Abstract
The study evaluated the effect of moderate heating temperatures on physical, mechanical, and spectral properties of bulk flaxseeds and pressed oils. The samples of bulk flaxseeds were measured at 60 mm pressing height and subjected to pretreatment temperatures between 40 °C and 60 °C at 5 °C intervals at a constant heating time of 30 min. The uniaxial compression process, comprising a pressing chamber of a diameter of 60 mm with a plunger, was used for extracting the oil under a load of 300 kN and a speed of 5 mm/min. Prior to the oil extraction, the moisture content of the flaxseeds samples was determined to be 8.15 ± 0.07% d.b., and that of oil content was 40.32 ± 0.02%. Based on the results obtained, porosity, density, oil yield, and oil expression efficiency significantly correlated positively (p-value < 0.05) with the increase in heating temperatures. However, kinematic and dynamic viscosities, compressive stress, deformation energy, and hardness did not significantly correlate (p-value > 0.05) with heating temperature. The study revealed that heating temperatures increased oil yield from 11.54% to 24.18% and oil expression efficiency from 28.62% to 59.96% with the corresponding deformation energy of 0.698 ± 0.011 kJ at 60 °C. The findings suggest that mild thermal pretreatment of flaxseeds improves oil recovery with minimal energy requirement under the linear compression process.
1. Introduction
Flaxseed (Linum usitatissimum L.) is a member of the Linaceae family. The seed is flat and oval with a pointed tip, and the color ranges from reddish-brown to a light yellow. The dimensions of the seed vary approximately from 3.0 to 6.4 mm in length, 1.8 to 3.4 mm in width, and 0.5 to 1.6 mm in thickness [1]. As the source of linen fiber, flax has been cultivated since at least 5000 BC, and, in the present day it is mainly grown for its oil, which is used for producing paints, varnishes, and linoleum due to its drying and hardening properties when exposed to air and sunlight [1,2,3]. Flaxseed oil is widely popular for its dietary benefits and has the potential to reduce the risk of chronic illnesses [4]. The oil is also utilized as a purgative for sheep and horses, and the meal is used as animal feed and human food. In poultry feeding, flaxseed meals increase the levels of omega-3 fatty acids in eggs [1,5]. In addition to the above benefits, the whole seed is used in the baking of multigrain bread, biscuits, confectionery industries, and organic human consumption products [1,6,7]. Flaxseed yields (30–45%), protein (20–30%), total dietary fiber (20–28%), moisture content (4–8%), and ash (3–4%), and the oil contains vitamins A, B, D, and E, minerals and amino acids, 9–10% of saturated fatty acids (palmitic and stearic), about 20% monounsaturated fatty acids (mainly oleic acid), and >70% alpha-linolenic acid [1,8].
The oil extraction technique is essential for recovering high-quality oil, and heat treatment is a crucial part in determining the oil’s final qualities and characteristics. Therefore, the heat treatment method is frequently used in the extraction process to enhance oil production and extraction efficiency. However, it can significantly change the oil’s mechanical, chemical, and physical properties such as density, oxidative stability, and viscosity, and it may break down delicate bioactive substances that are vital for the health benefits of the oil including antioxidants and polyunsaturated fatty acids [9,10,11,12,13]. In addition, the oxidation processes, namely peroxide and acid concentrations, are particularly relevant for analyzing oil quality. These chemical changes can be impacted by high temperatures, and it can result in the generation of peroxides and volatile fatty acids [14,15,16,17].
In the literature, various studies have observed the effects of heat treatment on flaxseed oil extraction, focusing on different temperatures and extraction techniques. Some studies have reported that heating flaxseeds between 40 °C and 150 °C reduced antioxidant activity [11]. Kabutey et al. [18] found that oil yield was improved by oven and vacuum pretreatments between 40 °C and 90 °C. Al-Madhagy et al. [8] examined raw and roasted flaxseeds between 90 °C and 120 °C using cold pressing, solvent extraction, and ultrasonic-assisted methods, and their results confirmed that although roasting increased oil yield, it also caused oxidative degradation. These findings show that while heat treatment can enhance extraction efficiency, excessive temperatures may degrade oil quality, underscoring the need for controlled heating strategies. Flaxseed oil is thermally unstable and may degrade at higher temperatures; therefore, an improved extraction technique is desirable [13]. On the other hand, most of these studies largely focused on solvent extraction and continuous mechanical screw pressing, with limited attention to linear compression pressing combined with moderate oven heating. Although solvent extraction ensures high oil recovery, it introduces chemical residues [8]. In contrast, cold pressing at ambient temperatures preserves oil quality but it thus limits oil extraction efficiency [19].
In view of the above observations, the impact of moderate heating temperatures below 60 °C on the mechanical properties of flaxseeds, such as force-deformation behavior, hardness, and energy demand, remains unexplored. Understanding these changes is crucial to optimizing oil recovery without compromising seed integrity and oil stability. Most importantly, the effectiveness of the oil extraction process relies on the knowledge of the complex nature of the mechanical behavior of oilseeds under a linear compression process [15]. In-depth knowledge of these factors in different heat treatment conditions is critical when designing energy-efficient and effective oil processing techniques. Therefore, the present study aims to fill these gaps by systematically analyzing the effect of controlled oven heating temperature on the physical (porosity, density, and viscosity), mechanical (oil yield, oil expression efficiency, hardness, and deformation energy) and spectral (absorbance-wavelength) properties of flaxseeds and pressed oils under the linear pressing method.
2. Materials and Methods
2.1. Determination of Moisture and Oil Content of Samples
Bulk flaxseeds purchased from Stredi, Prague, Czech Republic were used for the experiment. Before the experiments, the samples in sealed transparent plastic bags were stored under laboratory conditions. The moisture content of the samples was determined following the standard procedure [20] by drying the samples at 105 °C for 17 h using the hot-air oven (DS_Memmert Universal oven UF 110 (MEMMERT GmbH + Co. KG, Buechenbach, Germany). The moisture content of the samples was determined based on Equation (1) [21].
where is the moisture content of the sample (% d.b.), and are the masses of the samples before and after oven drying (g). The samples’ weights were measured using the electronic balance (KERN & SOHN 440–35, Balingen, Germany) with an accuracy of 0.01 g. The percentage oil content of the samples was also determined following the Soxhlet extraction procedure as reported in the literature [22,23,24].
2.2. Flaxseeds Samples Under Hot-Air Oven Pretreatment
The samples of flaxseeds (measured at 60 mm pressing height using the pressing vessel of diameter 60 mm with a graduated plunger) were pretreated at various heating temperatures between 40 and 60 °C with 5 °C intervals using the hot-air oven drying method for 30 min drying time. The air flap and fan of the hot-air oven were set at 30% to allow a uniform drying process. The laboratory temperature of 20 °C served as the control for the samples.
2.3. Pressed Oils Under Linear Compression Process
A universal compression testing machine with a maximum load capacity of 500 kN (TEMPOS spol. s.r.o., Opava, Czech Republic (Machine Service); ZDM 50, VEB Werkstoffprüfmaschinen, Leipzig, Germany) and a pressing vessel of 60 mm diameter with a plunger were used for extracting the flaxseeds oil (Figure 1a,b) at varying temperatures of 20, 40, 45, 50, 55, and 60 °C (Figure 1c,d; 1–6). Based on the cross-sectional area of the pressing vessel and the initial sample pressing height, the volume of the samples was calculated to be 16.965 × 10−5 m3 [25]. The input compression force of 300 kN and pressing rate of 5 mm/min were set prior to the oil extraction process. The output data from the compression process were the time, force (), and deformation (). This information was used to calculate the parameters described in Section 2.4 and Section 2.5, respectively. The compression tests were triplicated, and the data were presented as the mean, standard deviation, and percentage coefficient of variation.
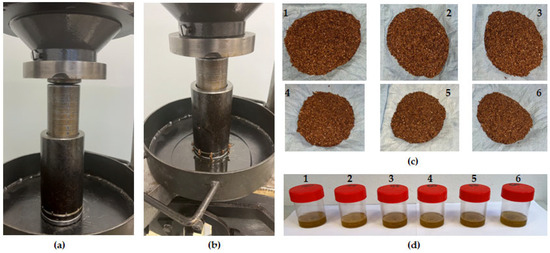
Figure 1.
(a): Linear compression process showing the initial pressing height of sample at 60 mm, (b) reduction in intial pressing height of sample to 30 mm for obtaining the pressed oil at 300 kN force and speed of 5 mm/min, (c1–c6) pressed flaxseed samples, and (d) pressed oils at control temperature at 20 °C (1) and heating tmperatures of 40, 45, 50, 55, and 60 °C (2–6).
2.4. Oil Yield, Oil Expression Efficiency, and Deformation Energy
The oil yield was determined using Equation (2) [26,27].
where is oil yield (%), is the mass of oil determined as the difference in mass of the seedcake and the initial mass of the sample (g). The oil expression efficiency was calculated using Equation (3) [28].
where is the oil expression efficiency (%) and is the percentage of oil content (%) in flaxseeds sample determined by the Soxhlet extraction procedure. The deformation energy demand was calculated using Equation (4) [25,29].
where is the deformation energy (kN·m = kJ), and are the compressive force (kN) and deformation (m), n is the number of data points, and i is the number of sections in which the axis deformation was divided.
2.5. Hardness and Compressive Stress
The hardness () and compressive stress () of the samples were calculated using Equations (5) and (6) [25,30].
where is the cross-sectional area of the pressing vessel, which was calculated to be 2827.4 mm2).
2.6. Porosity
The porosity, (%), is the fraction of the space in the bulk seeds which is not occupied by the seeds [1,31]. The porosity of bulk flaxseeds was calculated from the values of the true density and bulk density using Equation (7) [32].
where is the bulk density (kg/m3) and is the true density (kg/m3). The bulk density is the ratio of the mass of the sample to its total volume, whereas the true density is the ratio of the mass of the sample to its volume using the displacement method [1,33,34]. Coşkuner and Karababa [1] established the mathematical equations describing the bulk density, true density, and porosity of flaxseeds dependent on moisture content (% dry basis). The true density equation was used based on the determined moisture content, whereas the bulk density and the porosity values were determined experimentally.
2.7. Density, Viscosity, and Flow Rate of Pressed Oils
The density () is a measure of the mass per unit volume of that substance [35]. An improvised Saybolt universal viscosity technique was adopted [36]. The time taken for 60 mL of the oil samples was measured at each heating temperature, flowing through a channel orifice of 1.62 mm with an area of 2.061 mm2. The Saybolt universal seconds (t) can be converted to kinematic viscosity () in centistokes (cSt) using Equations (8)–(10) [36]. Equation (8) was used, where 1 cSt is equal to 10−6 m2/s or mm2/s.
The dynamic or absolute viscosity (μ) was calculated using Equation (11) [35].
The flow rate of a liquid is a measure of the speed at which the liquid flows from one point to another point at each time [37].
2.8. Absorbance and Transmittance Spectra Properties
The absorbance and transmittance curves were obtained using ATR-FTIR Alpha II spectroscopy using a zinc-selenide attenuated reflector (Nicolet, Madison, WI, USA). The spectra of the oil samples were recorded by 16 scans at 4 cm−1, and the background spectrum without a sample was recorded every 20 min to remove instrumental and atmospheric contributions to the spectrum of a sample [38,39]. The absorbance spectral curves of the extracted oil samples at the various temperatures were described in the wavelength range between 400 and 4000 cm−1. The measurements were carried out in triplicate, and the results were averaged.
2.9. Statistical Analyses
The experimental data were statistically evaluated by employing descriptive statistics, correlation, one-way ANOVA, and simple linear regression using STATISTICA 13 software [40]. Graphical illustrations were also performed by the above-mentioned software. The one-way ANOVA or repeated measures ANOVA is used to analyze designs in which responses on multiple dependent variables correspond to measurements at different levels of one or more varying factors. The simple regression is used to analyze designs with a single continuous predictor variable or regressor [40].
3. Results and Discussion
3.1. Effect of Heating Temperature on Physical Properties
The determined physical properties of flaxseeds were the moisture content, porosity, density, and viscosity. The moisture content of flaxseeds samples was calculated to be 8.15 ± 0.07 (% d.b.) before the hot-air pretreatment process. However, the thermal treatment at the various heating temperatures from 40 °C to 60 °C with 5 °C intervals at a drying time of 30 min might have caused a slight reduction in the samples’ moisture content. In general, thermal treatment induces changes in microstructure as well as physical and chemical properties. These factors have a significant impact on the organoleptic properties, physicochemical properties, and stability of the oil [41]. After thermal treatment, oilseed moisture content is reduced, hence oil yield increases; color, flavor, and texture are enhanced; accessibility of bioactive compounds such as polyphenol and tocopherol is increased; Maillard reaction products such as melanoidin are formed that contribute to antioxidant activity, which increases stability during storage and cooking [41,42,43,44,45]. In the literature, Coşkuner and Karababa [1] investigated the moisture content range from 6.09 to 16.81% (d.b.) on the physical properties of flaxseeds. Specifically, the authors reported that porosity increased with moisture content. Since porosity is dependent on bulk density and true density, the authors found that bulk density decreased with the increase in moisture content, indicating that the increase in volumetric expansion in the flaxseeds samples was greater than the weight. On the other hand, the true density of flaxseeds samples, as reported by the authors, increased with an increase in moisture content, indicating that the increase in weight gain in the flaxseeds samples was greater than the volume increase in the seeds. In this study, however, the true density of flaxseeds samples was calculated to be 1062.54 kg/m3 based on the established regression equation of true density of flaxseeds as a function of moisture content (% d.b.), which was reported by Coşkuner and Karababa [1]. The bulk density of the flaxseeds samples before the heating process was calculated to be 731.17 kg/m3. Using Equation (7), the porosity was calculated to be 31.19%. After the heating process and the subsequent compression tests of the flaxseeds’ samples, the amounts of bulk density and porosity were calculated as presented in Table 1. The bulk density values at various heating temperatures ranged between 554.390 ± 10.928 kg/m3 and 646.798 ± 11.333 kg/m3, whereas the porosity values ranged from 39.127 ± 1.067 to 47.824 ± 1.029%. It was observed that porosity increased along with the increase in temperature, but decreased at 50 °C. Broadly, as temperature increases, materials tend to expand since the expansion creates new voids. Temperature changes can affect the moisture content within the seeds. Higher temperatures can cause moisture to evaporate, potentially increasing porosity as the water leaves behind the voids. Higher temperatures can also cause structural changes in the seed material, such as the breakdown of cell walls, which also contribute to increased porosity. Heating pretreatment can also soften the seeds and increase their porosity, making it easier to extract the oil [46,47].

Table 1.
Calculated amounts of bulk density and porosity of flaxseeds under control and heating temperatures.
The mean, standard deviation, and percentage coefficient of variation in density, kinematic and dynamic viscosities, and flow rate of flaxseeds pressed oils in relation to temperature are presented in Table 2. The results showed that the density, kinematic, and dynamic viscosities of flaxseeds pressed oils decreased with an increase in heating temperature, whereas the flow rate increased along with the heating temperature. As the temperature increases, thermal fluctuations reduce intermolecular cohesion [37,48]. Polynomial and linear functions were used to describe the determined parameters in relation to temperature. Density–temperature dependence was described by a polynomial function of second order (R2 = 0.997) compared to a linear function (R2 = 0.886). The kinematic viscosity-temperature dependence was fitted by a polynomial function of second order (R2 = 0.999) compared to a linear function (R2 = 0.998). However, the dynamic viscoscity–temperature dependence was suitably described by both polynomial and linear functions (R2 = 0.999). The flow rate–temperature dependence was highly described by a polynomial function (R2 = 0.999) compared to a linear function (R2 = 0.995). Similar results were reported by Esteban et al. [35] and Davies [37] on some vegetable oils, including sunflower, soybean, and palm oils. The viscosity parameter is vital in determining the quality of vegetable oil, and it is sensitive to small changes in temperature, concentration, homogeneity, shape, and size of molecules [37]. Again, Davies [37] indicated that the greater the viscosity, the lower the flow rate. The statistical interpretation of the effect of temperature on the calculated parameters is provided in Section 3.4.

Table 2.
Calculated amounts of density, kinematic, and dynamic viscosities of flaxseeds oil samples under control and heating temperatures.
The oil content of flaxseeds was determined to be 40.32 ± 0.02%. The mean, standard deviation, and percentage coefficient of variation in the calculated amounts of oil yield (Equation (3)) and oil expression efficiency (Equation (4)) in relation to the varying temperatures are presented in Table 3. The oil yield values ranged from 11.539 ± 1.550 to 24.178 ± 1.495%, whereas the oil expression efficiency values ranged from 28.617 ± 3.844 to 59.959 ± 3.707%. Widely, the increase in temperature as a result of a decrease in moisture content thus increased the oil recovery. The statistical interpretation of the effect of temperature on the calculated parameters is provided in Section 3.4.

Table 3.
Calculated amounts of oil yield and oil expression efficiency of flaxseeds under control and heating temperatures.
3.2. Effect of Heating Temperature on Mechanical Properties
The calculated amounts of the mechanical properties of flaxseeds under the uniaxial compression process for extracting the flaxseed oil in relation to the varying temperatures from 20 to 60 °C were the force, deformation, deformation energy, hardness, and compressive stress as presented in Table 4. The amounts of force ranged from 112.450 ± 4.422 to 181.711 ± 8.106 kN, with the corresponding deformation values ranging from 27.903 ± 0.673 to 33.393 ± 0.199 mm. It is worth mentioning that beyond the maximum force-deformation limit was the ejection of the seedcake through the pressing vessel holes which is not energy efficient since there is no oil recovery. The determination of the maximum force-deformation limit of oilseeds under the linear compression process is relevant for designing optimal oil pressing technology [25,30]. The deformation energies ranged from 0.410 ± 0.018 to 0.723 ± 0.029 kN. The deformation energy is characterized by the area under the force-deformation curve (Figure 2a). The increase in the heating temperatures enlarged the area under the curve, which enhanced the oil recovery and the energy requirement. The values of hardness ranged from 4.034 ± 0.253 to 5.483 ± 0.175 kN/mm and that of the compressive stress ranged from 39.771 ± 1.564 to 64.267 ± 2.867. The results showed both increasing and decreasing trends with the temperature increment. The force-deformation curves at the various temperatures for determining the calculated amounts are shown in Figure 2a–f. The statistical interpretation of the effect of temperature on the calculated parameters is provided in Section 3.4.

Table 4.
Determined mechanical properties of bulk flax seeds (data are means ± standard deviations).
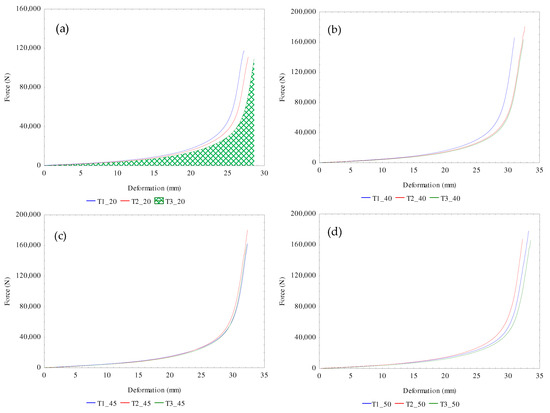
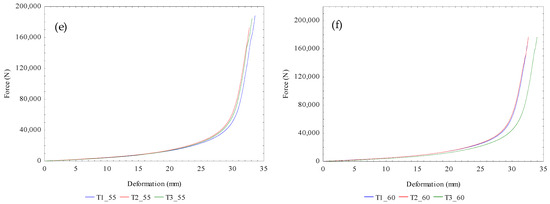
Figure 2.
(a–f): Experimental force-deformation curves of bulk flax seeds under control temperature of 20 °C through to 60 °C for three repeated tests (T1–T3).
3.3. Effect of Heating Temperature on Spectral Properties
The weighted means of the absorbance and transmittance in the wavelength range of 600 to 4000 cm−1 against the varying temperatures are presented in Table 5. It was observed that the absorbance values increased at temperatures from 20 °C to 45 °C but then showed both decreasing and increasing trends from 45 °C to 60 °C. The reciprocal of this observation represents the percent transmittance. The ANOVA univariate results (Table 6) indicated that the temperature had no significant effect (p-value > 0.05) on the absorbance and transmittance values.

Table 5.
Weighted means of absorbance and transmittance in relation to temperature effect.

Table 6.
Univariate results of absorbance and transmittance in relation to temperature effect.
The infrared absorbance and transmittance spectra in the wavelength of range of 600 to 4000 cm−1 of flaxseeds pressed oils at varying temperatures at a constant heating time of 30 min is shown in Figure 3 and Figure 4. The absorbance is the logarithm of the reciprocal of transmittance, and it is described by the Beer–Lambert law [49,50]. In terms of absorbance-wavelength peaks, intense absorbance peaks were observed from 720 to 1745 cm−1 and 2854 to 3008 cm−1. Similarities of absorbance spectra were observed at the varying temperatures, although there were slight differences in peak height. The similarity of the absorbance spectra compared to other vegetable oils is attributed to triglyceride, which is a significant component in all vegetable oils [51]. As the main compound in vegetable oils, triglycerides (95–98%) are composed of fatty acids, whose fatty chains can be saturated or poly-mono unsaturated depending on the botanical origin [39]. Similar observations were reported on soybean, canola or rapeseed, olive, sunflower, corn, and peanut [51,52,53,54]. The observed absorbance peaks’ position with their chemical bonds or functional groups has been described adequately in the literature [38,51,54,55,56,57,58,59,60]. For instance, Jiang et al. [61] reported that oxidative degradation products such as hydroperoxides (peroxide value), aldehydes, and ketones have absorption peaks in wavelength regions between 1730 and 1670 cm−1 whereas acid value has the absorption peak at 1711 cm−1. FTIR spectroscopy combined with principal component regression, multiple linear regression, and partial least squares have been used to determine the physicochemical properties such as acid value, peroxide value, and saponification value of vegetable oils [58,61]. This approach will be explored in future studies on flaxseeds pressed oils.
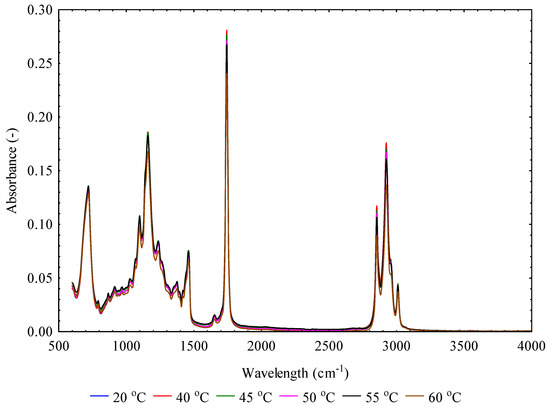
Figure 3.
Absorbance-wavelength spectra curves of flaxseeds oil at varying temperatures.
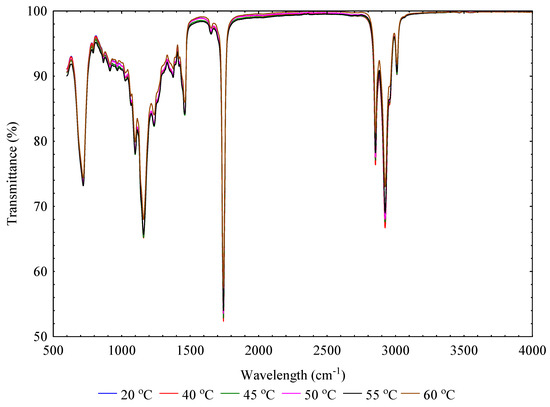
Figure 4.
Transmittance-wavelength spectra curves of flaxseeds oil at varying temperatures.
3.4. Established Linear Regression Models as a Function of Temperature
The results of the correlation analysis are presented in Table 7, Table 8 and Table 9. Porosity, density, oil yield, and oil expression efficiency significantly correlated positively (p-value < 0.05) with the increase in heating temperature. However, the kinematic and dynamic viscosities, compressive stress, deformation energy, and hardness did not correlate significantly (p-value > 0.05) with heating temperature. The results of the one-way ANOVA analysis proved the correlation results for compressive stress, hardness, and deformation energy, where the heating temperature had no significant effect compared to the porosity, density, kinematic and dynamic viscosities, oil yield, and oil expression efficiency, which were significantly influenced by the heating temperature. The coefficient of determination (R2) values ranged between 0.599 and 0.721. Nevertheless, the post hoc test based on the Duncan test showed that the means of the porosity, oil yield, and oil expression efficiency at heating temperatures from 50 °C to 60 °C at a 5 °C interval were not significantly different from each other. Similarly, the means of density at 40 °C and 50 °C were not significantly different from each other, but at 45 °C, 55 °C, and 60 °C, the means were significantly different from each other. Furthermore, the simple linear regression analysis confirmed the correlation results for compressive stress, hardness, deformation energy, and kinematic and dynamic viscosities where the heating temperature had no significant effect. The significant terms of the heating temperature and intercept describing porosity, density, oil yield, and oil expression efficiency are given in Equations (12)–(15), respectively, with their statistical evaluation metrics presented in Table 9. Their coefficients of determination (R2) values ranged 0.532 to 0.787.

Table 7.
Correlation analysis of the effect of heating temperature on calculated parameters.

Table 8.
Correlation analysis of the effect of heating temperature on the calculated.

Table 9.
Regression models estimate and statistical metrics in relation to the heating temperature effect.
4. Conclusions
The present study revealed that moderate heating temperatures did not cause increased mechanical resistance or hardness of the flaxseeds resulting in higher oil recovery. Similarities of the absorbance spectral peaks were observed at the various heating temperatures. The deformation energy under the force-deformation curve for recovering the pressed oils did not significantly correlate with heating temperature. Porosity of flaxseeds showed a strong correlation with heating temperature (R2 = 0.866), indicating that structural softening facilitated oil release. The regression models developed for porosity, density, oil yield, and oil expression efficiency as a function of temperature were statistically adequate for prediction with coefficients of determination (R2) values between 0.532 and 0.787. While pressed oils flow rate linearly increased with heating temperature, density and viscosity decreased linearly. The study contributes to the available knowledge on the flaxseeds oil extraction process towards the development of energy efficient technologies. In future studies, mechanical screw presses would be explored for processing flaxseeds pressed oils following moderate pretreatment conditions. In addition, FTIR spectroscopy combined with principal component regression, multiple linear regression, and partial least squares would be applied to determine the oil quality parameters of flaxseeds oil against titration techniques.
Author Contributions
A.K.: Conceptualization, Methodology, Supervision, Project administration, Funding acquisition, Formal analysis, Visualization, Validation, Writing—Original Draft, Writing—Review, and Editing. S.S.S.: Investigation, Data curation, Formal analysis, Writing—Original Draft, Writing—Review, and Editing. M.M.: Investigation, Data curation, Formal analysis, Writing—Original Draft, Writing—Review, and Editing. S.H.K.: Investigation, Data curation, Formal analysis, Writing—Original Draft, Writing—Review, and Editing. All authors have read and agreed to the published version of the manuscript.
Funding
The study was supported financially by the Internal Grant Agency of the Czech University of Life Sciences Prague (IGA Project Number—2024:31130/1312/3108).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The original contributions presented in the study are included in the article, further inquiries can be directed to the corresponding author.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Coşkuner, Y.; Karababa, E. Some physical properties of flaxseed (Linum usitatissimum L.). J. Food Eng. 2007, 78, 1067–1073. [Google Scholar] [CrossRef]
- Berglund, D.R. Flax: New Uses and Demands. In Trends in New Crops and New Uses; Janick, J., Whipkey, A., Eds.; ASHS Press: Alexandria, Egypt, 2002; pp. 358–360. [Google Scholar]
- Oomah, B.D. Flaxseed as a functional food source. J. Sci. Food Agric. 2001, 81, 889–894. [Google Scholar] [CrossRef]
- Broderick, C.H.; Dibrov, E.; Hirst, S.D.; Pierce, G.N. Physiological and pathological considerations for the use of flaxseed as a therapeutic dietary strategy. Rev. Cardiovasc. Med. 2023, 24, 149. [Google Scholar] [CrossRef]
- Rebolé, A.; Rodríguez, M.L.; Ortiz, L.T.; Alzueta, C.; Centeno, C.; Trevino, J. Mucilage in linseed: Effects on the intestinal viscosity and nutrient digestion in broiler chicks. J. Sci. Food Agric. 2002, 82, 1171–1776. [Google Scholar] [CrossRef]
- Chen, Z.Y.; Ratnayake, W.M.N.; Cunnane, S.C. Oxidative stability of flaxseed lipids during baking. Am. Oil Chem. Soc. 1994, 71, 629–632. [Google Scholar] [CrossRef]
- Goyal, A.; Sharma, V.; Upadhyay, N.; Gill, S.; Sihag, M. Flax and flaxseed oil: An ancient medicine & modern functional food. J. Food Sci. Technol. 2014, 51, 1633–1653. [Google Scholar] [CrossRef] [PubMed]
- Al-Madhagy, S.; Ashmawy, N.S.; Mamdouh, A.; Omayma, A.E.; Farag, M.A. A comprehensive review of the health benefits of flaxseed oil in relation to its chemical composition and comparison with other omega-3-rich oils. Eur. J. Med. Res. 2023, 28, 240. [Google Scholar] [CrossRef]
- Zhang, C.; Zhang, L.; Zhao, Y.; Cui, R.; Wu, H.; Xu, M.; Liu, W.; Liu, R.; Xu, L.; Song, L. Effect of different heating pretreatment methods on physicochemical properties of pressed walnut oils and functional properties of walnut protein isolates. LWT—Food Sci. Technol. 2025, 225, 117885. [Google Scholar] [CrossRef]
- Cheng, C.; Yu, X.; Huang, F.; Wang, L.; Zhu, Z.; Yang, J.; Chen, P.; Qianchun, D. Effect of heat-treated flaxseed lignan macromolecules on the interfacial properties and physicochemical stability of α-linolenic acid-enriched o/w emulsions. Food Funct. 2024, 15, 9524–9540. [Google Scholar] [CrossRef]
- Brestenský, M.; Nitrayová, S.; Patráš, P. The effect of heat treatment on the quality of fat in flaxseeds and chia seeds. Czech J. Food Sci. 2023, 41, 21–28. [Google Scholar] [CrossRef]
- Midhun, J.; Stephi, D.; Selvi, K.M.; Kameshwari, Y.; Swatika, S.K.; Sunil, C.K. Effect of emerging pretreatment methods on extraction and quality of edible oils: A review. Food Humanit. 2023, 1, 1511–1522. [Google Scholar] [CrossRef]
- Zhang, Z.-S.; Wang, L.-J.; Li, D.; Jiao, S.S.; Chen, X.D.; Mao, Z.-H. Ultrasound-assisted extraction of oil from flaxseed. Sep. Purif. Technol. 2008, 62, 192–198. [Google Scholar] [CrossRef]
- Sun, X.; Wang, Y.; Li, H.; Zhou, J.; Han, J.; Wei, C. Changes in the volatile profile, fatty acid composition and oxidative stability of flaxseed oil during heating at different temperatures. LWT—Food Sci. Technol. 2021, 151, 112137. [Google Scholar] [CrossRef]
- Petrů, M.; Novák, O.; Herák, D.; Simanjuntak, S. Finite element method model of the mechanical behaviour of Jatropha curcas L. seeds under compression loading. Biosyst. Eng. 2012, 111, 412–421. [Google Scholar] [CrossRef]
- Gutte, K.B.; Sahoo, A.K.; Ranveer, R.C. Effect of ultrasonic treatment on extraction and fatty acid profile of flaxseed oil. OCL 2015, 22, D606. [Google Scholar] [CrossRef]
- Losada-Barreiro, S.; Paiva-Martins, F.; Bravo-Díaz, C. Partitioning of antioxidants in edible oil–water binary systems and in oil-in-water emulsions. Antioxidants 2023, 12, 828. [Google Scholar] [CrossRef]
- Kabutey, A.; Kibret, S.H.; Kiros, A.W.; Afework, M.A.; Onwuka, M.; Raj, A. Comparative analysis of pretreatment methods for processing bulk flax and hemp oilseeds under uniaxial compression. Foods 2025, 14, 629. [Google Scholar] [CrossRef]
- Kabutey, A.; Herak, D.; Mizera, C. Assessment of quality and efficiency of cold-pressed oil from selected oilseeds. Foods 2023, 12, 3636. [Google Scholar] [CrossRef]
- IS:3579; Indian Standard Methods for Analysis of Oilseeds. Indian Standard Institute: New Delhi, India, 1996.
- Blahovec, J. Agromaterials Study Guide; Czech University of Life Sciences Prague: Prague, Czech Republic, 2008. [Google Scholar]
- Niu, L.; Li, J.; Chen, M.S.; Xu, Z.F. Determination of oil contents in Sacha inchi (Plukenetia volubilis) seeds at different developmental stages by two methods: Soxhlet extraction and time-domain nuclear magnetic resonance. Ind. Crop. Prod. 2014, 56, 187–190. [Google Scholar] [CrossRef]
- Danlami, J.M.; Arsad, A.; Zaini, M.A.A. Characterization and process optimization of castor oil (Ricinus communis L.) extracted by the Soxhlet method using polar and non-polar solvents. J. Taiwan Inst. Chem. Eng. 2015, 47, 99–104. [Google Scholar] [CrossRef]
- Gürdil, G.A.K.; Kabutey, A.; Selvi, K.Ç.; Mizera, Č.; Herák, D.; Fraňková, A. Evaluation of postharvest processing of hazelnut kernel oil extraction using uniaxial pressure and organic solvent. Processes 2020, 8, 957. [Google Scholar] [CrossRef]
- Herak, D.; Kabutey, A.; Choteborsky, R.; Petru, M.; Sigalingging, R. Mathematical models describing the relaxation behaviour of Jatropha curcas L. bulk seeds under axial compression. Biosyst. Eng. 2015, 131, 77–83. [Google Scholar] [CrossRef]
- Deli, S.; Farah Masturah, M.; Tajul Aris, Y.; Wan Nadiah, W.A. The effects of physical parameters of the screw press oil expeller on oil yield from Nigella sativa L. seeds. Int. Food Res. J. 2011, 18, 1367–1373. [Google Scholar]
- Chanioti, S.; Tzia, C. Optimization of ultrasound-assisted extraction of oil from olive pomace using response surface technology: Oil recovery, unsaponifiable matter, total phenol content and antioxidant activity. LWT—Food Sci. Technol. 2017, 79, 178–189. [Google Scholar] [CrossRef]
- Hernandez-Santos, B.; Rodriguez-Miranda, J.; Herman-Lara, E.; Torruco-Uco, J.G.; Carmona-Garcia, R.; Juarez-Barrientos, J.M.; Chavez-Zamudio, R.; Martinez-Sanchez, C.E. Effect of oil extraction assisted by ultrasound on the physicochemical properties and fatty acid profile of pumpkin seed oil (Cucurbita pepo). Ultrason. Sonochem. 2016, 31, 429–436. [Google Scholar] [CrossRef]
- Gupta, R.K.; Das, S.K. Fracture Resistance of Sunflower Seed and Kernel to Compressive Loading. J. Food Eng. 2000, 46, 1–8. [Google Scholar] [CrossRef]
- Chakespari, A.G.; Rajabipour, A.; Mobli, H. Strength Behavior Study of Apples (cv. Shafi Abadi & Golab Kohanz) under Compression Loading. Mod. Appl. Sci. 2010, 4, 173–182. [Google Scholar]
- Thompson, R.A.; Isaacs, G.W. Porosity determinations of grains and seeds with an air-comparison pycnometer. Trans. ASAE 1967, 10, 693–696. [Google Scholar] [CrossRef]
- Mohsenin, N.N. Physical Properties of Plants and Animal Materials; Gordon and Breach, Science Publishers, Inc. Taylor and Francis: New York, NY, USA, 1970. [Google Scholar]
- Olajide, J.O.; Ade-Omowaye, B.I.O.; Otunola, E.T. Some physical properties of shea kernel. J. Agric. Eng. Res. 2000, 76, 419–421. [Google Scholar] [CrossRef]
- Amin, M.N.; Hossain, M.A.; Roy, K.C. Effects of moisture content on some physical properties of lentil seeds. J. Food Eng. 2004, 65, 83–87. [Google Scholar] [CrossRef]
- Esteban, B.; Riba, J.-R.; Baquero, G.; Rius, A.; Puig, R. Temperature dependence of density and viscosity of vegetable oils. Biomass Bioenergy 2012, 42, 164–171. [Google Scholar] [CrossRef]
- Mihcakan, I.M.; Alkan, K.H.; Ugur, Z. Petroleum and Natural Gas Laboratory, Course Notes, I-Fluid Properties; ITU, Petroleum and Natural Gas Engineering: Istanbul, Turkey, 2001; pp. 2–7. [Google Scholar]
- Davies, R.M. Effect of the temperature on dynamic viscosity, density and flow rate of some vegetable oil. J. Sci. Res. Eng. Technol. 2016, 1, 14–24. [Google Scholar]
- Mohammadi, M.; Khorrami, M.; Vatani, A.; Ghasemzadeh, H.; Vatanparast, H.; Bahramian, A.; Fallah, A. Rapid determination and classification of crude oils by ATR-FTIR spectroscopy and chemometric methods. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2020, 232, 118157. [Google Scholar] [CrossRef] [PubMed]
- Yuan, L.; Meng, X.; Xin, K.; Ju, Y.; Zhang, Y.; Yin, C.; Hu, L. A comparative study on classification of edible vegetable oils by infrared, near infrared and fluorescence spectroscopy combined with chemometrics. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2023, 288, 122120. [Google Scholar] [CrossRef]
- Statsoft Inc. STATISTICA for Windows; Statsoft Inc.: Tulsa, OK, USA, 2013. [Google Scholar]
- Cai, Z.; Li, K.; Lee, W.J.; Reaney, M.T.J.; Zhang, N.; Wang, Y. Recent progress in the thermal treatment of oilseeds and oil oxidative stability: A review. Fundam. Res. 2021, 1, 767–784. [Google Scholar] [CrossRef]
- Ahmed, I.A.M.; Ozcan, M.M.; Uslu, N.; Juhaimi, F.A.L.; Osman, M.A.; Alqah, H.A.S.; Ghafoor, K.; Babiker, E.E. Effect of microwave roasting on color, total phenol, antioxidant activity, fatty acid composition, tocopherol and chemical composition of sesame seed and oils obtained from different countries. J. Food Process. Preserv. 2020, 44, e14807. [Google Scholar] [CrossRef]
- Hu, H.; Liu, H.; Shi, A.; Liu, L.; Fauconnier, M.L.; Wang, Q. The effect of microwave pretreatment on micronutrient contents, oxidative stability and flavor quality of peanut oil. Molecules 2019, 24, 62. [Google Scholar] [CrossRef]
- Ren, X.; Wang, L.; Xu, B.; Wei, B.; Liu, Y.; Zhou, C.; Ma, H.; Wang, Z. Influence of microwave pretreatment on the flavor attributes and oxidative stability of cold-pressed rapeseed oil. Dry. Technol. 2019, 37, 397–408. [Google Scholar] [CrossRef]
- Yang, K.; Hsu, F.; Chen, C.; Hsu, C.; Cheng, M. Quality characterization and oxidative stability of camelina seed oils produced with different roasting temperatures. J. Oleo Sci. 2018, 67, 389–396. [Google Scholar] [CrossRef]
- Wang, R.; Hou, N.-C.; Chen, Z.-M.; Liu, Y.-T.; Qin, Z.; Chang, Y.-L.; Qin, Z.; Liu, H.-M. Effect of heat pretreatment of safflower seeds on quality and polyphenol composition of extracted oil. LWT—Food Sci. Technol. 2025, 217, 117418. [Google Scholar] [CrossRef]
- Guo, X.; Wu, B.C.; Jiang, Y.; Zhang, Y.; Jiao, B.; Wang, Q. Improving enzyme accessibility in the aqueous enzymatic extraction process by microwave-induced porous cell walls to increase oil body and protein yields. Food Hydrocoll. 2024, 147, 109407. [Google Scholar] [CrossRef]
- Yerima, J.B.; Madugu, J.S.; Timter, P.; David, Y.M. Dependence of viscosity and density of Nigerian Lophiralanceolata Oil (Ochnaceae) on temperature. Phys. Rev. Res. 2012, 2, 125–132. [Google Scholar]
- Da Cunha, E.F.; Ferreira, F.D.O.; Maciel, G.D.F.; Kitano, C. Absorbance photometric technique to measure roll waves in a free surface of a non-Newtonian fluid flow. Measurement 2024, 235, 114880. [Google Scholar] [CrossRef]
- Parnis, J.; Oldham, K.B. Beyond the Beer-Lambert law: The dependence of absorbance on time in photochemistry. J. Photochem. Photobiol. A Chem. 2013, 267, 6–10. [Google Scholar] [CrossRef]
- Tsai, Y.-H.; Chiang, D.; Li, Y.-T.; Perng, T.-P.; Lee, S. Thermal degradation of vegetable oils. Foods 2023, 12, 1839. [Google Scholar] [CrossRef]
- Santana, K.D.S.; Tavares, M.I.B. Characterization of flaxseed oil for nuclear magnetic resonance and its encapsulation. Mater. Sci. Appl. 2022, 13, 279–299. [Google Scholar] [CrossRef]
- Song, F.-F.; Tian, S.-J.; Yang, G.-L.; Sun, X.-Y. Effect of phospholipid/flaxseed oi ratio on characteristics, structure change, and storage stability of liposomes. LWT—Food Sci. Technol. 2022, 157, 113040. [Google Scholar] [CrossRef]
- Shi, L.; Liu, Z.; Li, J.; Qin, Z. Analysis of edible vegetable oils by infrared absorption spectrometry. Adv. Eng. Res. 2017, 86, 286–289. [Google Scholar]
- Xiang, P.-F.; Zhang, Z.-S.; Le, W.; Wei, Y.-Y.; Li, B.-Z. Effect of thermal pretreatments on the quality attributes and irradiation makers of sesame extracted from sesame seeds without and with gamma irradiation. Food Chem. 2025, 463, 141401. [Google Scholar] [CrossRef]
- Cakmak-Arslan, G. Monitoring of hazelnut oil quality during thermal processing in comparison with extra virgin olive oil by using ATR-FTIR spectroscopy combined with chemometrics. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2022, 266, 120461. [Google Scholar] [CrossRef]
- Asemani, M.; Rabbani, A.R.; Sarafdokht, H. Evaluation of oil fingerprints similarity by a novel technique based on FTIR spectroscopy of asphaltenes: Modified moving window correction coefficient technique. Mar. Pet. Geol. 2020, 120, 104542. [Google Scholar] [CrossRef]
- Putri, A.R.; Rohman, A.; Setyaningsih, W.; Riyanto, S. Determination of acid, peroxide and saponification value in patin fish oil by FTIR spectroscopy combined with chemometrics. Food Res. 2020, 4, 1758–1766. [Google Scholar] [CrossRef]
- Tudorachi, N.; Mustata, F. Thermal degradation and evolved gas analysis of some vegetable oils using TG/FT-IR/MS technique. J. Therm. Anal. Calorim. 2015, 119, 1703–1711. [Google Scholar] [CrossRef]
- Rohman, A.; Man, Y.B.C. Application of FTIR spectroscopy for monitoring the stabilities of selected vegetable oils during thermal oxidation. Int. J. Food Prop. 2013, 16, 1594–1603. [Google Scholar] [CrossRef]
- Jiang, X.; Li, S.; Xiang, G.; Li, Q.; Fan, L.; He, L.; Gu, K. Determination of the acid values of edible oils via FTIR spectroscopy based on the O-H stretching band. Food Chem. 2016, 212, 585–589. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).