Abstract
Per- and polyfluoroalkyl substances (PFAS) are persistent environmental contaminants that tend to accumulate in solid matrices such as sewage sludge, raising concerns regarding their fate and potential ecological risks. This study aimed to develop and validate a robust analytical method for the accurate determination of PFAS in dehydrated sludge. A liquid chromatography–tandem mass spectrometry (LC–MS/MS) method was optimized for 28 PFAS, including perfluoroalkyl carboxylic acids (PFCAs) and sulfonic acids (PFSAs). Solid–liquid extraction with basic methanol was followed by cleanup using a cartridge packed with ferrite and sodium sulfate to remove moisture and particulate interferences. Chromatographic separation was performed with an Avantor® ACE® PFAS Delay column coupled to an Agilent triple quadrupole MS operating in negative electrospray ionization mode. The method achieved excellent sensitivity (MDL < 0.02 µg/g dry weight for most compounds), satisfactory precision (RSD < 15%), and recoveries between 80–118%. Optimization of mobile phase additives, gradient conditions, and MS parameters enhanced chromatographic resolution and signal-to-noise ratio. The validated method demonstrates high reliability for PFAS determination in complex solid matrices and can be applied as a valuable tool for environmental monitoring and risk assessment of sludge management practices.
1. Introduction
Per- and polyfluoroalkyl substances (PFAS) are a large class of synthetic organofluorine compounds used since the 1940s in products such as non-stick coatings, textiles, firefighting foams, and food packaging. Their strong carbon–fluorine bonds confer high chemical and thermal stability, leading to persistence and bioaccumulation in the environment, and are associated with toxicological effects such as carcinogenicity and endocrine disruption [1,2]. Over 12,000 PFAS have been registered to date [3], with global markets exceeding USD 28 billion and remediation costs reaching hundreds of billions of USD [4]. In response, regulatory initiatives from the U.S. Environmental Protection Agency (EPA)—including the draft Method 1633 for biosolids—and the European Chemicals Agency (ECHA) through the REACH PFAS restriction proposal are intensifying efforts to control PFAS contamination [5,6].
Analytical monitoring of PFAS relies primarily on liquid chromatography–tandem mass spectrometry (LC–MS/MS) for its sensitivity and selectivity in complex matrices [7,8]. However, quantification in solid matrices such as sewage sludge remains challenging due to strong adsorption to organic matter, background contamination, and trace-level concentrations [9,10]. Several international studies (e.g., U.S., Germany, Australia, Nordic countries) have reported PFAS in WWTP sludge at concentrations up to 7000 ng/g dw [11], underscoring the potential environmental risks linked to sludge reuse and PFAS transfer to soils and crops [12,13].
In Romania, the absence of a standardized analytical protocol for PFAS in sludge and the lack of a systematic national monitoring program represent major gaps in assessing environmental exposure. Although recent studies have reported low-to-moderate levels of PFOS, PFOA, and PFHxS in wastewater influents and effluents [14,15], no data is currently available for sewage sludge, despite its frequent use in agriculture under Directive 86/278/EEC [16]. To address this deficiency, the present study focuses on the development and validation of a simplified, high-sensitivity LC–MS/MS method for the determination of 28 PFAS in dehydrated sewage sludge. The approach adapts international best practices (EPA 1633, ISO 21675 [5,17]) to Romania’s analytical infrastructure, providing a practical tool to support future national PFAS monitoring and regulatory initiatives.
Although comprehensive protocols such as the United States Environmental Protection Agency’s Method 1633 and ISO 21675:2019 provide detailed procedures for PFAS determination in solid matrices, their implementation typically requires automated pressurized extraction systems, multi-sorbent cleanup (WAX/C18/ENVI-Carb), and advanced laboratory infrastructure, which are not always available in routine analytical laboratories. In this study, our objective was not to replace existing standardized methods but to develop and validate a simplified, robust LC–MS/MS approach that ensures comparable analytical performance while improving operational accessibility and efficiency.
The proposed method introduces several key advancements:
- A ferrite/sodium sulfate cleanup replacing multi-sorbent SPE, reducing analyte losses—particularly for short-chain PFAS—while maintaining recoveries of 70–130%;
- Ultrasound-assisted extraction (UAE) as a low-solvent, energy-efficient alternative to pressurized liquid extraction, improving sustainability and turnaround time;
- Validation on real Romanian sewage sludge, extending PFAS analytical capability to a matrix and regional context where standard protocols have not yet been fully implemented.
Its modular design, low resource demand, and reproducibility make this method a practical complement to EPA 1633 and ISO 21675, offering laboratories a cost-effective solution for routine PFAS monitoring in complex solid matrices while ensuring compliance with international analytical performance standards.
2. Materials and Methods
2.1. Standards and Reagents
Analytical-grade standards and reagents were used throughout the study to ensure high-precision quantification of PFAS compounds in environmental matrices. The native PFAS standards consisted of a certified analytical solution—EPA Method 533 Native Analyte PDS—containing 25 compounds at a concentration of 1000 ng/mL, purchased from Cambridge Isotope Laboratories, Inc. (Tewksbury, MA, USA). To expand the range of analyzed substances beyond those covered in EPA 533, the following additional native compounds were included: perfluorononanoic acid (PFNA), perfluorononanesulfonic acid (PFNS), perfluorodecanesulfonic acid (PFDS), and perfluorooctanesulfonamide (PFOSA), also acquired from Cambridge Isotope Laboratories, Inc., each with certified purity between 98 and 99%. For accurate quantification using isotope dilution, 13C-labeled internal standards were employed. Specifically, 2,3,4-13C3-sodium perfluoro-n-butyrate—(2,3,4-13C3)PFBA and 13C8-perfluorooctanoic acid (13C8-PFOA) were purchased from Cambridge Isotope Laboratories, Inc. and used to correct for matrix effects and instrumental variability. Working solutions of both native and labeled standards were prepared in methanol and stored at −20 °C in polypropylene containers to avoid contamination and degradation. The physicochemical properties of the analytes are given in Table S1.
All solvents used in sample preparation and chromatographic analysis were PFAS-free and of LC–MS-grade purity. Methanol was purchased from Merck (Darmstadt, Germany), while acetic acid, formic acid, and ammonium acetate, used for mobile phase preparation and pH control, along with anhydrous sodium sulfate and ferrite powder, were sourced from Sigma-Aldrich (Darmstadt, Germany). All reagents were used directly as supplied, without additional filtration, to avoid potential cross-contamination from filter membranes, following best practices for PFAS analysis as recommended in EPA Method 1633 and ISO 21675 [5,17]. All solvents were handled and stored under clean laboratory conditions to minimize background contamination during trace-level determinations.
2.2. Sample Preparation
Before conducting instrumental analysis, an efficient pretreatment protocol was developed and applied for extracting PFAS compounds from sewage sludge. This aims to maximize analyte recovery and ensure analytical reproducibility. The method utilized ultrasound-assisted extraction (UAE), a widely used technique in environmental analysis known for its simplicity, low solvent consumption, and high efficiency in extraction.
Municipal sewage sludge samples were collected by the authors from wastewater treatment plants in Bucharest (Romania), lyophilized, homogenized, and stored under clean laboratory conditions prior to use. The material was screened for PFAS using LC–MS/MS, and no target analytes were detected above the instrumental limits of detection, confirming its suitability as a blank matrix for method development and validation.
An aliquot of 0.5 g of dry sludge was spiked with a mixed PFAS standard solution at a concentration of 100 µg/L, equivalent to 0.2 µg/g dry weight. In addition, isotopically labeled internal standards were added to the matrix at a concentration of 150 µg/L (0.3 µg/g). The spiked material was allowed to impregnate and equilibrate with the added standards to simulate environmental contamination under controlled laboratory conditions. Following the spiking step, 10 mL of methanol was added to the sample, which was then vortexed for 5 min to ensure uniform dispersion of analytes.
The sample was subsequently subjected to ultrasonic treatment for 30 min at 30 °C, conditions optimized in-house based on a previously validated procedure for wastewater and sludge matrices [7]. After sonication, the mixture was centrifuged at 4000 rpm for 5 min. During the method optimization phase, solid-phase extraction (SPE) using multi-sorbent cartridges (WAX, C18, ENVI-Carb, PSA) was evaluated for potential matrix cleanup; however, this approach led to notable losses of highly polar, short-chain PFAS due to non-specific retention. Consequently, the simpler ferrite/sodium sulfate filtration procedure was selected as the final cleanup step, providing adequate matrix removal while preserving analyte recovery and method reproducibility. The cleanup was performed using glass cartridges (6 mL) packed with 1 g anhydrous sodium sulfate and 0.5 g ferrite powder, pre-rinsed with methanol to remove potential contaminants. PFAS-free blank tests confirmed no detectable background or analyte loss, demonstrating that the sorbents do not adsorb target PFAS or introduce contamination. To enhance extraction efficiency, the solid sludge residue was re-extracted by repeating the entire process with a fresh 10 mL aliquot of methanol. The two organic extracts were combined and subjected to evaporation to dryness under a gentle nitrogen stream at 40 °C. The dried residue was then reconstituted in 1 mL of a water/methanol mixture (20:80, v/v), ensuring compatibility with subsequent LC–MS/MS analysis. The reconstituted solutions were filtered through polypropylene syringe filters (0.45 µm) to remove particulates before injection. PTFE filters were deliberately avoided to prevent potential PFAS contamination, in line with recommendations from EPA Method 1633 and ISO 21675 [5,17].
2.3. Instrumentation
The chromatographic and mass spectrometric analysis of the target PFAS compounds was performed using an Agilent 1260 Infinity II LC system coupled to an Agilent 6410B triple quadrupole mass spectrometer (Agilent Technologies, Palo Alto, CA, USA). The LC configuration comprised a G1312C binary pump with integrated degasser, a G1329B autosampler, a G1316A thermostatted column compartment, and a G1330B sample cooler, enabling precise temperature and injection control for high reproducibility in complex environmental matrices. The system was optimized for high-sensitivity detection through the combination of an electrospray ionization (ESI) source in negative mode, scheduled MRM acquisition, and short dwell times, which together enhanced signal-to-noise ratios for trace-level PFAS quantification. Chromatographic separation was achieved using an Avantor® ACE® HTP-MS analytical column (50 × 2.1 mm, 2.0 µm particle size) (Avantor, Radnor, PA, USA), preceded by an Avantor® ACE® PFAS Delay column (10 × 2.1 mm, 2.0 µm) installed upstream of the injector to trap background PFAS originating from the LC system. The analytical column was responsible for compound separation, while the delay column served exclusively to prevent system-derived PFAS interference. Both columns incorporate ultra-clean hardware, metal-free PEEK-lined fittings, and PFAS-inert stationary phases, designed by the manufacturer to minimize background fluorinated contaminants and reduce adsorption of highly polar PFAS [18]. The analytical column was maintained at 40 °C, while the delay column operated at ambient temperature within the LC system flow path. Detection was achieved using the Agilent 6410B triple quadrupole MS equipped with a Jet Stream ESI source operating in negative ionization mode, with compound-specific MRM transitions optimized for all 28 PFAS and their isotopically labeled internal standards.
2.4. LC Conditions
The optimization of chromatographic and mass spectrometric parameters was a critical step in adapting the LC–MS/MS method for the simultaneous determination of 28 perfluoroalkyl substances (PFAS), along with two isotopically labeled internal standards, in complex sludge matrices. Compared to a previous method developed in our laboratory, which focused exclusively on nine perfluorosulfonic acids (PFSAs), this advanced approach required a comprehensive reconfiguration of the chromatographic setup to achieve baseline separation and improved analytical performance for a broader PFAS spectrum [14]. Two chromatographic columns were evaluated for their retention and resolution capabilities. A Zorbax Eclipse XDB-C18 column (2.1 × 100 mm, 3.5 µm particle size) (Agilent Technologies, Santa Clara, CA, USA) was initially employed to evaluate the chromatographic behavior and separation efficiency of perfluoroalkyl carboxylic acids (PFCAs) and sulfonic acids (PFSAs), both individually and in mixed solutions. However, improved resolution and reduced background contamination were obtained using a PFAS-dedicated configuration, consisting of an Avantor® ACE® PFAS Delay column (10 × 2.1 mm, 2.0 µm) positioned upstream of the injector to trap system-derived PFAS, and an Avantor® ACE® HTP-MS analytical column (50 × 2.1 mm, 2.0 µm) responsible for compound separation. This setup provided enhanced peak symmetry and retention stability, particularly for early-eluting short-chain PFAS.
2.5. MS Conditions and Optimization
The optimization of mass spectrometric detection parameters was essential for achieving low detection and quantification limits, required for the reliable determination of trace concentrations of perfluoroalkyl substances (PFAS). A systematic approach was employed to optimize the critical settings of the Agilent 6410B triple quadrupole mass spectrometer, with the goal of enhancing sensitivity, selectivity, and signal-to-noise ratios across all 30 analytes under investigation. Initially, the optimization involved testing the detector response for a standard PFAS mixture at 50 µg/L. Parameters such as fragmentor voltage, collision energy (CE), electron multiplier voltage (ΔEMV), resolution on quadrupoles Q1 and Q3, and dwell time per MRM transition were individually tuned. The objective was to identify the settings that produced the highest peak areas and the strongest signal-to-noise (S/N) values, while preserving ion stability and minimizing matrix-induced suppression.
2.6. Method Validation
The method validation followed the recommendations of the International Council for Harmonisation (ICH Q2(R2), 2022) and the U.S. Environmental Protection Agency (EPA, 2023, Method 1633) guidelines, evaluating linearity, MDL, MQL, accuracy, precision, matrix effects, and recovery [5,6,19]. All validation experiments were performed using a lyophilized sewage sludge matrix previously confirmed to be PFAS-free by LC–MS/MS screening performed in our laboratory before spiking. This approach is consistent with current EPA and ISO practices when certified blank sludge materials are not commercially available. Each parameter was evaluated through specific experimental protocols, as described below.
2.6.1. Linearity
Linearity was assessed by preparing matrix-matched calibration curves using seven concentration levels (0.02–0.6 µg/g). Each level was analyzed in triplicate, and the correlation coefficient (R2) was calculated for each analyte. A value of R2 > 0.995 was considered acceptable.
2.6.2. Method Detection (MDL) and Quantitation (MQL) Limits
MDL and MQL were determined empirically by serially diluting standards in extracted blank sewage sludge until the signal-to-noise (S/N) ratio reached approximately 3:1 (MDL) and 10:1 (MQL). These values were confirmed by injecting replicates at the threshold levels.
2.6.3. Accuracy
Accuracy was tested by analyzing pre-spiked sewage sludge samples (n = 3) at three concentration levels (e.g., 0.05, 0.25, and 0.5 µg/g) before extraction. The measured concentrations were compared with the nominal spiked values, and the percent accuracy was expressed as the ratio between the determined and theoretical concentrations. Acceptable accuracy was defined as recovery within 80–120% for most analytes, consistent with ICH Q2(R2) and EPA 1633 guidelines [5,19].
2.6.4. Method Precision
Precision, including repeatability (intra-day) and intermediate precision (inter-day), was assessed by analyzing spiked samples (n = 6) at a mid-range concentration (50 µg/L) on the same day and across three separate days by different analysts. Relative standard deviation (RSD%) values were calculated and compared to acceptance thresholds (<15%).
2.6.5. Matrix Effects
Matrix effects (ME) were assessed using a method called post-extraction addition. After extracting blank sewage sludge, we added analytes and compared their peak areas to those of equal concentrations in pure solvents. We expressed matrix effects as a percentage ratio. Values near 100% indicated little suppression or enhancement of the signal.
To calculate the matrix effect, we used a formula that compares the signal produced by the analytes in the extracted sample to the signal from a standard solution. The result is shown as a percentage (% ME) according to Equation (1). Additionally, we extracted three sludge samples using ultrasound-assisted extraction (UAE) and spiked the resulting extracts with per- and polyfluoroalkyl substances (PFAS) at a concentration of 0.2 µg/g.
Matrix Effect (%) = (Peak area in the sample/Peak area in the standard solution) × 100
2.6.6. Method Recovery
Recovery was assessed independently from accuracy to evaluate analyte losses during extraction and cleanup. Recovery was calculated by comparing the peak areas of pre-spiked samples (spiked before extraction) with those of post-spiked samples (spiked after extraction but before instrumental analysis) at the same nominal concentrations. This approach isolates the efficiency of the extraction step from matrix and instrumental variability. Three replicates were analyzed at each concentration level, and recoveries ranged between 60% and 127%, depending on analyte polarity. These results demonstrate that the developed extraction procedure effectively recovers both short- and long-chain PFAS from complex sludge matrices.
2.6.7. Method Robustness
Robustness was assessed by varying critical method parameters slightly (e.g., flow rate ±0.02 mL/min, column temperature ±2 °C, injection volume ±1 µL) and observing the impact on retention time, peak area, and resolution. The method was considered robust if performance metrics remained within acceptable ranges.
2.7. Quality Assurance and Quality Control (QA/QC)
To ensure the reliability, reproducibility, and integrity of analytical results, a comprehensive quality assurance and quality control (QA/QC) protocol was implemented throughout all stages of sample preparation and LC–MS/MS analysis [5,17]. All reagents and solvents used were PFAS-free and of LC-MS-grade purity.
Glassware and polypropylene labware were pre-cleaned with methanol and ultrapure water to minimize background contamination. Procedural blanks (PFAS-free sludge), matrix spikes, quality control samples, and replicates were included in each batch to monitor contamination, recovery, and analytical variability. Two isotopically labeled internal standards (13C3–PFBA and 13C8–PFOA) were added to each sample before extraction to correct for recovery and matrix effects. The selection was based on commercial availability in Romania, where isotope-labeled PFAS standards remain limited.
Instrumental calibration was performed daily using matrix-matched calibration standards (0.02–0.6 µg/g). Calibration curves were accepted only if the correlation coefficient (R2) was ≥0.995, and the response factor variation between calibration levels was ≤20%. Recoveries from matrix spikes were required to fall within 70–130%, and relative standard deviations (RSDs) among replicates were ≤15% for intra-day precision. Matrix effects (ME), evaluated by comparing post-extraction spiked samples with solvent-based standards, were considered acceptable when within ±20%. Method blanks were analyzed after every ten samples and between analytical batches to verify the absence of target PFAS above 1/3 of the method detection limit (MDL). Retention times were accepted within ±0.1 min of the calibration standard, and quantification was based on the primary MRM transition with a confirmatory ion meeting a signal ratio tolerance of ±30%. All sample preparations and instrument runs were conducted under PFAS-controlled laboratory conditions, meaning that materials known to leach fluorinated compounds (PTFE-coated vessels, fluoropolymer tubing, etc.) were avoided. Only polypropylene 0.45 µm filters were used before LC–MS/MS injection to remove particulates.
3. Results
3.1. Chromatographic Behavior and Column Performance
The chromatographic analysis of perfluoroalkyl substances (PFAS) presents unique analytical challenges due to the physicochemical diversity of these compounds, including high polarity, amphiphilicity, and the presence of multiple isomeric forms. Additionally, PFAS compounds tend to adsorb onto metallic components and fluoropolymer tubing commonly used in LC systems, leading to peak broadening, tailing, low recovery, and irreproducible retention, particularly for short-chain analytes [20]. Therefore, optimizing the chromatographic configuration and materials was essential for reliable detection at trace levels.
Two reversed-phase columns were evaluated to determine the most effective stationary phase for separating a mixture of 28 native PFAS compounds. The Zorbax Eclipse XDB-C18 column (2.1 × 100 mm, 1.8 μm), a conventional C18 phase, demonstrated moderate retention for long-chain PFAS but failed to resolve critical isobaric and isomeric analyte pairs fully. Substantial co-elution was observed for short-chain species such as PFBA, PFPeA, and PFHxA, often accompanied by broad and asymmetric peak shapes. Furthermore, significant retention drift and signal suppression were observed under high-aqueous mobile phase conditions, likely due to nonspecific interactions with residual silanols or metal surfaces.
To minimize these effects, a PFAS-compatible LC configuration was employed, comprising an Avantor® ACE® PFAS Delay column (10 × 2.1 mm, 2.0 µm) installed between the pump and injector to trap background PFAS originating from the LC system, a guard column (ACE® UltraCore 5 SuperC18, 10 × 2.1 mm) to protect the analytical column from matrix residues, and an ACE® HTP-MS analytical column (50 × 2.1 mm, 2.0 µm) responsible for the chromatographic separation of all 28 target PFAS. As illustrated in Figure 1, the PFAS-specific column system produced sharper peaks, excellent peak symmetry, and baseline resolution for all 28 analytes across both carboxylic and sulfonic acid subclasses. Even structurally similar isomers, such as PFHpA vs. PFHpS or PFHxA vs. PFHxS, were fully resolved under the optimized gradient. Retention time reproducibility was outstanding, with RSDs <0.1% over 150 consecutive injections, and no evidence of carryover or memory effects was detected. Improved baseline stability, sharper peak resolution, and significantly enhanced signal intensity—up to 105 counts for short-chain PFAS (PFBA–PFPeA) compared to approximately 103 counts using the Zorbax Eclipse column—are evident in (b) following implementation of the PFAS Delay column. These results confirm the column’s suitability for high-throughput, trace-level analysis of environmental PFAS.
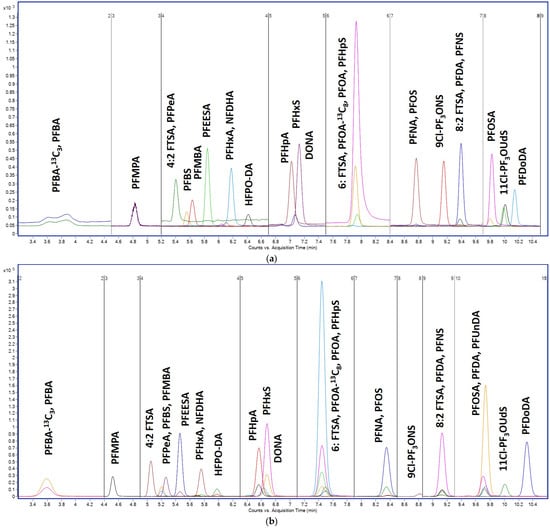
Figure 1.
Comparison of chromatographic separation of 28 PFAS obtained using (a) Zorbax Eclipse XDB-C18 column (2.1 × 100 mm, 3.5 µm) and (b) Avantor® ACE® PFAS-dedicated configuration consisting of a PFAS Delay column (10 × 2.1 mm, 2.0 µm) and an HTP-MS analytical column (50 × 2.1 mm, 2.0 µm). Y-axis: signal intensity (counts or peak area); X-axis: acquisition time (min).
Chromatographic retention behavior correlated strongly with chain length and analyte hydrophobicity. Short-chain perfluorocarboxylic acids (e.g., PFBA, PFPeA) eluted early, within 3.2–4.5 min, while longer-chain species such as PFDA, PFUnA, and PFDoA were retained beyond 7.5 min. A similar elution trend was observed for sulfonic acids, with PFBS eluting earliest and PFDS eluting last. This separation profile enabled an efficient time-segmented MRM acquisition strategy, minimizing transition overlaps and maximizing dwell time during mass spectrometric detection.
To reduce system contamination and PFAS memory effects, stainless steel tubing in the LC system was replaced with PEEK where feasible. The PFAS Delay column also functioned as an efficient trap for residual background PFAS leaching from valve components or autosampler needles. Baseline noise in the early elution window was significantly reduced, and re-equilibration was reliably achieved within a 7 min post-run phase. These features collectively ensured high system robustness and analytical reproducibility.
3.2. Effect of Mobile Phase Composition
The mobile phase composition plays a pivotal role in determining both chromatographic separation efficiency and electrospray ionization (ESI) performance for PFAS analysis by LC–MS/MS. Because PFAS compounds ionize predominantly in negative mode, the selection of suitable mobile phase modifiers directly impacts ion generation, spray stability, and analyte signal intensity.
Initially, aqueous mobile phases containing 0.5% acetic acid were evaluated in combination with methanol. Although acetic acid slightly improved peak shapes by promoting better elution of ionic species, the buffering capacity was insufficient to support stable and efficient ionization. Under these conditions, the MS signals for low-mass PFAS (e.g., PFBA, PFPeA) were weak and inconsistent, while sulfonic acids exhibited reduced peak symmetry and increased baseline noise.
To address these limitations, ammonium acetate was evaluated as a mobile phase modifier at concentrations of 2, 5, 10, and 20 mM. The use of 2 mM ammonium acetate in the aqueous phase significantly improved ESI performance, with an average 2–3× increase in signal intensity for most PFAS compared to acetic acid (Figure 2). Peak areas were notably enhanced for mid- and long-chain PFAS such as PFOA, PFNA, PFOS, and PFDS, all of which require stable deprotonation for effective detection (Figure 2).
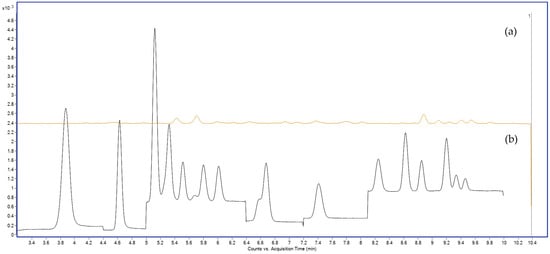
Figure 2.
Total ion chromatograms (TICs) acquired in MRM mode showing PFAS separation obtained with different aqueous mobile phases: (a) 0.1% acetic acid and (b) 2 mM ammonium acetate. Y-axis: signal intensity (counts or peak area); X-axis: acquisition time (min).
Interestingly, a lower concentration of 2 mM ammonium acetate further enhanced signal intensity for many analytes (Figure 3), particularly for early-eluting compounds. The reduced ionic strength likely minimized ion suppression in the electrospray plume, facilitating more efficient droplet desolvation and ion transfer into the MS.
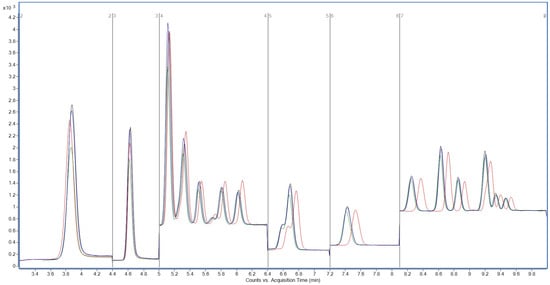
Figure 3.
Total ion chromatograms (TICs) acquired in MRM mode, showing PFAS separation using different ammonium acetate concentrations in the aqueous mobile phase: black, 2 mM; blue, 5 mM; green, 10 mM; black, 20 mM. Y-axis: signal intensity (counts or peak area); X-axis: acquisition time (min).
In addition to aqueous-phase optimization, the addition of 2 mM ammonium acetate to the methanol organic phase resulted in enhanced analytical signal and peak shape stability for all compounds (Figure 4), especially those eluting late in the gradient, such as PFUnA, PFDoA, and PFDS.
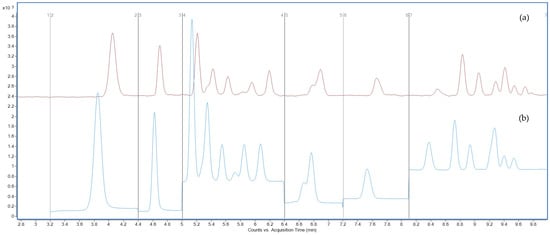
Figure 4.
Total ion chromatograms (TICs) acquired in MRM mode showing PFAS separation using 2 mM ammonium acetate in the aqueous phase, (a) methanol, (b) methanol containing 2 mM ammonium acetate as the organic modifier. Y-axis: signal intensity (counts or peak area); X-axis: acquisition time (min).
The final optimized mobile phase consisted of 2 mM ammonium acetate in both water (A) and methanol (B), providing an ideal compromise between buffering capacity and ionization efficiency. Gradient conditions were also systematically optimized to provide baseline separation for all 30 PFAS. The selected program (Table S2) featured a progressive increase in methanol from 5% to 80% over 8.5 min, followed by a 7 min column re-equilibration. This approach ensured efficient elution of highly retained analytes while minimizing carryover and maintaining robust retention time reproducibility.
To exploit the chromatographic resolution achieved, time-segmented MRM acquisition was implemented using 11 acquisition windows (Table S3). This strategy allowed selective detection of PFAS within their respective retention time intervals, improving dwell time allocation and reducing signal suppression. A switching valve was used to divert early eluting solvents and late-gradient impurities to waste, protecting the MS source and enhancing long-term stability. Together, the optimized mobile phase composition and gradient profile enabled excellent analytical performance across all PFAS classes, demonstrating high reproducibility, low noise, and reliable quantification. These findings confirm the critical importance of tailored mobile phase strategies when developing sensitive, multi-residue LC–MS/MS methods for PFAS determination in complex environmental samples.
3.3. MS/MS Detection Optimization
The optimization of tandem mass spectrometry (MS/MS) parameters was crucial for achieving sensitive and accurate detection of perfluoroalkyl substances (PFAS) in complex environmental matrices such as sewage sludge. Given the wide variability in chain lengths, polarities, and ionization efficiencies of PFAS, method development focused on fine-tuning each aspect of MS performance, ranging from precursor ion selection to ion source conditions.
The analytical strategy employed negative electrospray ionization (ESI−) coupled with multiple reaction monitoring (MRM), providing high sensitivity and selectivity across 28 PFAS analytes and two isotopically labeled internal standards (Table 1). Total ion chromatograms (TICs) acquired in MRM mode were used to assess ionization behavior and tuning efficiency (Figure 5 and Figure 6). Each compound was monitored using specific precursor-to-product ion transitions optimized through product-ion scans (50 µg/L standard). In accordance with the European Commission Decision 2002/657/EC, two transitions—quantifier (Q) and qualifier (q)—were used whenever possible to ensure both quantification and confirmation. However, for PFBA-13C3, PFBA, PFMPA, PFPeA, 9Cl-PF3ONS, and PFOSA, only a single quantifier transition was monitored due to the lack of secondary fragment ions with sufficient signal-to-noise (S/N > 10). Identification for these compounds was verified by retention time and isotopic pattern consistency.

Table 1.
Multiple reaction monitoring (MRM) transitions for target PFAS compounds, including quantifier (Q) and qualifier (q) ions, and optimized MS operating conditions.
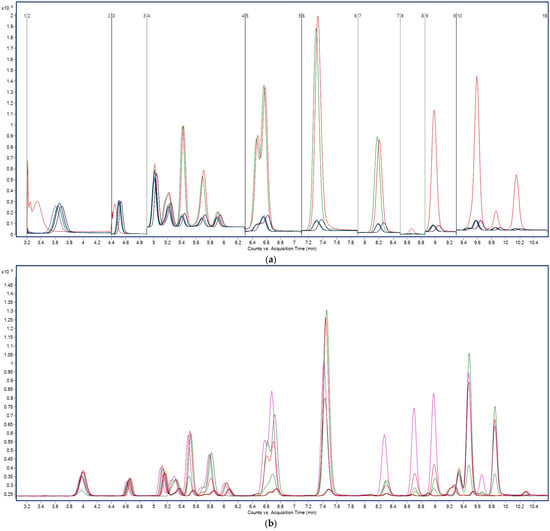
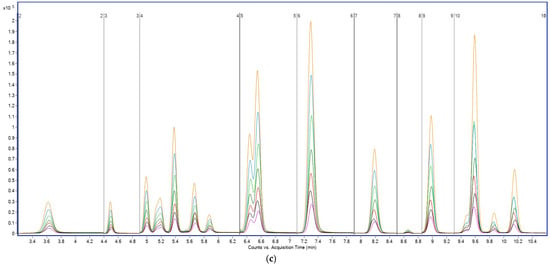
Figure 5.
Total ion chromatograms (TICs) acquired in MRM mode during MS detector optimization, showing the effect of (a) fragmentor voltage, (b) collision energy (CE), and (c) electron multiplier voltage (ΔEMV) on PFAS signal intensity and ionization efficiency. Y-axis: signal intensity (counts or peak area); X-axis: acquisition time (min).
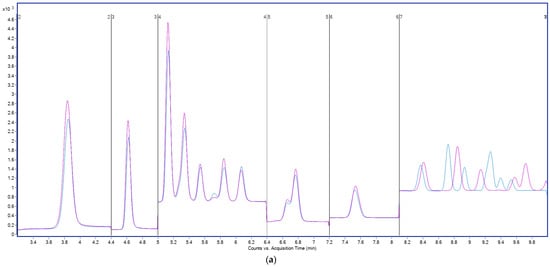
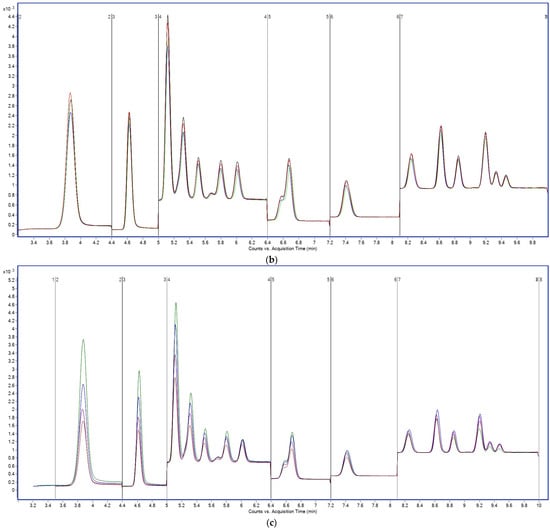
Figure 6.
Total ion chromatograms (TICs) acquired in MRM mode during ESI source optimization for PFAS detection, showing the influence of (a) drying gas flow, (b) nebulizer pressure, and (c) capillary voltage on signal intensity and spray stability. Y-axis: signal intensity (counts or peak area); X-axis: acquisition time (min).
Fragmentor voltages and collision energies (CE) were systematically optimized to generate the most abundant product ion. Short-chain PFAS such as PFBA and PFPeA responded well to low CE values (5–10 eV), while long-chain PFAS like PFDA and PFUnA required higher CE values (15–25 eV) to yield the appropriate product ions. These effects are illustrated in Figure 5, which presents TIC-MRM chromatograms comparing responses at different collision energies.
To further improve ionization efficiency, the electrospray source parameters were optimized. The drying gas flow was tested at 8 and 9 L/min, with 9 L/min yielding a more stable and intense ion spray. Nebulizer pressure was examined at 15, 30, 45, and 50 psi, with 30 psi providing the best compromise between droplet formation and spray stability. Capillary voltage, an important factor for desolvation and ion release, varied from 1000 V to 2500 V in 500 V steps. The optimal value was determined to be 1500 V, as it resulted in enhanced signal intensity without compromising stability. The resulting improvements are shown in Figure 6.
Quantification was performed using an Extracted Internal Standard (EIS) approach, in which target PFAS were quantified relative to the isotopically labeled compound with the closest chemical similarity and retention behavior. Specifically, 13C3-PFBA was used as the internal standard for short-chain PFAS (C4–C6), while 13C8-PFOA was used for medium- and long-chain PFAS (C7–C14). Because isotopically labeled analogs were not available for all 28 analytes, the method does not constitute true isotope dilution (ID) as defined in EPA Method 1633. Instead, the quantification strategy corresponds to the EIS-based procedure described in Section 10.3 of EPA 1633 and is consistent with the surrogate-based calibration approach permitted by ISO 21675. The correspondence between analytes and their assigned internal standards is summarized in Supplementary Table S1. Time-segmented MRM acquisition (11 retention-time windows; Table S3) was used to increase dwell times and enhance sensitivity for late-eluting compounds.
3.4. Extraction and Cleanup Performance
The extraction of per- and polyfluoroalkyl substances (PFAS) from complex solid matrices, such as dehydrated sewage sludge, presents substantial analytical challenges. These arise primarily from the strong adsorption of PFAS to organic and particulate matter, the complex matrix composition, and the risk of co-extracted interferents affecting ionization during LC–MS/MS analysis. To overcome these limitations and enable accurate quantification of a broad spectrum of PFAS, a robust and efficient sample preparation protocol was developed and optimized.
The selected approach employed ultrasound-assisted extraction (UAE) on 0.5 g of freeze-dried and finely homogenized sludge. Samples were spiked with a mixture of 28 native PFAS at a concentration of 0.2 µg/g and two isotopically labeled internal standards at 0.3 µg/g. After an equilibration period, 10 mL of methanol was added to each sample. The mixture was vortexed and subjected to 30 min of sonication at 30 °C to promote analyte desorption and release from solid binding sites. To enhance extraction efficiency, a two-step protocol was implemented. Following centrifugation, the supernatant was collected, and the residue was re-extracted under the same conditions with an additional 10 mL of methanol. The combined extracts were purified using a ferrite/sodium sulfate column to remove particulates and residual moisture. The eluates were evaporated to dryness under a gentle nitrogen stream and then reconstituted in 1 mL of a 20:80 (v/v) water/methanol solution. Final extracts were filtered through 0.45 µm PTFE membranes and transferred to LC vials for instrumental analysis. This protocol proved highly effective for a wide range of PFAS, including both short-chain and long-chain compounds. Methanol, combined with ultrasonic agitation, facilitated the disruption of hydrophobic and electrostatic interactions, as previously reported in the literature [20,21]. Moreover, the moderate solvent-to-solid ratio (10 mL:0.5 g) provided efficient extraction while limiting dilution effects.
Although solid-phase extraction (SPE) using multi-sorbent cartridges (e.g., WAX, C18, ENVI-Carb, PSA) was preliminarily evaluated during method development, it was ultimately excluded from the final protocol due to significant analyte losses—particularly for short-chain PFAS—resulting from non-specific retention. Therefore, a simpler and more reproducible cleanup approach based on ferrite and sodium sulfate filtration was adopted, ensuring minimal matrix interference while maintaining high recovery rates across all PFAS classes. The overall method demonstrated good reproducibility, with relative standard deviations (RSDs) below 10% across three independent replicates. Slightly higher variability was observed for early-eluting, short-chain PFAS. Matrix effects, assessed by comparing the response of post-extraction spiked samples to neat standards, remained within ±15% for most compounds, aligning with regulatory performance thresholds [22,23,24].
3.5. Method Validation Parameters
The developed LC–MS/MS method for determining per- and polyfluoroalkyl substances (PFAS) in dehydrated sewage sludge was thoroughly validated against internationally recognized analytical standards. Key performance parameters—including linearity, precision, selectivity, matrix effects, recovery, and sensitivity (limits of detection and quantification)—were systematically evaluated. The results demonstrate the method’s robustness, accuracy, and suitability for trace-level PFAS analysis in complex environmental matrices, ensuring reliable data for environmental monitoring, regulatory compliance, and risk assessment purposes.
3.5.1. Linearity Assessment
Linearity is a fundamental criterion for quantitative analytical methods, ensuring that the detector response is directly proportional to the analyte concentration within a defined working range. In this study, the linearity of the LC–MS/MS method was evaluated for all 28 PFAS compounds over a concentration range of 0.02–0.6 µg/g (equivalent to 10–300 µg/L in the final extract), using seven calibration levels: 0.02, 0.05, 0.1, 0.2, 0.3, 0.4, and 0.6 µg/g, All calibration standards were prepared in PFAS-free sludge matrix and processed through the whole extraction and clean-up procedure. A fixed concentration of internal standard (IS) at 0.3 µg/g was used to normalize instrumental variability and correct for potential signal drift. Calibration curves were constructed using ordinary least squares (OLS) regression. The homoscedasticity of residuals across the working range (10–300 µg/L) was verified, and no significant variance heteroscedasticity was observed; therefore, unweighted regression was applied for all analytes. Calibration linearity was assessed according to ISO 17025:2017 and ICH Q2(R2) (2022), using the following acceptance criteria: coefficient of determination (R2) ≥ 0.995 (Table S4), residuals ≤ 20% of the predicted response, and consistent slope and intercept values across independent calibration curves.
All analytes fulfilled these criteria, confirming strong proportionality between concentration and detector response. Compounds such as PFEESA and DONA exhibited the steepest slopes (0.021 and 0.033, respectively), indicating a higher detector response per unit concentration and suggesting superior ionization efficiency or favorable fragmentation behavior in MS/MS conditions. In contrast, compounds with shallower slopes may require careful optimization to maintain sufficient sensitivity at lower concentration levels.
The consistency of slope values across multiple replicates and calibration sets also confirmed the stability of the detector response. In contrast, the low intercept values (<0.001) indicated minimal baseline signal or background interference in the sludge matrix. These findings collectively support the method’s suitability for quantitative applications, such as environmental contamination surveys, regulatory compliance, or risk assessment studies targeting PFAS in complex solid matrices.
3.5.2. Precision
Precision is a key performance criterion in analytical method validation, as it reflects the method’s reproducibility under both repeatability and intermediate precision conditions. In this study, the precision of the LC–MS/MS method was assessed at two concentration levels, 0.02 µg/g and 0.1 µg/g, representative of trace-level PFAS contamination in environmental sludge. Repeatability (intra-day precision) was evaluated by six replicate injections of a single extract for each concentration level. Intermediate precision (inter-day) was assessed by analyzing six subsamples prepared on different days by different analysts, following the whole sample preparation procedure. The results (Table 2) show that relative standard deviations (RSDr) were generally below 5% for most PFAS compounds across both concentration levels, indicating excellent method reproducibility. For example, PFOA, PFDA, and PFOSA exhibited RSDr values consistently below 2% at both tested levels, demonstrating outstanding stability of the method under controlled conditions. Compounds such as PFNA, PFPeA, and PFHpS also showed low variability, with RSDr values under 3%. Slightly higher RSDs (up to ~5%) were observed for more complex or less stable analytes such as PFOS, DONA, and PFHxS, which may be attributed to matrix interactions or minor fluctuations in ionization efficiency during electrospray operation. At the lower concentration level (0.02 µg/g), variability increased for a few compounds (e.g., PFMPA, PFHxA, PFDS) yet remained within acceptable thresholds (<15%) according to ECHA and EPA guidelines [5,6].

Table 2.
Repeatability and intermediate precision for 28 PFAS at two spiking levels (0.02 and 0.1 µg/g). Average and sr are expressed in µg/g; RSDr (intra-day repeatability) and RSDR (intermediate precision) are expressed in %RSD. sr = repeatability standard deviation; RSDr = relative standard deviation under repeatability conditions; RSDR = relative standard deviation under intermediate precision conditions.
Notably, a moderate increase in RSDs was seen for inter-day precision across all analytes, as expected when multiple analysts and time points are involved. Despite this, values remained well within acceptable limits, confirming the method’s robustness and suitability for routine application in environmental monitoring programs. These precision results support the method’s reliability for the quantification of PFAS in complex matrices such as sewage sludge, even at ultra-trace concentrations. Combined with the method’s accuracy and low detection limits, the high precision achieved underscores its applicability for regulatory compliance and risk assessment purposes in environmental studies.
3.5.3. Selectivity
Selectivity is a critical performance parameter for analytical methods, especially in complex environmental matrices such as sewage sludge, which may contain a multitude of interfering compounds. To assess the selectivity of the developed LC–MS/MS method for the determination of target per- and polyfluoroalkyl substances (PFAS), the complete sample preparation protocol—including extraction and concentration steps—was applied to three independent PFAS-free sludge blanks (n = 3). The analysis of these blanks revealed no detectable peaks at the retention times corresponding to the target analytes. Furthermore, any background signals present in the chromatograms exhibited signal-to-noise (S/N) ratios below 3, which is below the established detection threshold. These results confirmed that no significant co-eluting compounds interfered with the quantification or confirmation transitions of the PFAS analytes.
To illustrate the method’s specificity, Figure 7 presents a comparison between a chromatogram obtained from a blank sludge extract and that from a standard solution containing 10 µg/L of the PFAS mixture. The absence of interfering peaks in the blank sample further validates the method’s capacity to distinguish the target compounds from potential matrix interferences.
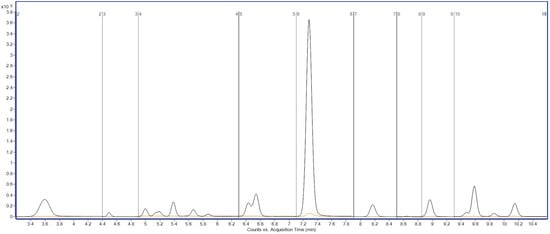
Figure 7.
Comparative total ion chromatograms (TICs) acquired in MRM mode: 10 µg/L PFAS standard vs. blank extract. Y-axis: signal intensity (counts or peak area); X-axis: acquisition time (min).
This method’s high selectivity is vital for trace-level environmental monitoring, where false positives or overlapping signals can distort contamination levels. The findings demonstrate the method’s reliability in analyzing complex solid matrices, like wastewater treatment sludge. This supports its use in regulatory monitoring and risk assessment of PFAS pollutants, ensuring accurate data for environmental safety.
3.5.4. Matrix Effects Evaluation
Matrix effects (MEs) represent a significant challenge in LC–MS/MS analysis, particularly when analyzing complex environmental samples such as sewage sludge. These effects arise from co-eluting matrix components that can suppress or enhance the ionization of target analytes in the electrospray ionization (ESI) source, ultimately affecting the accuracy, sensitivity, and reproducibility of the method. Therefore, evaluating and quantifying matrix effects is essential to ensure the reliability of quantitative results, especially when analytes are present at trace levels. In this study, matrix effects were assessed using the post-extraction addition method and expressed as a percentage (% ME), as described in Equation (1).
Analyzing the data, significant variations in matrix effects were observed among different compounds. For instance, PFMBA (150%), PFHxA (142%), and PFEESA (136%) exhibited the highest matrix effect values, suggesting that the sludge matrix may enhance the ionization of these compounds in the ESI source or that specific interactions within the matrix contribute to more efficient detection. Other compounds, such as PFHpA (129%), PFNS (131%), and PFBS (122%), were also positively affected by matrix effects, confirming that certain matrix conditions can facilitate analyte ionization (Table 3). Conversely, compounds like NFDHA (77%) and PFDS (80%) showed significantly lower matrix effect values, indicating ion suppression that could negatively impact their detection. This suggests that the matrix exerts an adverse influence on these analytes, requiring further optimization of extraction procedures or analytical conditions.

Table 3.
Instrumental detection limit (IDL), method detection limits (MDL), and quantitation (MQL) recovery and matrix effects (ME) for the 28 PFAS analytes.
Evaluating matrix effects is crucial for optimizing analytical methodologies. Identifying compounds that are more susceptible to matrix influence can guide the refinement of analytical protocols and support method validation in complex environmental matrices [22,23]. These observations are particularly relevant in the context of monitoring environmental pollutants in water, with direct implications for risk assessment and environmental regulation.
3.5.5. Recovery
Recovery performance is an important parameter for method validation, particularly when analyzing trace-level contaminants in complex matrices like sewage sludge. In this study, absolute recoveries for the 28 PFAS analytes were determined to evaluate the efficiency of the developed UAE-based extraction protocol. Recoveries were calculated by comparing the peak areas of post-extraction spiked samples (corrected for any background signal in blanks) to those of equivalent concentration calibration standards prepared in pure solvent. The results, presented in Table 3, demonstrated high extraction efficiency for both short- and long-chain PFAS compounds.
Short-chain PFAS, such as PFBA and PFPeA, which are typically challenging to extract due to their high polarity and low retention on sorbents, showed favorable recovery values. This underscores the method’s capability to extract even more mobile and hydrophilic compounds efficiently. At the same time, long-chain PFAS like PFOS, PFUnDA, and PFDA—known for their strong affinity to organic matter—also exhibited consistent recoveries, indicating that the method effectively disrupts analyte–matrix interactions. These findings validate the use of methanol as an extraction solvent and support the effectiveness of sonication in enhancing analyte release from complex solid phases. The combination of physical (UAE) and chemical (solvent) actions proved essential for achieving quantitative extraction across a structurally diverse group of PFAS.
The recovery results confirm that the method satisfies the accuracy and extraction efficiency criteria required for quantitative LC–MS/MS methods, as defined by current international standards and regulatory frameworks such as US EPA Method 1633 and EN ISO 21675:2021 [5,16]. Furthermore, the methodology demonstrates sufficient flexibility to accommodate emerging PFAS classes and future monitoring needs.
3.5.6. Sensitivity—Limits of Detection and Quantification
The sensitivity of the developed LC–MS/MS method was assessed by determining the method detection limits (MDL) and method quantification limits (MQL) for all 28 target PFAS compounds in dehydrated sewage sludge. MDLs and MQLs were calculated based on signal-to-noise (S/N) ratios of 3 and 10, respectively, using serial dilutions of PFAS standards spiked into matrix extracts to simulate real analytical conditions. The final values reported account for the entire analytical workflow, including sample preparation, extraction, and concentration, thereby reflecting the actual method sensitivity in the presence of matrix interferences. The method demonstrated excellent sensitivity across a broad range of PFAS, with MDLs ranging from 0.0004 to 0.0013 µg/g, and MQLs from 0.0012 to 0.0037 µg/g (Table 3). These values confirm the method’s capability to detect PFAS at ultra-trace levels in complex solid waste matrices. Notably, the compound PFEESA exhibited the lowest MQL value, highlighting the high efficiency of ionization and low background interference for these analytes. In contrast, slightly higher MQLs were observed for long-chain or highly functionalized PFAS, such as PFUnDA and PFDS, likely due to reduced ionization efficiency or more substantial matrix effects. The low MDL and MQL values achieved for the full suite of PFAS analytes confirm the method’s suitability for monitoring trace-level contamination in sludge, supporting environmental surveillance, regulatory compliance, and risk assessment efforts.
3.5.7. Robustness
Robustness testing is essential to confirm the stability and reliability of an analytical method when subject to minor, intentional variations in experimental conditions. In this study, the robustness of the LC–MS/MS method for PFAS quantification in dehydrated sewage sludge was evaluated by slightly modifying key instrumental parameters during chromatographic separation and injection.
The tested variations included changes in flow rate (±0.02 mL/min from the nominal 0.3 mL/min), column temperature (±2 °C from the optimized 40 °C), and injection volume (±1 µL around the standard 10 µL) (Figure 8). These conditions were selected to simulate minor fluctuations that may occur during routine laboratory operation due to instrument drift or operator variability. Each modified condition was applied independently to samples spiked with a PFAS mixture at 0.1 µg/g, and the impact on retention time, peak area, and chromatographic resolution was monitored for all analytes. Across all experiments, retention time shifts remained below ±3%, and no significant changes in peak symmetry or baseline separation were observed. Similarly, peak areas varied within ±10%, and no loss of analytical signal or co-elution issues were detected. The method was considered robust under all tested conditions, as performance metrics remained within generally accepted thresholds for LC–MS/MS quantitative analysis. In addition, broader robustness—reflecting real-laboratory variability—was demonstrated through the intermediate precision tests (Section 3.5.2), which involved independently prepared subsamples analyzed on different days by different analysts. The consistent recoveries (70–130%) and inter-day RSDR values ≤ 15% confirm that the method maintains reliability across variations in operators, days, and sludge matrices.
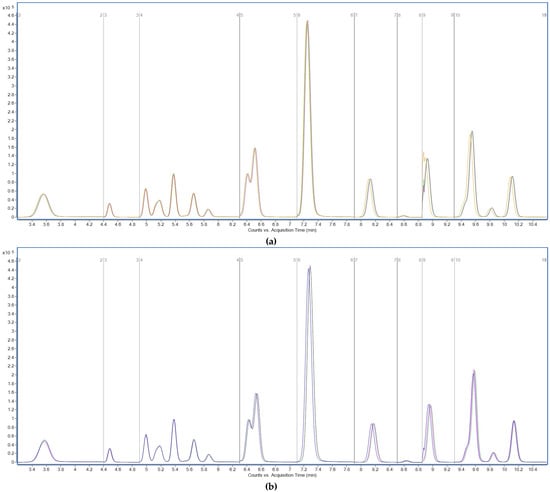
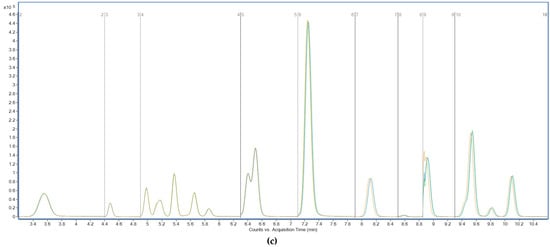
Figure 8.
Total ion chromatograms recorded in MRM mode during on robustness assessment. Effect of minor changes in flow rate (a), temperature (b), and injection volume (c) on LC–MS/MS method performance. Y-axis: signal intensity (counts or peak area); X-axis: acquisition time (min).
Overall, the robustness of the developed method ensures reliable performance during extended monitoring campaigns and across different operators or instrumentation. This characteristic is particularly important in the context of environmental PFAS surveillance, where reproducibility and data comparability are critical for regulatory and scientific reporting.
3.6. Comparative Performance of Analytical Methods
A comparative analysis of published methods for PFAS determination in solid environmental matrices, particularly sewage sludge and soil, reveals significant variability in analytical strategies, influenced by regional standards, instrumentation, and targeted PFAS classes [5,25,26,27,28,29,30,31,32,33,34,35,36,37,38,39,40]. Table 4 consolidates relevant data from 2020–2024 studies, providing insight into differences in extraction techniques, cleanup procedures, instrumentation, and detection performance.

Table 4.
PFAS analysis methods in sludge: sample preparation and detection limits.
Extraction of PFAS from sludge remains a critical step due to the matrix’s complexity and heterogeneity. Our method, which uses a two-step ultrasound-assisted extraction (UAE) in methanol followed by ferrite/sodium sulfate cleanup, offers a robust and reproducible protocol, with minimal matrix interferences and high recovery across a broad range of PFAS. Compared to more aggressive techniques such as alkaline digestion combined with ion-pair extraction [28], our method avoids the use of caustic reagents while achieving comparable or superior recovery for both short- and long-chain PFAS. Other studies employed oxidative strategies to quantify precursors, including heat-activated persulfate oxidation [29,30], often integrated into Total Oxidizable Precursor (TOP) assays. While these methods offer valuable information on the total PFAS burden, including unknown precursors, they add considerable complexity, increase analysis time, and can lead to overestimation or degradation of thermally unstable PFAS.
A key differentiator across methods lies in the sample cleanup stage. SPE-WAX and graphitized carbon black (GCB) sorbents [32] are commonly used to remove co-extracted organics but have been associated with analyte losses, particularly for ultra-short-chain PFAS. Our use of a ferrite/sodium sulfate combination provides efficient matrix removal without compromising recovery for polar or volatile compounds. Some studies apply sequential SPE strategies or dispersive solid-phase extraction (dSPE) with ENVI-Carb [32,34]. While effective, these methods require additional manual steps and conditioning, increasing the risk of variability and analyte loss, particularly in field applications or inter-laboratory comparisons.
Most reviewed studies applied LC–MS/MS, LC–QTOF/MS, or CIC techniques, covering between 10 and 73 PFAS depending on analytical scope. The EPA Method 1633, the current international benchmark, quantifies 40 PFAS with MDLs of 0.00005–0.0004 µg/g. In comparison, the method developed in this study achieved MDLs of 0.0004–0.0013 µg/g and MQLs of 0.0012–0.0037 µg/g, comparable to or lower than those reported for sludge analyses in other regions (e.g., Germany, Canada). These results demonstrate high analytical sensitivity and confirm the method’s suitability for trace-level PFAS monitoring in sewage sludge.
Several published methods prioritize either targeted PFAS analysis or nontarget screening via high-resolution mass spectrometry (HRMS). While these strategies are valuable for discovery studies, they may lack the quantification precision and reproducibility required for regulatory compliance. Our method aligns with international validation protocols [5,17], offering a practical balance between analytical depth and operational feasibility. Moreover, although methods based on combustion ion chromatography (CIC) enable estimation of total organic fluorine, they do not provide compound-specific data necessary for risk assessment or regulatory reporting. Our method, by contrast, is designed to fulfill these specific requirements, offering both quantitative accuracy and compound-level resolution.
3.7. Application of the LC–MS/MS Method to Real Sludge Samples
To evaluate the practical applicability of the developed LC–MS/MS method, six dehydrated sewage sludge samples were collected from municipal wastewater treatment plants (WWTPs) in Romania during the spring of 2024. Each sample underwent triplicate analysis to ensure consistency, reproducibility, and robustness of the results under routine conditions. The samples were processed following the optimized ultrasound-assisted extraction (UAE) protocol, which was previously demonstrated to provide effective release of both short- and long-chain PFAS compounds from solid matrices. The extracts were subjected to solid-phase cleanup and subsequently analyzed using the validated LC–MS/MS method targeting 28 perfluoroalkyl substances.
The analytical results revealed that all six samples exhibited PFAS concentrations below the established limits of quantification (MQLs) for the method. These MQLs ranged between 0.005 and 0.037 µg/g, as previously determined during method validation. The absence of PFAS signals above the MQL does not necessarily indicate that the analytes were present below the method detection limits (MDLs). Instead, concentrations may also fall within the interval between MDL and MQL (MDL < X < MQL), where PFAS are detectable but cannot be quantified with the validated accuracy and precision. Therefore, the non-quantifiable findings in this study reflect concentrations below the validated quantification capability of the method (MQL), while the method’s analytical sensitivity remains defined by the established MDL values (0.004–0.018 μg/g). This interpretation is further supported by the satisfactory recoveries (70–130%) and precision (RSD ≤ 15%) obtained during method validation, confirming that the analytical protocol provides adequate sensitivity for trace-level quantification in solid matrices. Thus, the non-detects observed are most plausibly attributed to genuinely low PFAS burdens in the analyzed sludge rather than method-related limitations.
These findings reflect the effectiveness of the wastewater treatment technologies employed at the source WWTPs, which potentially removed or degraded PFAS compounds before sludge dewatering. Additionally, the nature of the influent wastewater, which may vary depending on industrial, domestic, or seasonal contributions, could significantly influence the occurrence and concentration of PFAS in biosolids. Furthermore, the type and efficiency of the treatment processes—such as biological activated sludge, tertiary filtration, or chemical oxidation—may play a decisive role in PFAS fate during wastewater processing. Another possible explanation is that the selected WWTPs were relatively small facilities serving limited populations and with low industrial inputs, which may have resulted in reduced PFAS loading to both influent and sludge. This contrasts with our previous study [7], which investigated nine PFAS in samples from five large Romanian WWTPs, including sludge, and where quantifiable levels of target compounds were detected. The discrepancy likely reflects differences in plant scale, influent composition, and treatment efficiency rather than methodological sensitivity. The current findings are consistent with recent European studies reports showing minimal or non-detectable PFAS in sludge from advanced treatment systems operating under optimized conditions [11,28,29].
Despite the non-detectable levels in the current dataset, it is important to emphasize that this outcome does not imply the absence of PFAS in the tested samples but rather reflects concentrations below the quantifiable range of the applied analytical protocol. To further improve detection capability, future applications may consider implementing additional sample concentration steps, employing selective preconcentration methods, or enhancing signal response through increased injection volumes. Such strategies could reveal trace-level contamination and further delineate PFAS occurrence patterns in environmental solids. In addition, further monitoring across different seasons is recommended to capture possible temporal variations. In future work, selected samples will also be submitted to an accredited laboratory for independent verification. These additional optimizations, combined with continuous monitoring programs, would enable more comprehensive evaluation of PFAS fate and persistence in Romanian sewage sludge and contribute to aligning national datasets with European and international monitoring frameworks.
In summary, the developed LC–MS/MS method proved to be robust, reproducible, and sensitive, demonstrating reliable quantification capability in complex matrices. Its application to real-world samples confirms its analytical performance, while acknowledging that broader validation through external proficiency testing would be beneficial. These results underline the method’s potential for integration into routine PFAS surveillance programs in Romania, where data on PFAS in biosolids remain scarce.
3.8. Analytical Figures of Merit
The optimized analytical method presented in this study offers a robust, high-throughput platform for the determination of 28 PFAS compounds in dehydrated sewage sludge, combining efficient chromatographic separation, sensitive MS/MS detection, and effective sample preparation. The key analytical figures of merit demonstrate the method’s applicability for both regulatory monitoring and environmental research purposes. The total chromatographic runtime was 15.5 min per sample, including column re-equilibration. Within this window, all analytes were successfully separated with baseline resolution, including structural isomers and compounds with closely related retention behavior such as PFHxA/PFHxS and PFHpA/PFHpS. The use of a PFAS-specific stationary phase and gradient optimization ensured narrow peak widths and high chromatographic efficiency, even for short-chain acids eluting early in the gradient.
Reproducibility of retention times was excellent across over 150 sequential injections, with relative standard deviations below 0.1% for all analytes. No significant drift or carryover was observed, confirming the method’s stability and suitability for batch analysis. This level of performance is critical when analyzing large sets of environmental samples with low expected PFAS concentrations and potential matrix complexity. Sensitivity was a core advantage of the method. Limits of detection (MDL) were typically below 0.015 µg/g dw, and limits of quantification (MQL) ranged between 0.013 and 0.037 µg/g dw, depending on analyte structure and ionization behavior. These values are well below proposed regulatory thresholds for PFAS in biosolids, such as those discussed in the EU Strategy for Sustainable Chemicals and the U.S. EPA’s risk assessments on PFAS in wastewater residuals [5,34]. The inclusion of isotopically labeled internal standards allowed for accurate quantification by correcting for analyte losses during extraction and cleanup, as well as for ion suppression during ESI. The precision of the method was further supported by low intra-day and inter-day variability (RSD <10% and <15%, respectively), even when applied to sludge samples with diverse organic and inorganic content. No significant deterioration in column performance or loss of analyte recovery was observed after repeated analysis, confirming the method’s robustness over time. This was achieved without additional rinsing cycles or frequent hardware maintenance, underscoring the method’s applicability for high-throughput laboratories.
The method combines high resolution, speed, sensitivity, and reliability, making it well-suited for monitoring PFAS in solid environmental matrices. Its compatibility with existing regulatory standards and its potential scalability to automated workflows further support its use in routine analytical laboratories.
3.9. Strengths and Limitations of the Method
The analytical method developed in this study demonstrates several notable strengths, particularly in addressing the complex analytical challenges associated with the quantification of PFAS in solid matrices such as dehydrated sewage sludge collected from Romanian wastewater treatment plants. Unlike multi-sorbent SPE-based protocols, the method integrates a simplified ferrite/sodium sulfate cleanup step, which provides reproducible recoveries and reduces matrix interference while remaining cost-effective and compatible with local laboratory resources. First, the use of a PFAS-specific chromatographic column, in combination with a carefully optimized gradient and mobile phase composition, enabled complete baseline separation of 28 target analytes, including short- and long-chain perfluorinated acids and sulfonates. This level of resolution is critical for avoiding quantification errors arising from co-elution or isomeric interference, especially in complex environmental samples.
Another major strength is the method’s sensitivity and broad dynamic range. Detection limits below 0.015 µg/g dw were achieved for most analytes without the need for pre-concentration or derivatization, and quantification was possible below the limits proposed by current and upcoming regulatory frameworks. The method’s use of isotopically labeled internal standards further enhanced its quantitative accuracy and corrected for variations in recovery, matrix effects, and instrument response drift. In addition, the sample preparation protocol, involving mild alkaline extraction followed by optimized ferrite/sodium sulfate cleanup, provided acceptable recoveries for all PFAS classes with minimal matrix interference and no significant analyte degradation. This simplified and reproducible cleanup strategy represents a practical alternative to multi-sorbent SPE procedures, particularly for regional laboratories that may lack advanced automated extraction systems.
The method also demonstrated excellent robustness over time. More than 150 consecutive injections were carried out without column fouling, retention time shifts, or significant signal loss, confirming the method’s suitability for high-throughput applications in environmental monitoring.
Despite these strengths, several limitations should be acknowledged. Short-chain PFAS, such as PFBA and PFPeA, exhibited slightly elevated recoveries, likely due to mild matrix-induced ionization enhancement or incomplete sorbent-analyte interactions during cleanup. While these effects were mitigated using internal standard correction, they may limit the method’s suitability for analyte profiling in matrices with highly variable composition, such as compost, sludge from industrial wastewater treatment plants, or samples with extreme pH or organic content.
Another limitation is the method’s dependence on high-quality LC–MS/MS instrumentation with negative electrospray capability and sufficient dwell time control to handle scheduled MRM acquisition. Laboratories without access to triple quadrupole systems or those using older instrumentation may not achieve the same sensitivity and reproducibility, particularly for low-abundance PFAS in complex solids. Lastly, while the method covers 28 common PFAS, it does not include ultra-short chain PFAS (e.g., trifluoroacetic acid) or emerging replacement compounds such as GenX and ADONA, which may require different extraction or chromatographic conditions. Expansion of the method to include these compounds would require further validation and likely adjustments to the chromatographic and mass spectrometric parameters.
The method offers a practical, sensitive, and reproducible platform for PFAS analysis in sewage sludge and similar matrices, while recognizing the need for careful calibration and potential method adaptation depending on sample type and analytical scope.
4. Conclusions
This study presents a validated LC–MS/MS method for the determination of 28 per- and polyfluoroalkyl substances (PFAS) in dehydrated sewage sludge. The method combines ultrasound-assisted methanolic extraction with a simplified ferrite/sodium sulfate cleanup, achieving recoveries between 70–130% and precision (RSD) below 15% across all tested analytes. The optimized chromatographic setup—using an Avantor® ACE® PFAS Delay column coupled to an HTP-MS analytical column—provided complete separation of short- and long-chain PFAS with stable retention times and excellent reproducibility over 150 injections. The method achieved method detection limits (MDLs) of 0.0004–0.0013 µg/g and quantification limits (MQLs) of 0.0011–0.0037 µg/g, enabling trace-level analysis compliant with current international performance requirements. Application to six Romanian WWTP sludge samples yielded PFAS concentrations below LOQs, suggesting low environmental burdens rather than analytical limitations.
This outcome supports the effectiveness of current wastewater treatment technologies and highlights the need for continued national monitoring to track PFAS fate in biosolids. The method’s robustness, cost-efficiency, and compatibility with standard LC–MS/MS platforms make it suitable for regulatory and research laboratories, and it can be further adapted to other solid matrices such as soils and sediments.
Future work should extend validation to emerging PFAS analogs (e.g., GenX, ADONA, ultra-short chains) and include inter-laboratory testing and seasonal sampling to strengthen representativeness and comparability across monitoring programs.
Supplementary Materials
The following supporting information can be downloaded at https://www.mdpi.com/article/10.3390/analytica6040049/s1, Table S1: Physicochemical properties of PFAS compounds; Table S2: The gradient program used to separate the 28 compounds; Table S3: Acquisition windows set for the most sensitive detection of the investigated analytes; Table S4: Calibration curve parameters for the 28 PFAS compounds (slope, intercept, R2).
Author Contributions
Conceptualization, V.A.P. and F.L.C.; methodology, L.F.P., V.I.I., and I.A.C.; software, L.F.P. and V.I.I.; validation, V.I.I., V.A.P., and F.L.C.; formal analysis, L.F.P. and I.A.C.; investigation, V.A.P. and F.L.C.; resources, L.F.P. and V.I.I.; data curation, L.F.P. and I.A.C.; writing—original draft preparation, V.A.P., L.F.P., V.I.I., I.A.C., and F.L.C.; writing—review and editing, V.A.P., L.F.P., V.I.I., I.A.C., and F.L.C.; visualization, L.F.P. and F.L.C.; supervision, L.F.P. and F.L.C.; project administration, F.L.C.; funding acquisition, V.I.I. and I.A.C. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Data Availability Statement
The original contributions presented in this study are included in the article/Supplementary Materials. Further inquiries can be directed to the corresponding authors.
Acknowledgments
This work was carried out through the “Nucleu” Program within the National Research Development and Innovation Plan 2022–2027 with the support of the Romanian Ministry of Research, Innovation and Digitalization, Contract No. 3N/2022, Project Codes PN 23 22 01 01. During the preparation of this manuscript, the authors used AI technology to assist with language editing and text refinement. The authors have thoroughly reviewed and edited the AI-generated content and take full responsibility for the final version of the manuscript.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Giesy, J.P.; Kannan, K. Perfluorochemical surfactants in the environment. Environ. Sci. Technol. 2002, 36, 146A–152A. [Google Scholar] [CrossRef] [PubMed]
- Grandjean, P.; Clapp, R. Perfluorinated Alkyl Substances: Emerging Insights into Health Risks. New Solut. 2015, 25, 147–163. [Google Scholar] [CrossRef]
- OECD. Toward a New Comprehensive Global Database of PFAS. 2021. Available online: https://www.oecd.org/chemicalsafety/portal-perfluorinated-chemicals/ (accessed on 22 August 2025).
- ChemSec—International Chemical Secretariat. The Top 12 PFAS Producers in the World and the Staggering Societal Costs of PFAS Pollution. ChemSec Report, Gothenburg, Sweden. 2023. Available online: https://chemsec.org/reports/the-top-12-pfas-producers-in-the-world-and-the-staggering-societal-costs-of-pfas-pollution/ (accessed on 20 August 2025).
- USEPA. Method 1633, Revision A—Analysis of Per- and Polyfluoroalkyl Substances (PFAS) in Aqueous, Solid, Biosolids, and Tissue Samples by LC-MS/MS; EPA 820-R-24-007; USEPA: Washington, DC, USA, 2024. Available online: https://www.epa.gov/system/files/documents/2024-12/method-1633a-december-5-2024-508-compliant.pdf (accessed on 22 August 2025).
- ECHA. Universal PFAS Restriction Proposal Under REACH. 2023. Available online: https://echa.europa.eu/restrictions-under-consideration (accessed on 22 August 2025).
- Chiriac, F.L.; Pirvu, F.; Paun, I.; Petre, V.A. Perfluoroalkyl substances in Romanian wastewater treatment plants: Transfer to surface waters, environmental and human risk assessment. Sci. Total Environ. 2023, 892, 164576. [Google Scholar] [CrossRef] [PubMed]
- Pascu, L.F.; Petre, V.A.; Cimpean, I.A.; Paun, I.; Pirvu, F.; Chiriac, F.L. Managing PFAS in Sewage Sludge: Exposure Pathways, Impacts, and Treatment Innovations. J. Xenobiotics 2025, 15, 135. [Google Scholar] [CrossRef] [PubMed]
- Ghisi, R.; Vamerali, T.; Manzetti, S. Accumulation of perfluorinated alkyl substances (PFAS) in agricultural plants: A review. Environ. Res. 2019, 169, 326–341. [Google Scholar] [CrossRef] [PubMed]
- David, V.; Medvedovici, A. Modern sample preparation for LC–MS analysis of pharmaceuticals in environmental and biological matrices. Bioanalysis 2013, 5, 1993–2011. [Google Scholar]
- Srivastava, P.; Macdonald, B. PFAS in biosolids: Insights into current and future challenges. J. Hazard. Mater. Lett. 2025, 6, 100163. [Google Scholar] [CrossRef]
- Venkatesan, A.K.; Halden, R.U. National inventory of perfluoroalkyl substances in archived U.S. biosolids. Environ. Sci. Technol. 2013, 47, 525–533. [Google Scholar]
- Vierke, L.; Berger, U.; Cousins, I.T.; Filipovic, M.; Glynn, A.; Scheringer, M.; Bignert, A. Transfer of perfluoroalkyl substances (PFASs) from contaminated soils to plants. Environ. Pollut. 2012, 158, 3134–3141. [Google Scholar]
- Chiriac, F.L.; Petre, V.A.; Cimpean, I.A.; Cojocaru, V.C.; Paun, I.; Pirvu, F.; Iancu, V.I. New LC-MS/MS method for the determination of unconventional organic pollutants: Perfluoroalkyl sulfonic acids in wastewater, surface water, and drinking water. Rom. J. Ecol. Environ. Chem. 2024, 6, 7–20. [Google Scholar] [CrossRef]
- Chiriac, F.L.; Petre, V.A.; Paun, I.; Pirvu, F. Occurrence of PFAS in Romanian WWTP effluents: Implications for downstream aquatic ecosystems. Sci. Total Environ. 2023, 884, 163875. [Google Scholar]
- EC. Council Directive 86/278/EEC on the Protection of the Environment When Sewage Sludge Is Used in Agriculture. Off. J. L 1986, 0006–0012. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/?uri=CELEX:31986L0278 (accessed on 22 August 2025).
- ISO 21675:2019; Water Quality—Determination of Perfluoroalkyl and Polyfluoroalkyl Substances (PFAS) in Water—Method Using Solid Phase Extraction and Liquid Chromatography-Tandem Mass Spectrometry (LC-MS/MS). ISO: Geneva, Switzerland, 2019.
- Avantor ACE Technical Note, 2023. PFAS Analysis Using ACE PFAS Delay and Analytical Columns. Avantor® Technical Publication, Avantor Sciences, UK. Available online: https://www.ace-hplc.com (accessed on 23 March 2025).
- International Council for Harmonisation (ICH). ICH Harmonised Guideline Q2(R2): Validation of Analytical Procedures; ICH: Geneva, Switzerland, 2022; Available online: https://www.ich.org (accessed on 23 March 2025).
- Fredriksson, H.; Eriksson, U.; Kärrman, A.; Haglund, P. Perfluorinated chemicals and polybrominated diphenyl ethers in sewage sludge and leachate from landfill in Sweden. Chemosphere 2011, 84, 1353–1359. [Google Scholar]
- Ozelcaglayan, O.; van der Veen, I.; van Leeuwen, S.; de Boer, J. Optimization of a method for PFAS analysis in sewage sludge and validation for the determination of 26 PFAS. Sci. Total Environ. 2020, 705, 135840. [Google Scholar]
- Mattila, J.M.; Li, E.Y.; Offenberg, J.H. Tubing material considerably affects measurement delays of gas-phase oxygenated per- and polyfluoroalkyl substances. J. Air Waste Manag. Assoc. 2023, 73, 335–344. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- David, V.; Medvedovici, A. Advanced sample preparation techniques for PFAS analysis in complex environmental matrices. J. Chromatogr. A 2013, 1294, 1–14. [Google Scholar]
- Tănase, I.G.; Petre, J.; David, V. Validarea Metodelor Analitice—Aplicații în Spectrometrie de Masă și Cromatografie; Ed. Printech: București, Romania, 2016. [Google Scholar]
- Idowu, I.G.; Ekpe, O.D.; Megson, D.; Bruce-Vanderpuije, P.; Sandau, C.D. A systematic review of methods for the analysis of total per- and polyfluoroalkyl substances (PFAS). Sci. Total Environ. 2025, 967, 178644. [Google Scholar] [CrossRef]
- Alukkal, C.R.; Modiri, M.; Ruiz, R.A.; Choi, Y.J.; Lee, L.S. Evaluation of PFAS extraction and analysis methods for biosolids. Talanta 2025, 286, 127485. [Google Scholar] [CrossRef] [PubMed]
- Dennis, N.M.; Braun, A.J.; Gan, J. A high-throughput analytical method for complex contaminant mixtures in biosolids. Environ. Pollut. 2024, 345, 123517. [Google Scholar] [CrossRef] [PubMed]
- Aro, R.; Eriksson, U.; Kärrman, A.; Chen, F.; Wang, T.; Yeung, L.W.Y. Fluorine mass balance analysis of effluent and sludge from Nordic countries. ACS EST Water 2021, 1, 2087–2096. [Google Scholar] [CrossRef]
- Liu, S.; Zhou, A.; Fan, Y.; Duan, Y.; Liu, Z.; He, Z.; Yue, X. Using heat-activated persulfate to accelerate short-chain fatty acids production from waste activated sludge fermentation triggered by sulfate-reducing microbial consortium. Sci. Total Environ. 2023, 861, 160795. [Google Scholar] [CrossRef] [PubMed]
- Shojaei, M.; Kumar, N.; Chaobol, S.; Wu, K.; Crimi, M.; Guelfo, J. Enhanced recovery of per- and polyfluoroalkyl substances (PFASs) from impacted soils using heat activated persulfate. Environ. Sci. Technol. 2021, 55, 9805–9816. [Google Scholar] [CrossRef] [PubMed]
- Müller, V.; Kindness, A.; Feldmann, J. Fluorine mass balance analysis of PFAS in communal waters at a wastewater plant from Austria. Water Res. 2023, 244, 120501. [Google Scholar] [CrossRef] [PubMed]
- Vogel, C.; Roesch, P.; Wittwer, P.; Piechotta, C.; Lisec, J.; Sommerfeld, T.; Kluge, S.; Herzel, H.; Huthwelker, T.; Borca, C.; et al. Levels of per- and polyfluoroalkyl substances (PFAS) in various wastewater-derived fertilizers—Analytical investigations from different perspectives. Environ. Sci. Adv. 2023, 2, 1436–1445. [Google Scholar] [CrossRef]
- Spaan, K.M.; Seilitz, F.; Plassmann, M.M.; de Wit, C.A.; Benskin, J.P. Pharmaceuticals account for a significant proportion of the extractable organic fluorine in municipal wastewater treatment plant sludge. Environ. Sci. Technol. Lett. 2023, 10, 328–336. [Google Scholar] [CrossRef]
- Kärrman, A.; Yeung, L.W.Y.; Spaan, K.M.; Lange, F.T.; Nguyen, M.A.; Plassmann, M.; De Wit, C.A.; Scheurer, M.; Awad, R.; Benskin, J.P. Can determination of extractable organofluorine (EOF) be standardized? First interlaboratory comparisons of EOF and fluorine mass balance in sludge and water matrices. Environ. Sci. Process. Impacts 2021, 23, 1458–1465. [Google Scholar] [CrossRef]
- Roesch, P.; Vogel, C.; Huthwelker, T.; Wittwer, P.; Simon, F.-G. Investigation of per- and polyfluoroalkyl substances (PFAS) in soils and sewage sludges by fluorine K-edge XANES spectroscopy and combustion ion chromatography. Environ. Sci. Pollut. Res. Int. 2021, 29, 26889–26899. [Google Scholar] [CrossRef]
- Nickerson, A.; Rodowa, A.E.; Adamson, D.T.; Field, J.A.; Kulkarni, P.R.; Kornuc, J.J.; Higgins, C.P. Spatial trends of anionic, zwitterionic, and cationic PFASs at an AFFF-impacted site. Environ. Sci. Technol. 2020, 55, 313–323. [Google Scholar] [CrossRef]
- OECD. Toward a New Comprehensive Global Database of Per- and Polyfluoroalkyl Substances (PFASs); MONO(2018)7; OECD: Paris, France, 2018. [Google Scholar] [CrossRef]
- Spaan, K.M.; Yuan, B.; Plassmann, M.M.; Benskin, J.P.; de Wit, C.A. Characterizing the Organohalogen Iceberg: Extractable, Multihalogen Mass Balance Determination in Municipal Wastewater Treatment Plant Sludge. Environ. Sci. Technol. 2023, 57, 9309–9320. [Google Scholar] [CrossRef]
- Hutchinson, S.; Rieck, T.; Wu, X. Advanced PFAS precursor digestion methods for biosolids. Environ. Chem. 2020, 17, 558–567. [Google Scholar] [CrossRef]
- Kim, J.; Xin, X.; Mamo, B.T.; Hawkins, G.L.; Li, K.; Chen, Y.; Huang, Q.; Huang, C.-H. Occurrence and fate of ultrashort-chain and other per- and polyfluoroalkyl substances (PFAS) in wastewater treatment plants. ACS EST Water 2022, 2, 1380–1390. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).