Early Chondrogenic Differentiation of Spheroids for Cartilage Regeneration: Investigation of the Structural and Biological Role of a Lactose-Modified Chitosan
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of CTLc-Spheroids, MF-Spheroids, and CTLs-Spheroids
2.3. Spheroid Formation in the Presence of Blocking Antibodies and Cytoskeletal Inhibitory Compounds
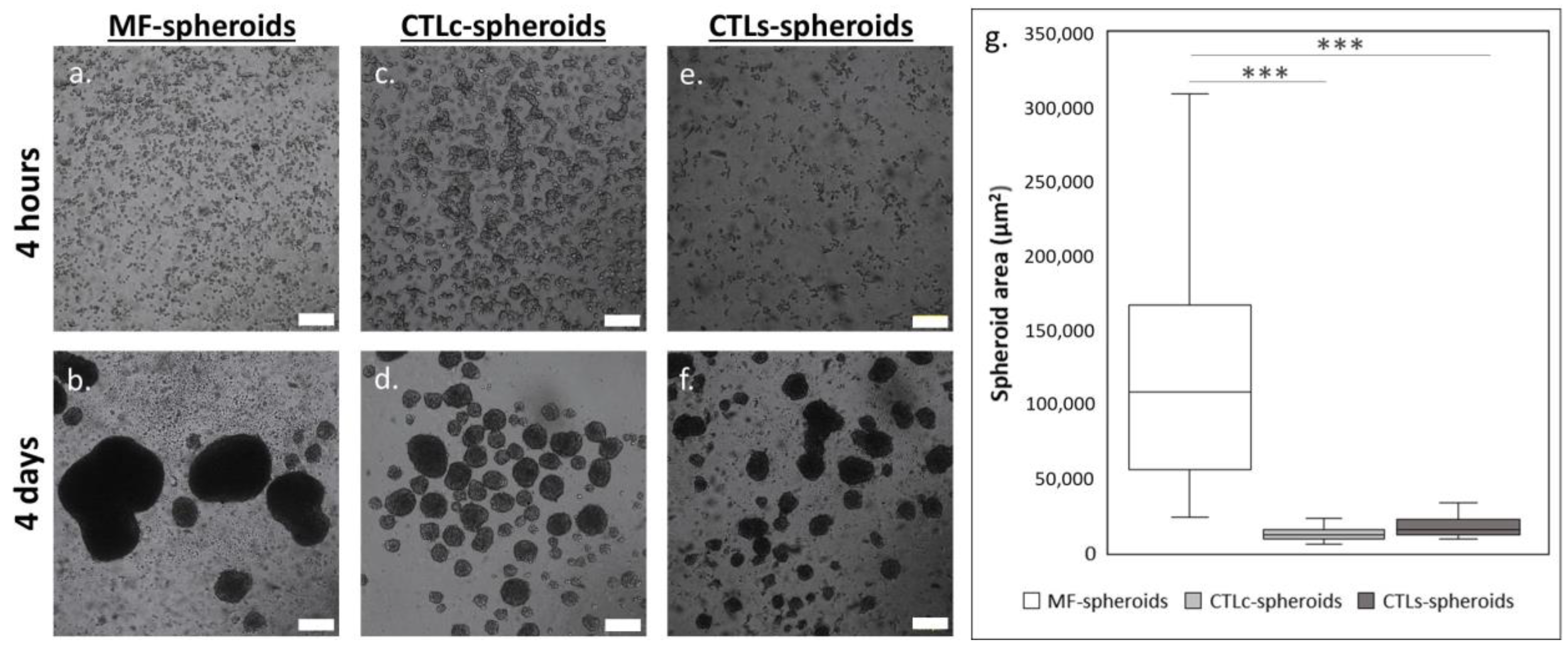
2.4. Analysis of Spheroid Dimensions
2.5. Synthesis of CTL Labeled with Fluorescein Isothiocyanate (FITC)
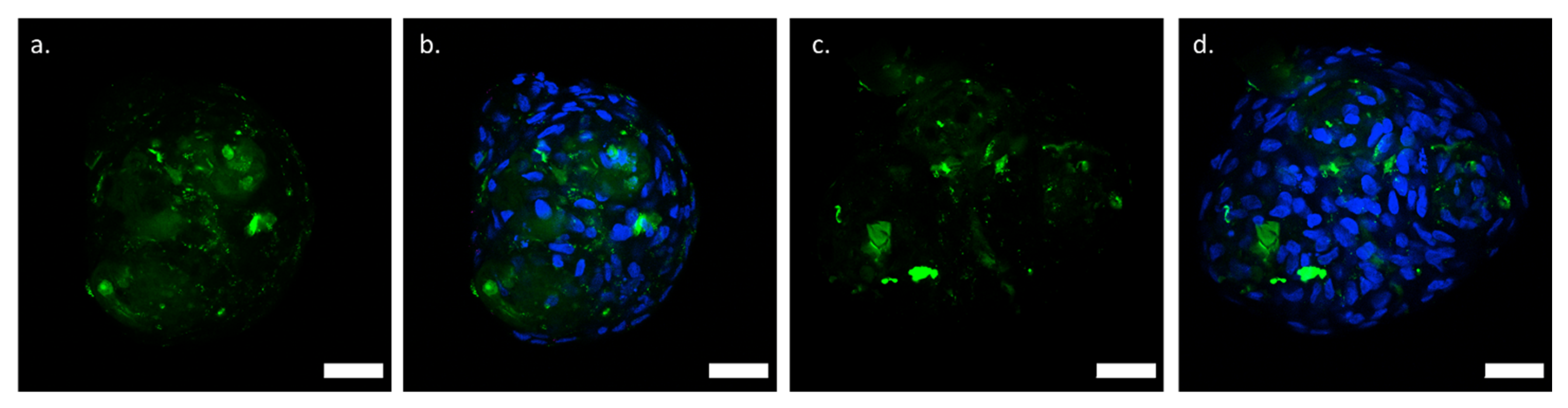
2.6. Localization of Labeled Polymer in CTLc-Spheroids and CTLs-Spheroids
2.7. Immunohistochemistry of Integrins and Cadherins on Spheroids
2.8. Real-Time PCR
2.9. Light Microscopy Analyses on Spheroids
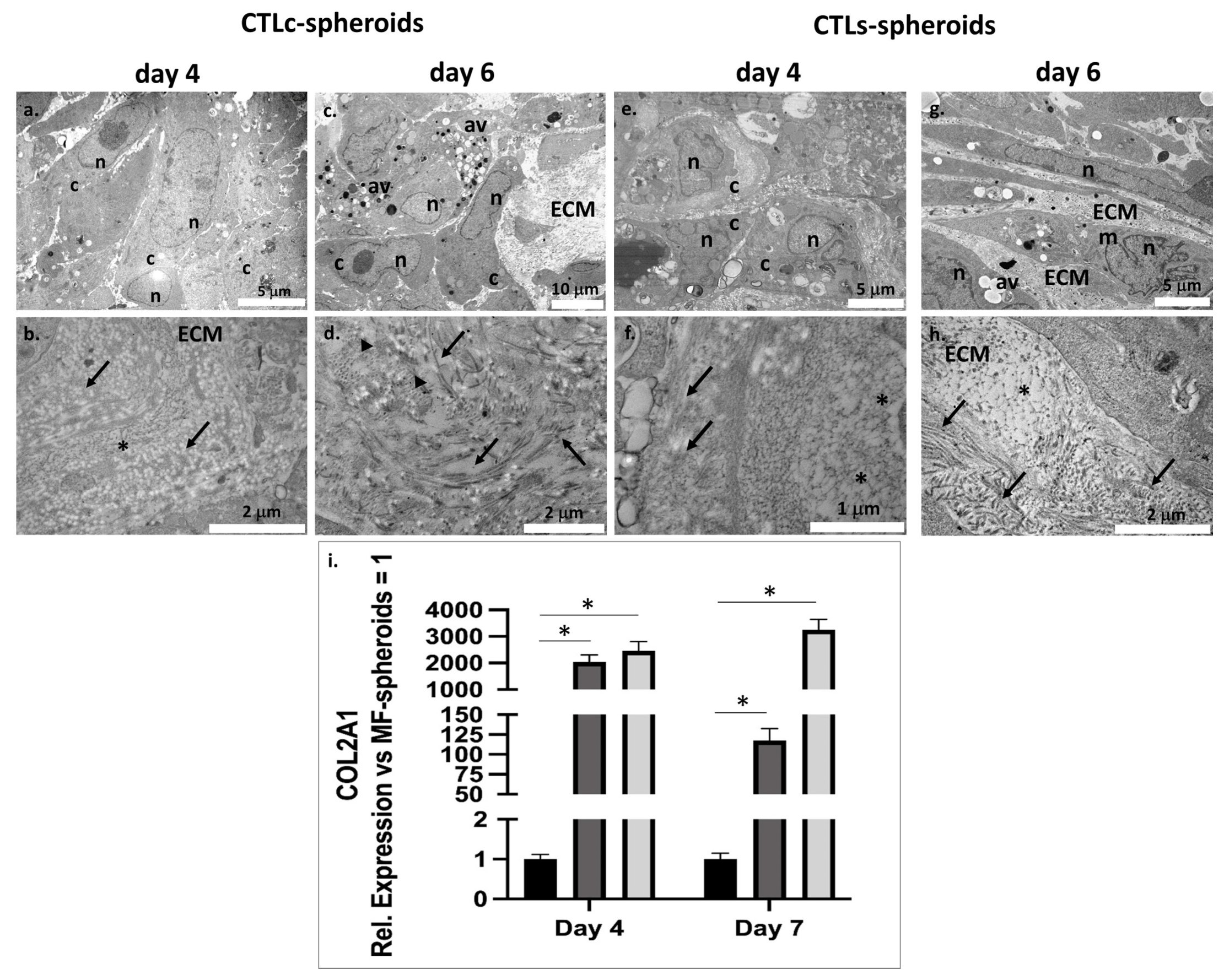
2.10. Transmission Electron Microscopy (TEM) on Spheroids
3. Results
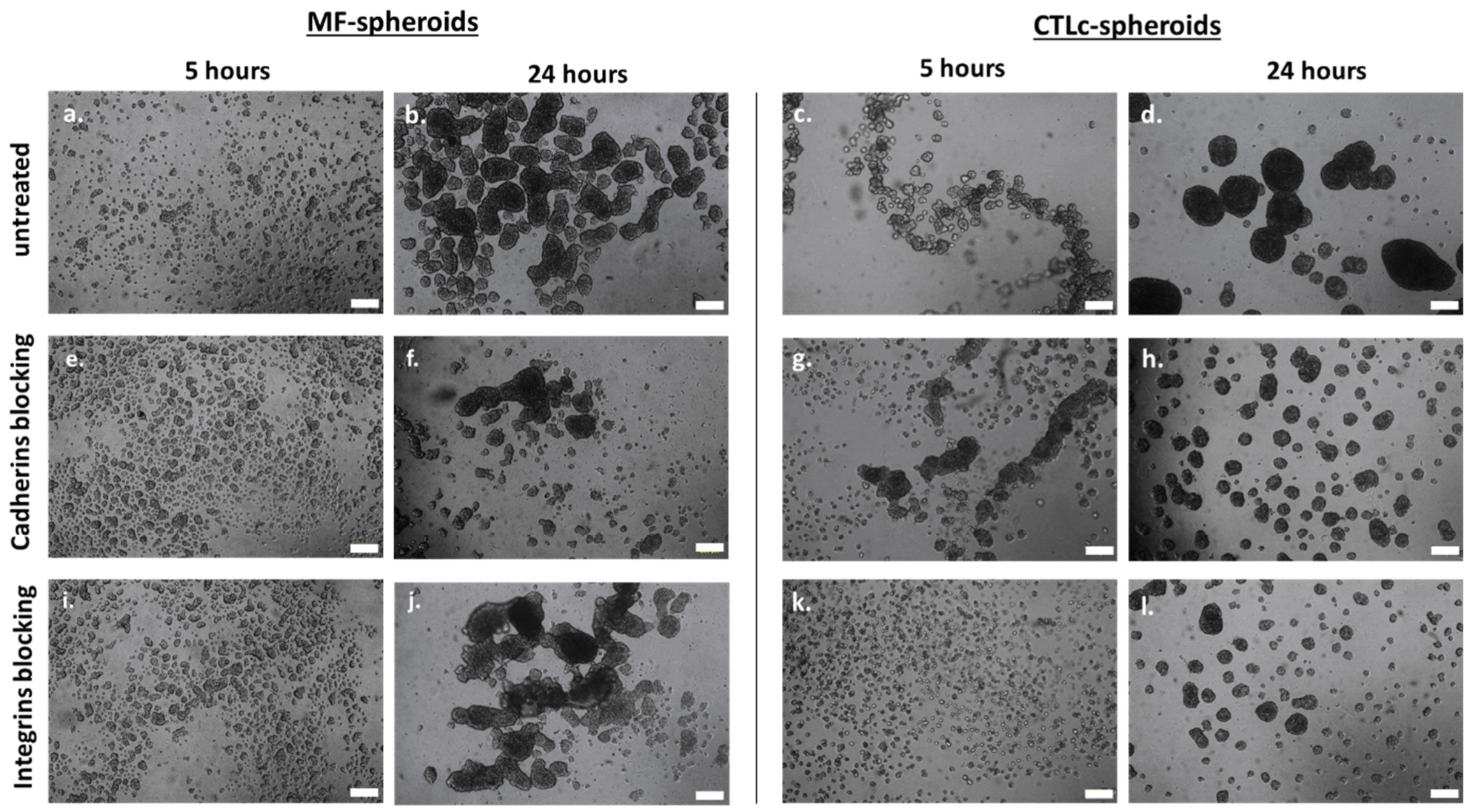
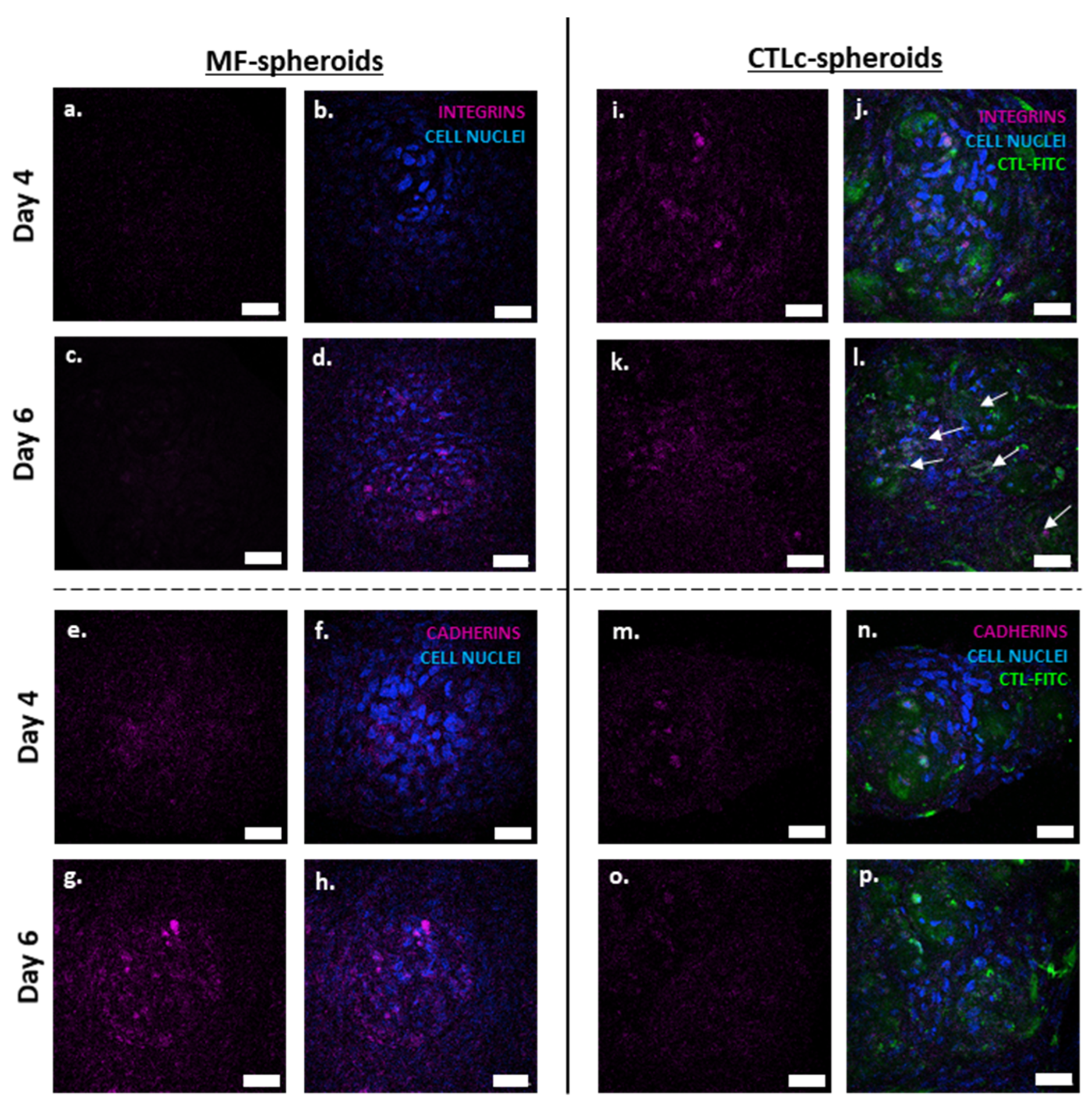
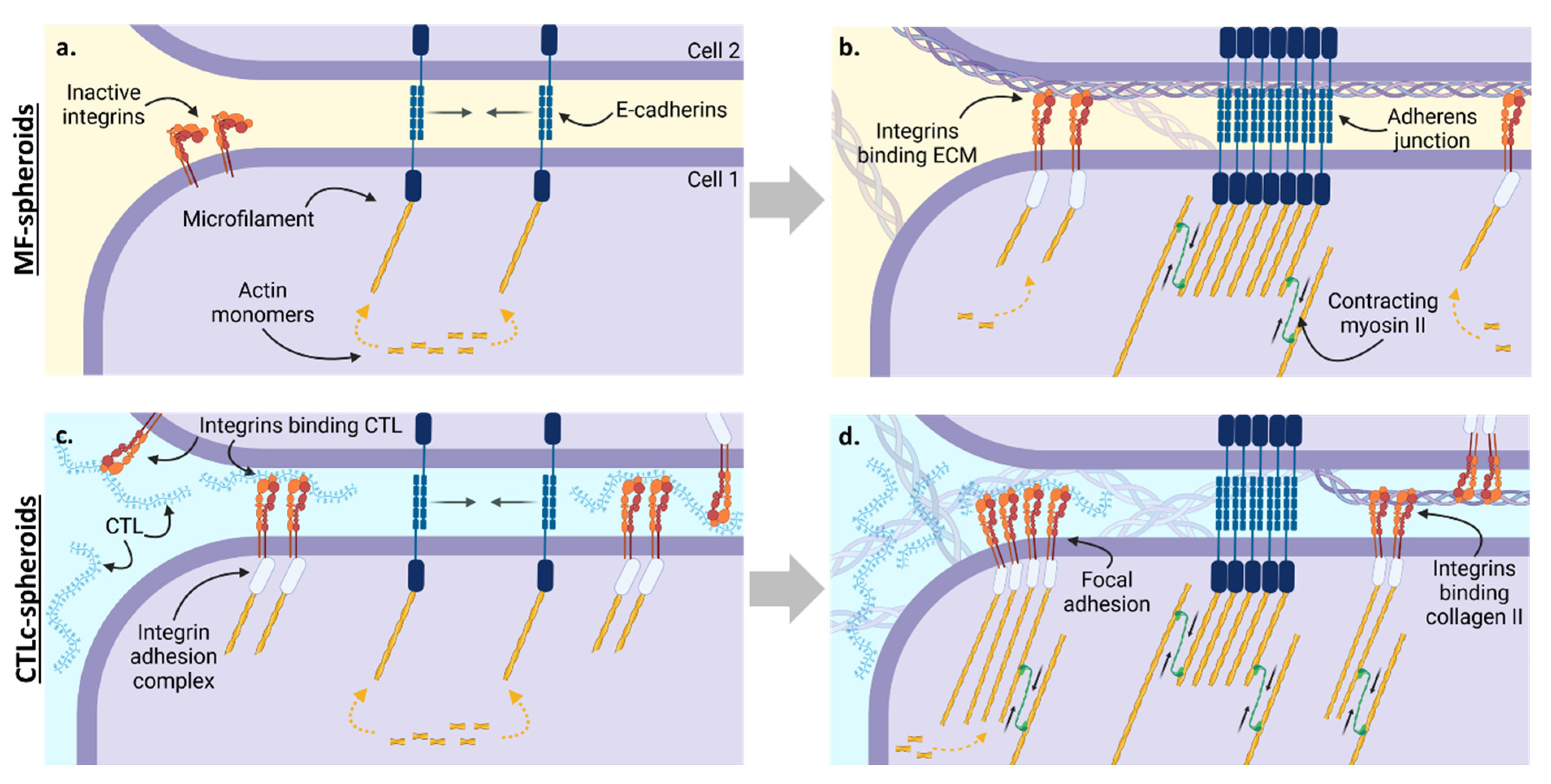
3.1. The Involvement of Integrins and Cadherins in the Dynamics of CTL-Spheroid and MF-Spheroid Formation
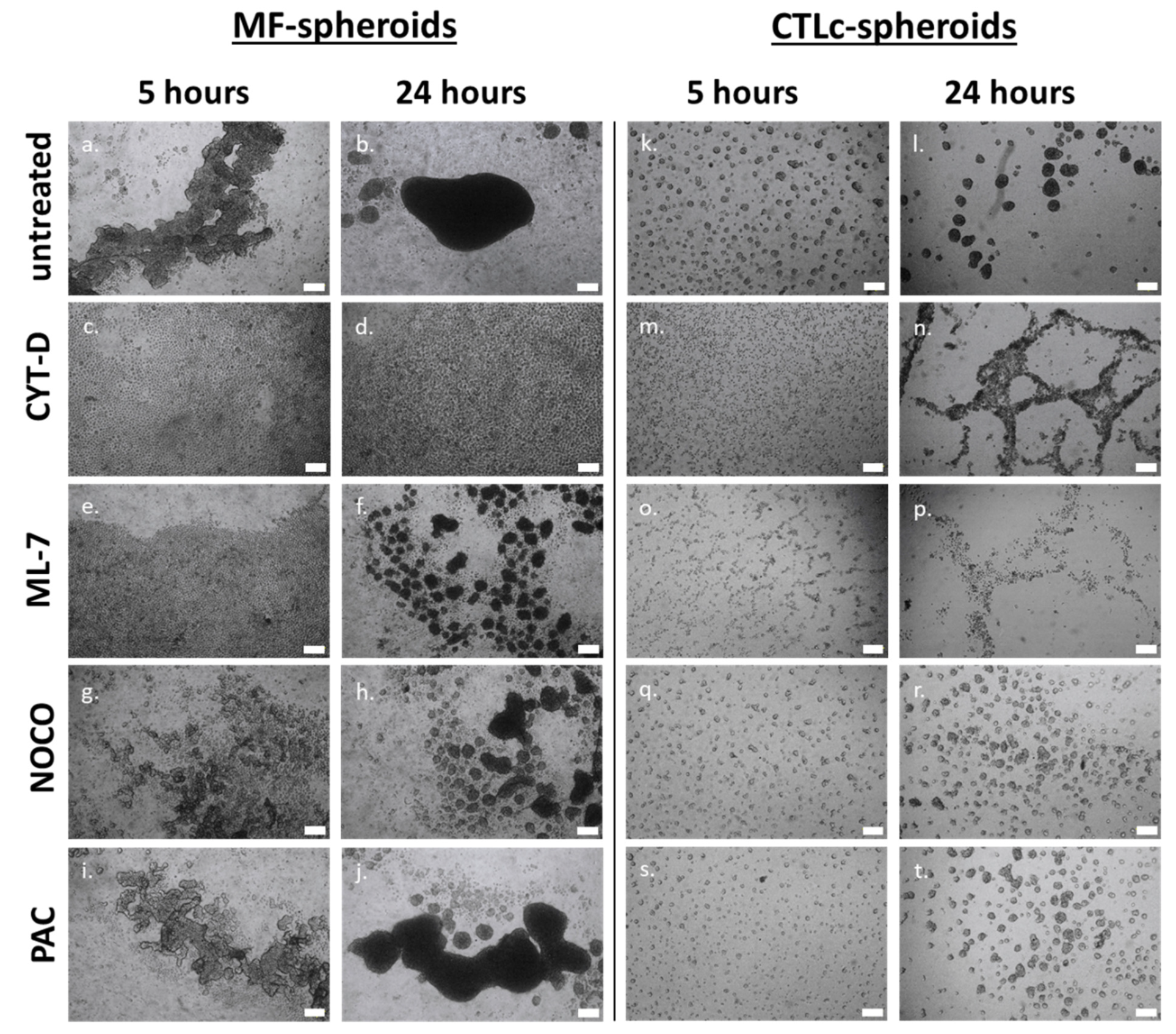
3.2. Actin, Myosin, and Microtubules in the Process of Spheroid Formation
3.3. Structural Features and Biological Response of Spheroids in Presence of CTL in Solution
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chen, M.; Jiang, Z.; Zou, X.; You, X.; Cai, Z.; Huang, J. Advancements in Tissue Engineering for Articular Cartilage Regeneration. Heliyon 2024, 10, e25400. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Yin, H.; Yan, Z.; Li, H.; Wu, J.; Wang, Y.; Wei, F.; Tian, G.; Ning, C.; Li, H.; et al. The Immune Microenvironment in Cartilage Injury and Repair. Acta Biomater. 2022, 140, 23–42. [Google Scholar] [CrossRef] [PubMed]
- Casey, R.C.; Burleson, K.M.; Skubitz, K.M.; Pambuccian, S.E.; Oegema, T.R.; Ruff, L.E.; Skubitz, A.P.N. β1-Integrins Regulate the Formation and Adhesion of Ovarian Carcinoma Multicellular Spheroids. Am. J. Pathol. 2001, 159, 2071–2080. [Google Scholar] [CrossRef] [PubMed]
- Lin, R.-Z.; Chou, L.-F.; Chien, C.-C.M.; Chang, H.-Y. Dynamic Analysis of Hepatoma Spheroid Formation: Roles of E-Cadherin and β1-Integrin. Cell Tissue Res. 2006, 324, 411–422. [Google Scholar] [CrossRef]
- Pillai, V.V.; Koganti, P.P.; Kei, T.G.; Gurung, S.; Butler, W.R.; Selvaraj, V. Efficient Induction and Sustenance of Pluripotent Stem Cells from Bovine Somatic Cells. Biol. Open 2021, 10, bio058756. [Google Scholar] [CrossRef]
- Yun, C.; Kim, S.H.; Kim, K.M.; Yang, M.H.; Byun, M.R.; Kim, J.-H.; Kwon, D.; Pham, H.T.M.; Kim, H.-S.; Kim, J.-H.; et al. Advantages of Using 3D Spheroid Culture Systems in Toxicological and Pharmacological Assessment for Osteogenesis Research. Int. J. Mol. Sci. 2024, 25, 2512. [Google Scholar] [CrossRef]
- Ko, J.-Y.; Lee, E.; Park, J.-W.; Kim, J.; Im, G.-I. Enhancement of Cartilage Regeneration Efficiency with Human Adipose Stem Cell Three Dimensional Spheroid. Osteoarthr. Cartil. 2020, 28, S515–S516. [Google Scholar] [CrossRef]
- Ahmad, T.; Lee, J.; Shin, Y.M.; Shin, H.J.; Madhurakat Perikamana, S.K.; Park, S.H.; Kim, S.W.; Shin, H. Hybrid-Spheroids Incorporating ECM like Engineered Fragmented Fibers Potentiate Stem Cell Function by Improved Cell/Cell and Cell/ECM Interactions. Acta Biomater. 2017, 64, 161–175. [Google Scholar] [CrossRef]
- Gonzalez-Fernandez, T.; Tenorio, A.J.; Saiz, A.M., Jr.; Leach, J.K. Engineered Cell-Secreted Extracellular Matrix Modulates Cell Spheroid Mechanosensing and Amplifies Their Response to Inductive Cues for the Formation of Mineralized Tissues. Adv. Healthc. Mater. 2022, 11, 2102337. [Google Scholar] [CrossRef]
- Scognamiglio, F.; Travan, A.; Borgogna, M.; Donati, I.; Marsich, E. Development of Biodegradable Membranes for the Delivery of a Bioactive Chitosan-Derivative on Cartilage Defects: A Preliminary Investigation. J. Biomed. Mater. Res. A 2020, 108, 1534–1545. [Google Scholar] [CrossRef]
- Scognamiglio, F.; Travan, A.; Donati, I.; Borgogna, M.; Marsich, E. A Hydrogel System Based on a Lactose-Modified Chitosan for Viscosupplementation in Osteoarthritis. Carbohydr. Polym. 2020, 248, 116787. [Google Scholar] [CrossRef] [PubMed]
- Pizzolitto, C.; Scognamiglio, F.; Sacco, P.; Lipari, S.; Romano, M.; Donati, I.; Marsich, E. Immediate Stress Dissipation in Dual Cross-Link Hydrogels Controls Osteogenic Commitment of Mesenchymal Stem Cells. Carbohydr. Polym. 2023, 302, 120369. [Google Scholar] [CrossRef] [PubMed]
- Scognamiglio, F.; Pizzolitto, C.; Romano, M.; Teti, G.; Zara, S.; Conz, M.; Donati, I.; Porrelli, D.; Falconi, M.; Marsich, E. A Lactose-Modified Chitosan Accelerates Chondrogenic Differentiation in Mesenchymal Stem Cells Spheroids. Biomater. Adv. 2024, 160, 213849. [Google Scholar] [CrossRef] [PubMed]
- Pizzolitto, C.; Scognamiglio, F.; Baldini, G.; Bortul, R.; Turco, G.; Donati, I.; Nicolin, V.; Marsich, E. Bioactive Lactose-Modified Chitosan Acts as a Temporary Extracellular Matrix for the Formation of Chondro-Aggregates. ACS Appl. Polym. Mater. 2023, 5, 504–516. [Google Scholar] [CrossRef]
- Sacco, P.; Piazza, F.; Pizzolitto, C.; Baj, G.; Brun, F.; Marsich, E.; Donati, I. Regulation of Substrate Dissipation via Tunable Linear Elasticity Controls Cell Activity. Adv. Funct. Mater. 2022, 32, 2200309. [Google Scholar] [CrossRef]
- Greco, S.; D’agostino, E.; Manfrin, C.; Gaetano, A.S.; Furlanis, G.; Capanni, F.; Santovito, G.; Edomi, P.; Giulianini, P.G.; Gerdol, M. RNA-Sequencing Indicates High Hemocyanin Expression as a Key Strategy for Cold Adaptation in the Antarctic Amphipod Eusirus Cf. Giganteus Clade G3. BIOCELL 2021, 45, 1611–1619. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of Relative Gene Expression Data Using Real-Time Quantitative PCR and the 2−ΔΔCT Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Ernest, N.J.; Habela, C.W.; Sontheimer, H. Cytoplasmic Condensation Is Both Necessary and Sufficient to Induce Apoptotic Cell Death. J. Cell Sci. 2008, 121, 290–297. [Google Scholar] [CrossRef]
- Amano, M.; Nakayama, M.; Kaibuchi, K. Rho-Kinase/ROCK: A Key Regulator of the Cytoskeleton and Cell Polarity. Cytoskeleton 2010, 67, 545–554. [Google Scholar] [CrossRef]
- Smyrek, I.; Mathew, B.; Fischer, S.C.; Lissek, S.M.; Becker, S.; Stelzer, E.H.K. E-Cadherin, Actin, Microtubules and FAK Dominate Different Spheroid Formation Phases and Important Elements of Tissue Integrity. Biol. Open 2019, 8, bio037051. [Google Scholar] [CrossRef]
- DeLise, A.M.; Fischer, L.; Tuan, R.S. Cellular Interactions and Signaling in Cartilage Development. Osteoarthr. Cartil. 2000, 8, 309–334. [Google Scholar] [CrossRef] [PubMed]
- Song, E.K.; Park, T.J. Integrin Signaling in Cartilage Development. Anim. Cells Syst. 2014, 18, 365–371. [Google Scholar] [CrossRef]
- Ryu, N.-E.; Lee, S.-H.; Park, H. Spheroid Culture System Methods and Applications for Mesenchymal Stem Cells. Cells 2019, 8, 1620. [Google Scholar] [CrossRef] [PubMed]
- Tuli, R.; Tuli, S.; Nandi, S.; Huang, X.; Manner, P.A.; Hozack, W.J.; Danielson, K.G.; Hall, D.J.; Tuan, R.S. Transforming Growth Factor-β-Mediated Chondrogenesis of Human Mesenchymal Progenitor Cells Involves N-Cadherin and Mitogen-Activated Protein Kinase and Wnt Signaling Cross-Talk. J. Biol. Chem. 2003, 278, 41227–41236. [Google Scholar] [CrossRef]
- Nelson, W.J. Regulation of Cell–Cell Adhesion by the Cadherin–Catenin Complex. Biochem. Soc. Trans. 2008, 36 Pt 2, 149–155. [Google Scholar] [CrossRef]
- LaFlamme, S.E.; Mathew-Steiner, S.; Singh, N.; Colello-Borges, D.; Nieves, B. Integrin and Microtubule Crosstalk in the Regulation of Cellular Processes. Cell. Mol. Life Sci. 2018, 75, 4177–4185. [Google Scholar] [CrossRef]
- Li, X.; Goult, B.T.; Ballestrem, C.; Zacharchenko, T. The Structural Basis of the Talin–KANK1 Interaction That Coordinates the Actin and Microtubule Cytoskeletons at Focal Adhesions. Open Biol. 2023, 13, 230058. [Google Scholar] [CrossRef]
- Yu, M.; Le, S.; Ammon, Y.-C.; Goult, B.T.; Akhmanova, A.; Yan, J. Force-Dependent Regulation of Talin–KANK1 Complex at Focal Adhesions. Nano Lett. 2019, 19, 5982–5990. [Google Scholar] [CrossRef]
- Li, J.X.H.; Tang, V.W.; Brieher, W.M. Actin Protrusions Push at Apical Junctions to Maintain E-Cadherin Adhesion. Proc. Natl. Acad. Sci. USA 2020, 117, 432–438. [Google Scholar] [CrossRef]
- Maître, J.-L.; Heisenberg, C.-P. Three Functions of Cadherins in Cell Adhesion. Curr. Biol. 2013, 23, R626–R633. [Google Scholar] [CrossRef]
- Rafiq, N.B.M.; Nishimura, Y.; Plotnikov, S.V.; Thiagarajan, V.; Zhang, Z.; Shi, S.; Natarajan, M.; Viasnoff, V.; Kanchanawong, P.; Jones, G.E.; et al. A Mechano-Signalling Network Linking Microtubules, Myosin IIA Filaments and Integrin-Based Adhesions. Nat. Mater. 2019, 18, 638–649. [Google Scholar] [CrossRef] [PubMed]
- Gauthier-Rouvière, C.; Causeret, M.; Comunale, F.; Charrasse, S. Cadherin-Mediated Cell-Cell Adhesion and the Microtubule Network. In Rise and Fall of Epithelial Phenotype: Concepts of Epithelial-Mesenchymal Transition; Savagner, P., Ed.; Springer: Boston, MA, USA, 2005; pp. 288–296. [Google Scholar]
- Devanny, A.J.; Vancura, M.B.; Kaufman, L.J. Exploiting Differential Effects of Actomyosin Contractility to Control Cell Sorting among Breast Cancer Cells. Mol. Biol. Cell 2021, 32, ar24. [Google Scholar] [CrossRef] [PubMed]
- Napolitano, A.P.; Chai, P.; Dean, D.M.; Morgan, J.R. Dynamics of the Self-Assembly of Complex Cellular Aggregates on Micromolded Nonadhesive Hydrogels. Tissue Eng. 2007, 13, 2087–2094. [Google Scholar] [CrossRef] [PubMed]
- Ng, D.H.J.; Humphries, J.D.; Byron, A.; Millon-Frémillon, A.; Humphries, M.J. Microtubule-Dependent Modulation of Adhesion Complex Composition. PLoS ONE 2014, 9, e115213. [Google Scholar] [CrossRef]
- Francis, D.V.; Rajeswari, A.J.; Stephen, J.B.; Parasuraman, G.; Lisha, J.J.; Livingston, A.; Rani, S.; Daniel, A.J.; Sathishkumar, S.; Vinod, E. An Ultrastructural Report of Human Articular Cartilage Resident Cells in Correlation with Their Phenotypic Characteristics. J. Histotechnol. 2024, 47, 23–38. [Google Scholar] [CrossRef]
- Goessler, U.; Bugert, P.; Bieback, K.; Stern-Straeter, J.; Bran, G.; Hörmann, K.; Riedel, F. Integrin Expression in Stem Cells from Bone Marrow and Adipose Tissue during Chondrogenic Differentiation. Int. J. Mol. Med. 2008, 21, 271–279. [Google Scholar] [CrossRef]
- Wang, L.; Zheng, F.; Song, R.; Zhuang, L.; Yang, M.; Suo, J.; Li, L. Integrins in the Regulation of Mesenchymal Stem Cell Differentiation by Mechanical Signals. Stem Cell Rev. Rep. 2022, 18, 126–141. [Google Scholar] [CrossRef]
- Zhang, T.; Wen, F.; Wu, Y.; Goh, G.S.H.; Ge, Z.; Tan, L.P.; Hui, J.H.P.; Yang, Z. Cross-Talk between TGF-Beta/SMAD and Integrin Signaling Pathways in Regulating Hypertrophy of Mesenchymal Stem Cell Chondrogenesis under Deferral Dynamic Compression. Biomaterials 2015, 38, 72–85. [Google Scholar] [CrossRef]
- Mathieu, P.S.; Loboa, E.G. Cytoskeletal and Focal Adhesion Influences on Mesenchymal Stem Cell Shape, Mechanical Properties, and Differentiation down Osteogenic, Adipogenic, and Chondrogenic Pathways. Tissue Eng. Part B Rev. 2012, 18, 436–444. [Google Scholar] [CrossRef]
- Kanazawa, T.; Furumatsu, T.; Hachioji, M.; Oohashi, T.; Ninomiya, Y.; Ozaki, T. Mechanical Stretch Enhances COL2A1 Expression on Chromatin by Inducing SOX9 Nuclear Translocalization in Inner Meniscus Cells. J. Orthop. Res. 2012, 30, 468–474. [Google Scholar] [CrossRef]
- Chiquet, M.; Koch, M.; Matthisson, M.; Tannheimer, M.; Chiquet-Ehrismann, R. Regulation of Extracellular Matrix Synthesis by Mechanical Stress. Biochem. Cell Biol. 1996, 74, 737–744. [Google Scholar] [CrossRef]

| Biological Mechanism | Role in MF-Spheroid Dynamics | Role in CTLc-spheroid Dynamics | |
|---|---|---|---|
| E-cadherin | Cell–cell interaction | Mediate cell–cell aggregation; primary role in early stages (Figure 1 and Figure 2) | Mediate cell–cell aggregation and spheroid coalescence in early and late stages (Figure 1 and Figure 2) |
| β1–β3 integrins | Cell–ECM interaction | Exert a minor role in spheroid formation (Figure 1 and Figure 2) | Mediate cell–ECM aggregation and spheroid coalescence in early and late stages (Figure 1 and Figure 2) |
| Actin fibers | Assembly and disassembly | Generate pushing forces on cadherins that allows cell aggregation and increase cell cohesion (Figure 3) | Generate pushing forces on cadherins that allows cell aggregation; No impact on integrin-binding activity (Figure 3) |
| MLCK | Actomyosin contractility | Accumulation of cadherins at cell–cell interface which accounts for spheroid coalescence and compactness (Figure 3) | Progressive formation and strengthening of cell–cell interactions, with a densification of the spheroidal structures and further coalescence (Figure 3) |
| Microtubules | Assembly and disassembly | Microtubule depolymerization holds a minor role in the regulation of spheroid coalescence (Figure 3) | Microtubule dynamics participate in spheroid coalescence (Figure 3) |
| ROCK | Myosin light chain phosphorylation | ---- | -- |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Conz, M.; Scognamiglio, F.; Donati, I.; Zara, S.; Teti, G.; Romano, M.; Marsich, E. Early Chondrogenic Differentiation of Spheroids for Cartilage Regeneration: Investigation of the Structural and Biological Role of a Lactose-Modified Chitosan. Polysaccharides 2025, 6, 47. https://doi.org/10.3390/polysaccharides6020047
Conz M, Scognamiglio F, Donati I, Zara S, Teti G, Romano M, Marsich E. Early Chondrogenic Differentiation of Spheroids for Cartilage Regeneration: Investigation of the Structural and Biological Role of a Lactose-Modified Chitosan. Polysaccharides. 2025; 6(2):47. https://doi.org/10.3390/polysaccharides6020047
Chicago/Turabian StyleConz, Marco, Francesca Scognamiglio, Ivan Donati, Susi Zara, Gabriella Teti, Maurizio Romano, and Eleonora Marsich. 2025. "Early Chondrogenic Differentiation of Spheroids for Cartilage Regeneration: Investigation of the Structural and Biological Role of a Lactose-Modified Chitosan" Polysaccharides 6, no. 2: 47. https://doi.org/10.3390/polysaccharides6020047
APA StyleConz, M., Scognamiglio, F., Donati, I., Zara, S., Teti, G., Romano, M., & Marsich, E. (2025). Early Chondrogenic Differentiation of Spheroids for Cartilage Regeneration: Investigation of the Structural and Biological Role of a Lactose-Modified Chitosan. Polysaccharides, 6(2), 47. https://doi.org/10.3390/polysaccharides6020047











