 for yields).
Anionic σ-adducts 2c,g,i,k (general procedure). To a solution of dinitro compound 1 in 2-nitropropane (5 mL), t-BuOK (0.112 g, 1 mmol) was added. The mixture was stirred for 1 h at 20 °C, poured in ether (50 mL), and the resulting precipitate was filtered off, washed with ether and dried to give target adduct (see Table 1 for yields).
Oxidation of adducts 2 (general procedure). To a solution of the corresponding adduct 2 (1 mmol) in 5 mL of H2O, a solution of (NH4)2Ce(NO3)4 (1.1 g, 2 mmol) in H2O (5 mL) was added. The mixture was stirred for 10 min at 20 °C and extracted with CHCl3 (3 × 10 mL). Organic layers were washed with brine, dried over Na2SO4 and evaporated to give target compound 3 which was then purified by column chromatography (SiO2, CHCl3) (see Table 1 for yields).
4. Conclusions
Thus, a series of the previously unknown dinitrobenzoazoles and azines functionalized in a benzene ring were synthesized using stable anionic σ-adducts as key intermediates of the C-H functionalization of π-deficient nitroarenes.
Author Contributions
Conceptualization, A.S. and M.B.; methodology, A.S., M.B. and V.K.; investigation, A.S. and V.K.; writing—original draft preparation, A.S.; writing—review and editing, M.B.; project administration, V.K. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Russian Science Foundation, grant number 19-73-20259.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Terrier, F.; Kizilian, E.; Halle, J.C.; Buncel, E. 4,6-Dinitrobenzofuroxan: A stronger electrophile than the p-nitrobenzenediazonium cation and proton. J. Am. Chem. Soc. 1992, 114, 1740–1742. [Google Scholar] [CrossRef]
- Remennikov, G.Y.; Kempf, B.; Ofial, A.R.; Polborn, K.; Mayr, H. 5-Methoxyfuroxano[3,4-d]pyrimidine: A highly reactive neutral electrophile. J. Phys. Org. Chem. 2003, 16, 431–437. [Google Scholar] [CrossRef]
- Remennikov, G.Y.; Pirozhenko, V.V.; Vdovenko, S.I.; Kravchenko, S.A. σ-Complexes in the pyrimidine series. 13. Reaction of 7- and 5-methoxyfuroxano[3,4-d]pyrimidines with some C-nucleophiles. Chem. Heterocycl. Compd. 1998, 34, 104–110. [Google Scholar] [CrossRef]
- Starosotnikov, A.M.; Shkaev, D.V.; Bastrakov, M.A.; Fedyanin, I.V.; Shevelev, S.A.; Dalinger, I.L. Nucleophilic dearomatization of 4-aza-6-nitrobenzofuroxan by CH-acids in the synthesis of pharmacology-oriented compounds. Beilstein J. Org. Chem. 2017, 13, 2854–2861. [Google Scholar] [CrossRef] [PubMed]
- Starosotnikov, A.M.; Shkaev, D.V.; Bastrakov, M.A.; Fedyanin, I.V.; Shevelev, S.A.; Dalinger, I.L. Dearomatization of oxa- or selenadiazolopyridines with neutral nucleophiles as an efficient approach to pharmacologically relevant nitrogen compounds. Mendeleev Commun. 2018, 28, 638–640. [Google Scholar] [CrossRef]
- Starosotnikov, A.M.; Ilkov, K.V.; Bastrakov, M.A.; Fedyanin, I.V.; Kokorekin, V.A. Mild and efficient addition of carbon nucleophiles to condensed pyridines: Influence of structure and limits of applicability. Chem. Heterocycl. Compd. 2020, 56, 92–100. [Google Scholar] [CrossRef]
- Pesin, V.G.; Khaletskii, A.M.; Sergeev, V.A. Studies in field of 2,1,3-thiodiazoles. 22. niitration of derivatives of benz-2,1,3-thiodiazoles. J. Gen. Chem. USSR 1963, 33, 1714–1719. [Google Scholar]
- Elvidge, J.A.; Newbold, G.T.; Percival, A.; Senciall, I.R. 3-Nitro-o-phenylenediamines: A new route. J. Chem. Soc. 1965, 5119–5120. [Google Scholar] [CrossRef]
- Starosotnikov, A.M.; Nikol’skiy, V.V.; Borodulya, A.N.; Kachala, V.V.; Bastrakov, M.A.; Solkan, V.N.; Shevelev, S.A. Synthesis and functionalization of 5,7-dinitroquinoline and its N-oxide. Asian J. Org. Chem. 2016, 5, 685–690. [Google Scholar] [CrossRef]
- Renfrow, R.A.; Strauss, M.J.; Terrier, F. Stability of carbon-bonded anionic sigma-complexes. 3. Decomposition in aqueous acidic media. J. Org. Chem. 1980, 45, 471–475. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).