The Role of Molecular and Structural Characteristics of Starch, Hydrocolloids, and Gluten in Bread In Vitro Digestibility
Abstract
1. Introduction
2. Materials and Methods
2.1. Preparation of Starches, Hydrocolloids, and Gluten Mixtures
2.2. Experimental Design and Specific Ratios
2.3. Chemical Characterization
2.4. Free Sugars
2.5. Starch and Amylose Contents
2.6. Starch Crystallinity
2.7. Starch and Protein In Vitro Digestibility
2.8. Bread Formulation and Substitution Design
2.9. Molecular Characterization of Starch, Gums, and Proteins
2.9.1. Molecular Characteristics of Starch
2.9.2. Molecular Characterization of Gums
2.9.3. Molecular Characterization of Proteins
2.9.4. Amylopectin Debranching and Chain Length Distribution
2.9.5. FTIR Analysis of Bread Digestion Residues
2.10. Statistical Analyses
3. Results and Discussion
- Starch and protein digestion
- Molecular structure characteristics of mixtures
- Principal component analysis of mixtures
- Bread chemical composition
- Bread digestibility
- Principal component and cluster statistical analyses
- Bread molecular characteristics during in vitro digestion
4. Conclusions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Copeland, L.; Blazek, J.; Salman, H.; Tang, M.C. Form and functionality of starch. Food Hydrocoll. 2009, 23, 1527–1534. [Google Scholar] [CrossRef]
- Gómez, M.; Ronda, F.; Blanco, C.A.; Caballero, P.A.; Apesteguía, A. Effect of dietary fibre on dough rheology and bread quality. Eur. Food Res. Technol. 2003, 216, 51–56. [Google Scholar] [CrossRef]
- Funami, T. Next target for food hydrocolloid studies: Texture design of foods using hydrocolloid technology. Food Hydrocoll. 2011, 25, 1904–1914. [Google Scholar] [CrossRef]
- Rosell, C.M.; Rojas, J.A.; de Barber, C.B. Influence of hydrocolloids on dough rheology and bread quality. Food Hydrocoll. 2001, 15, 75–81. [Google Scholar] [CrossRef]
- AACC International. Approved Methods of Analysis, 11th ed.; AACC International: St. Paul, MN, USA, 2010. [Google Scholar]
- Kohajdová, Z.; Karovičová, J. Application of hydrocolloids as baking improvers. Chem. Pap. 2009, 63, 26–38. [Google Scholar] [CrossRef]
- de la Rosa-Millán, J. Physicochemical, molecular, and digestion characteristics of annealed and heat–moisture treated starches under acidic, neutral, or alkaline pH. Cereal Chem. 2017, 94, 770–779. [Google Scholar] [CrossRef]
- Englyst, K.N.; Liu, S.; Englyst, H.N. Nutritional characterization and measurement of dietary carbohydrates. Eur. J. Clin. Nutr. 2007, 61, S19–S39. [Google Scholar] [CrossRef]
- Goñi, I.; Garcia-Alonso, A.; Saura-Calixto, F. A starch hydrolysis procedure to estimate glycemic index. Nutr. Res. 1997, 17, 427–437. [Google Scholar] [CrossRef]
- Hsu, H.W.; Vavak, D.L.; Satterlee, L.; Miller, G.A. A multienzyme technique for estimating protein digestibility. J. Food Sci. 1977, 42, 1269–1273. [Google Scholar] [CrossRef]
- AOAC. Official Methods of Analysis, 15th ed.; Association of Official Analytical Chemists: Washington, DC, USA, 1990. [Google Scholar]
- Yoo, S.H.; Jane, J.L. Molecular weights and gyration radii of amylopectins determined by high-performance size-exclusion chromatography equipped with multi-angle laser-light scattering and refractive index detectors. Carbohydr. Polym. 2002, 49, 307–314. [Google Scholar] [CrossRef]
- Ao, Z.; Simsek, S.; Zhang, G.; Venkatachalam, M.; Reuhs, B.L.; Hamaker, B.R. Starch with a slow digestion property produced by altering its chain length, branch density, and crystalline structure. J. Agric. Food Chem. 2007, 55, 4540–4547. [Google Scholar] [CrossRef] [PubMed]
- Susi, H.; Byler, D.M. Protein structure by Fourier transform infrared spectroscopy: Second derivative spectra. Biochem. Biophys. Res. Commun. 1983, 115, 391–397. [Google Scholar] [CrossRef] [PubMed]
- Yu, P.; McKinnon, J.J.; Christensen, C.R.; Christensen, D.A. Using synchrotron-based FTIR microspectroscopy to reveal chemical features of feather protein secondary structure: Comparison with other feed protein sources. J. Agric. Food Chem. 2004, 52, 7353–7361. [Google Scholar] [CrossRef]
- Vernon-Carter, E.J.; Hernandez-Jaimes, C.; Meraz, M.; Lara, V.H.; Lobato-Calleros, C.; Alvarez-Ramirez, J. Physico-chemical characterization and in vitro digestibility of gelatinized corn starch dispersion fractions obtained by centrifugation. Starch/Staerke 2015, 67, 701–708. [Google Scholar] [CrossRef]
- Singh, J.; Dartois, A.; Kaur, L. Starch digestibility in food matrix: A review. Trends Food Sci. Technol. 2010, 21, 168–180. [Google Scholar] [CrossRef]
- Denardin, C.C.; da Silva, L.P. Starch granules structure and its regards with physicochemical properties [Estrutura dos grânulos de amido e sua relação com propriedades físico-químicas]. Cienc. Rural 2009, 39, 945–954. [Google Scholar] [CrossRef]
- Lazaridou, A.; Biliaderis, C.G. Molecular aspects of cereal β-glucan functionality: Physical properties, technological applications and physiological effects. J. Cereal Sci. 2007, 46, 101–118. [Google Scholar] [CrossRef]
- Nugent, A.P. Health properties of resistant starch. Nutr. Bull. 2005, 30, 27–54. [Google Scholar] [CrossRef]
- Atkinson, F.S.; Foster-Powell, K.; Brand-Miller, J.C. International tables of glycemic index and glycemic load values: 2008. Diabetes Care 2008, 31, 2281–2283. [Google Scholar] [CrossRef]
- Gilani, G.S.; Xiao, C.W.; Cockell, K.A. Impact of antinutritional factors in food proteins on the digestibility of protein and the bioavailability of amino acids and protein quality. Br. J. Nutr. 2012, 108, S315–S332. [Google Scholar] [CrossRef]
- Yaskin Harush, M.; Shani Levi, C.; Lesmes, U. Potential of Process-Induced Modification of Potato Starch to Modulate Starch Digestibility and Levels of Resistant Starch Type III. Foods 2025, 14, 880. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Copeland, L.; Wang, S. Molecular disassembly of starch granules during gelatinization and its effect on starch digestibility: A review. Food Funct. 2015, 6, 3360–3372. [Google Scholar] [CrossRef] [PubMed]
- Scott, G.; Awika, J. Effect of protein–starch interactions on starch retrogradation and implications for food product quality. Compr. Rev. Food Sci. Food Saf. 2023, 22, 2081–2111. [Google Scholar] [CrossRef]
- Yamul, Y.K.; Navarro, A.S. Effect of hydrocolloids on structural and functional properties of wheat/potato (50/50) flour dough. Food Struct. 2020, 24, 100138. [Google Scholar] [CrossRef]
- Tsai, P.-C.; Lai, L.-S. In Vitro Starch Digestibility, Rheological, and Physicochemical Properties of Water Caltrop Starch Modified with Cycled Heat-Moisture Treatment. Foods 2021, 10, 1687. [Google Scholar] [CrossRef]
- Zhu, P.; Wang, M.; Du, X.; Chen, Z.; Liu, C.; Zhao, H. Morphological and physicochemical properties of rice starch dry heated with whey protein isolate. Food Hydrocoll. 2020, 109, 106091. [Google Scholar] [CrossRef]
- Ronda, F.; Perez-Quirce, S.; Lazaridou, A.; Biliaderis, C.G. Effect of barley and oat β-glucan concentrates on gluten-free rice-based doughs and bread characteristics. Food Hydrocoll. 2015, 48, 197–207. [Google Scholar] [CrossRef]
- Miao, M.; Jiang, B.; Cui, S.W.; Zhang, T.; Jin, Z. Slowly digestible starch—A review. Crit. Rev. Food Sci. Nutr. 2015, 55, 1642–1657. [Google Scholar] [CrossRef]
- Fardet, A.; Leenhardt, F.; Lioger, D.; Scalbert, A.; Rémésy, C. Parameters controlling the glycaemic response to breads. Nutr. Res. Rev. 2006, 19, 18–25. [Google Scholar] [CrossRef]
- Fennema, O.R. Food Chemistry, 3rd ed.; Marcel Dekker, Inc.: New York, NY, USA, 1996. [Google Scholar]
- Schwenzer, A.-K.; Kruse, L.; Jooß, K.; Neusüß, C. Capillary electrophoresis-mass spectrometry for protein analyses under native conditions: Current progress and perspectives. Proteomics 2024, 24, e2300135. [Google Scholar] [CrossRef]
- Raigond, P.; Ezekiel, R.; Raigond, B. Resistant starch in food: A review. J. Sci. Food Agric. 2015, 95, 1968–1978. [Google Scholar] [CrossRef] [PubMed]
- Zhong, Y.; Tai, L.; Blennow, A.; Ding, L.; Herburger, K.; Qu, J.; Xin, A.; Guo, D.; Hebelstrup, K.; Xinxun, L. High-amylose starch: Structure, functionality and applications. Crit. Rev. Food Sci. Nutr. 2022, 63, 8568–8590. [Google Scholar] [CrossRef] [PubMed]
- Gómez, M.; Ronda, F.; Caballero, P.A.; Blanco, C.A.; Rosell, C.M. Functionality of different hydrocolloids on the quality and shelf-life of yellow layer cakes. Food Hydrocoll. 2007, 21, 575–581. [Google Scholar] [CrossRef]
- Jenkins, D.J.; Wolever, T.M.; Taylor, R.H.; Barker, H.; Fielden, H.; Baldwin, J.M.; Bowling, A.C.; Newman, H.C.; Jenkins, A.L.; Goff, D.V. Glycemic index of foods: A physiological basis for carbohydrate exchange. Am. J. Clin. Nutr. 1981, 34, 362–366. [Google Scholar] [CrossRef]
- Foster-Powell, K.; Holt, S.H.; Brand-Miller, J.C. International table of glycemic index and glycemic load values: 2002. Am. J. Clin. Nutr. 2002, 76, 5–56. [Google Scholar] [CrossRef]
- Wang, S.; Zhang, X.; Wang, S.; Copeland, L. Changes of multi-scale structure during mimicked DSC heating reveal the nature of starch gelatinization. Sci. Rep. 2016, 6, 28271. [Google Scholar] [CrossRef]
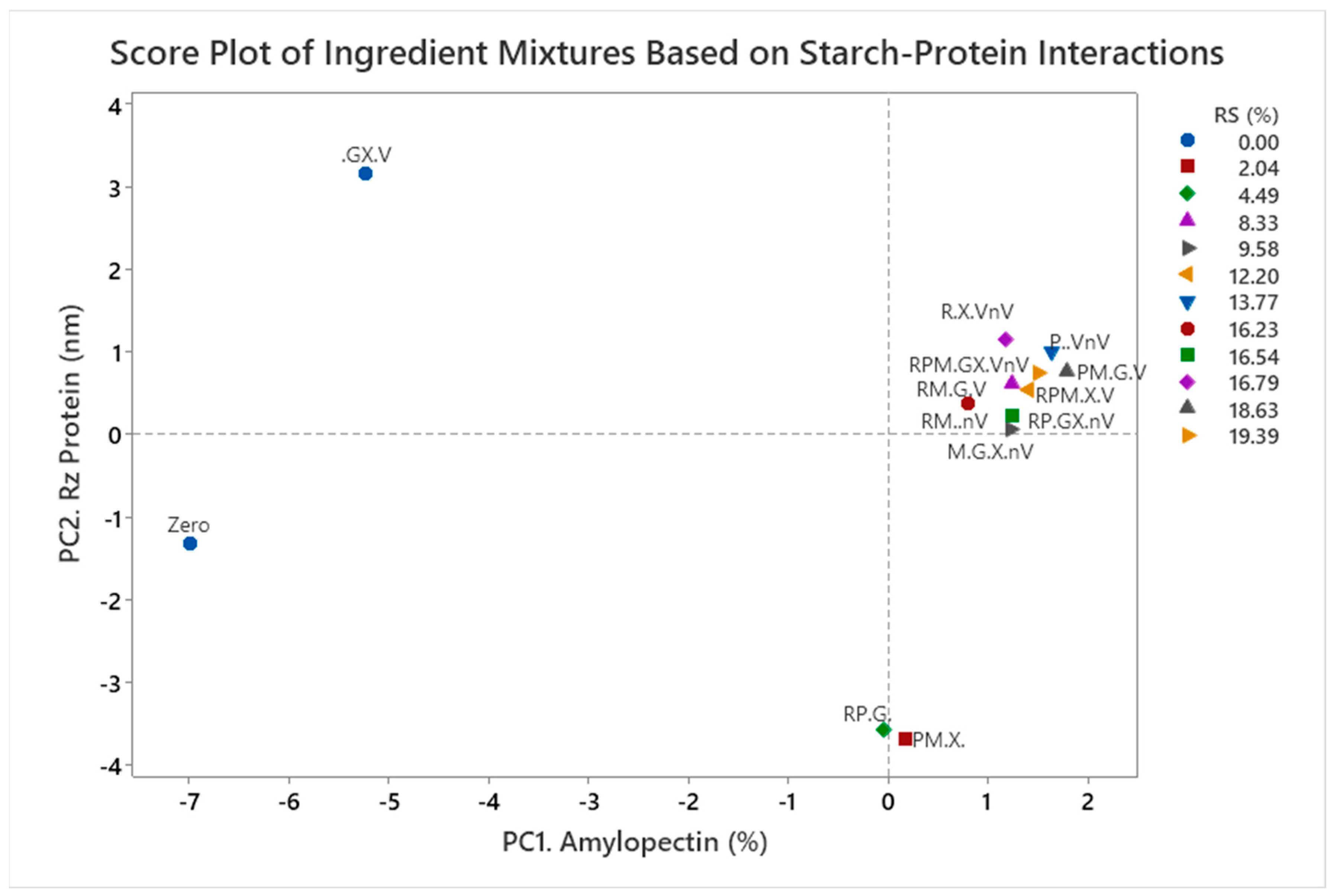
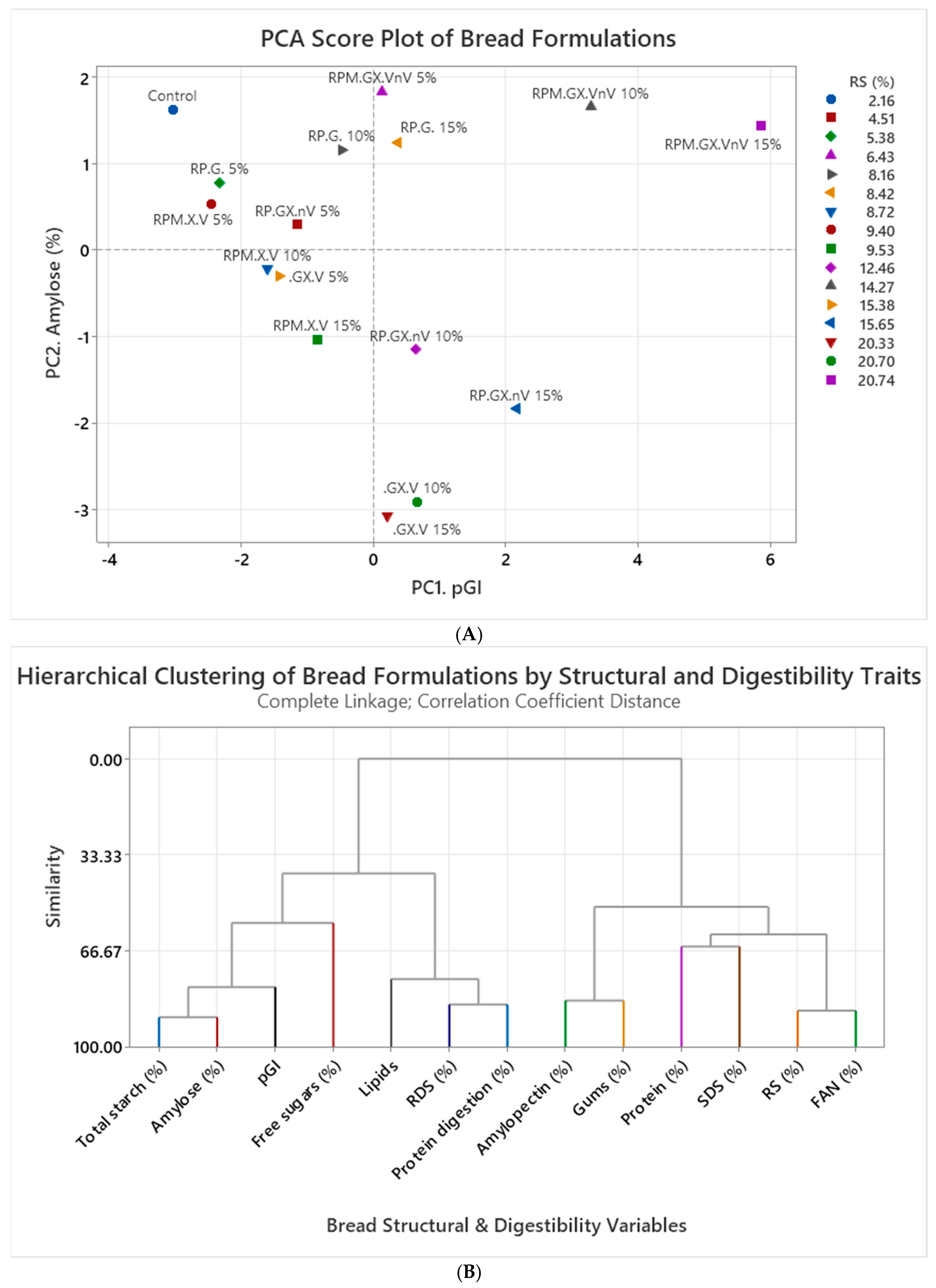
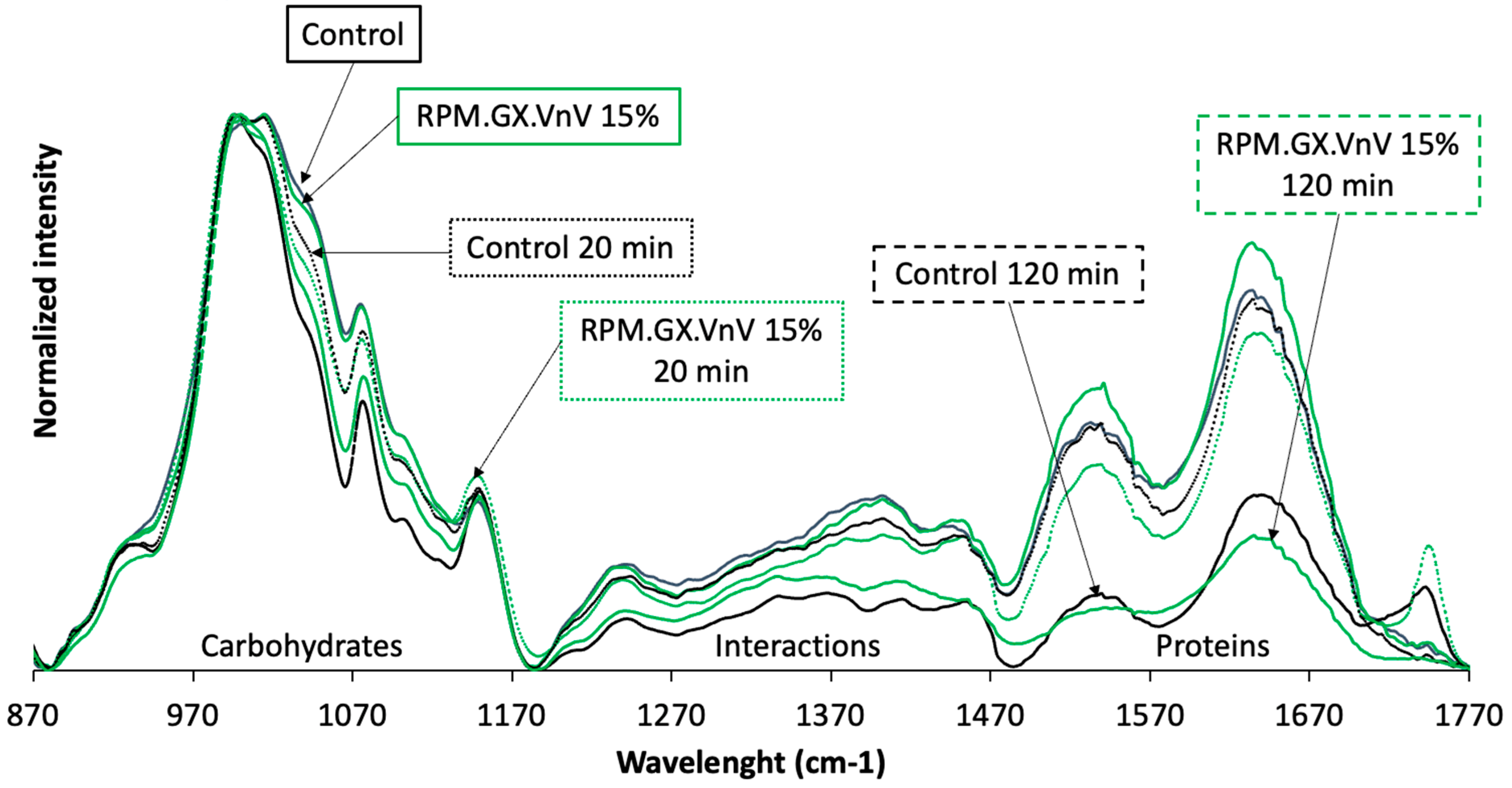
| Experiment | Rice (%) | Potato (%) | Maize (%) | Guar Gum (%) | Xanthan Gum (%) | Vital Gluten (%) | Non-Vital Gluten (%) | Sum (%) |
|---|---|---|---|---|---|---|---|---|
| RP.GX.nV | 20.00 | 20.00 | 0.00 | 20.00 | 20.00 | 0.00 | 20.00 | 100 |
| PM.G.V | 0.00 | 20.00 | 20.00 | 20.00 | 0.00 | 20.00 | 20.00 | 100 |
| RM.G.V | 25.00 | 0.00 | 25.00 | 25.00 | 0.00 | 25.00 | 0.00 | 100 |
| RPM.GX.VnV | 14.29 | 14.29 | 14.29 | 14.29 | 14.29 | 14.29 | 14.29 | 100 |
| R.X.VnV | 25.00 | 0.00 | 0.00 | 0.00 | 25.00 | 25.00 | 25.00 | 100 |
| .GX.V | 0.00 | 0.00 | 0.00 | 33.33 | 33.33 | 33.33 | 0.00 | 100 |
| P..VnV | 0.00 | 33.33 | 0.00 | 0.00 | 0.00 | 33.33 | 33.33 | 100 |
| PM.X. | 0.00 | 33.33 | 33.33 | 0.00 | 33.33 | 0.00 | 0.00 | 100 |
| M.G.X.nV | 0.00 | 0.00 | 25.00 | 25.00 | 25.00 | 0.00 | 25.00 | 100 |
| RPM.X.V | 20.00 | 20.00 | 20.00 | 0.00 | 20.00 | 20.00 | 0.00 | 100 |
| RM..nV | 33.33 | 0.00 | 33.33 | 0.00 | 0.00 | 0.00 | 33.33 | 100 |
| Zero | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0 |
| RP.G. | 33.33 | 33.33 | 0.00 | 33.33 | 0.00 | 0.00 | 0.00 | 100 |
| Sample | Total Starch (%) | Amylose (%) | Amylopectin (%) | Protein (%) | Gums (%) |
|---|---|---|---|---|---|
| RP.GX.nV | 37.78 ± 0.26 e | 23.45 ± 0.61 b | 77.27 ± 0.51 b | 20.08 ± 0.57 g | 40.00 ± 0.30 c |
| PM.G.V | 38.03 ± 0.86 e | 24.75 ± 0.56 b | 76.92 ± 0.19 b | 36.81 ± 0.85 c | 20.00 ± 0.08 g |
| RM.G.V | 47.38 ± 0.16 c | 21.65 ± 0.31 c | 79.85 ± 0.70 a | 24.00 ± 0.81 f | 25.00 ± 0.07 f |
| RPM.GX.VnV | 40.56 ± 0.29 d | 23.10 ± 0.45 b | 77.64 ± 0.78 b | 27.71 ± 0.85 e | 28.57 ± 0.26 e |
| R.X.VnV | 23.52 ± 0.69 g | 20.49 ± 0.56 c | 80.98 ± 0.92 a | 45.44 ± 1.00 b | 25 ± 0.89 f |
| .GX.V | --- | --- | --- | 30.84 ± 0.53 d | 66.67 ± 0.98 a |
| P..VnV | 31.80 ± 0.34 f | 26.11 ± 0.20 a | 75.17 ± 0.28 c | 58.95 ± 0.13 a | --- |
| PM.X. | 63.53 ± 0.69 a | 24.60 ± 0.11 b | 76.56 ± 0.72 b | 3.25 ± 0.68 h | 33.33 ± 0.78 d |
| M.G.X.nV | 24.80 ± 0.87 g | 22.90 ± 0.04 b | 77.84 ± 0.90 b | 24.48 ± 0.90 f | 50.00 ± 0.63 b |
| RPM.X.V | 56.98 ± 0.73 b | 22.86 ± 0.37 b | 77.78 ± 0.27 b | 19.37 ± 0.22 g | 20.00 ± 0.76 g |
| RM..nV | 62.83 ± 0.65 a | 21.48 ± 0.40 c | 79.07 ± 0.24 a | 31.62 ± 0.69 d | --- |
| Zero | --- | --- | --- | --- | --- |
| RP.G. | 62.49 ± 0.42 a | 23.69 ± 0.20 b | 77.46 ± 0.09 b | 3.07 ± 0.46 h | 33.33 ± 0.55 d |
| Sample | RDS (%) | SDS (%) | RS (%) | pGI | Protein Digestion (%) |
|---|---|---|---|---|---|
| RP.GX.nV | 52.29 ± 0.52 d | 31.17 ± 0.53 e | 16.54 ± 0.57 c | 83.71 ± 0.35 b | 74.42 ± 0.59 d |
| PM.G.V | 50.11 ± 0.34 e | 31.26 ± 0.63 e | 18.63 ± 0.49 b | 83.08 ± 0.59 b | 77.75 ± 0.16 c |
| RM.G.V | 53.36 ± 0.38 c | 38.31 ± 0.87 b | 8.33 ± 0.49 f | 84.02 ± 0.23 b | 81.38 ± 0.63 a |
| RPM.GX.VnV | 46.49 ± 0.73 f | 34.12 ± 0.16 d | 19.39 ± 0.95 a | 82.04 ± 0.82 c | 78.42 ± 0.40 b |
| R.X.VnV | 55.07 ± 0.37 b | 28.14 ± 0.37 f | 16.79 ± 0.37 c | 84.52 ± 0.28 b | 78.56 ± 0.01 b |
| .GX.V | --- | --- | --- | --- | 81.84 ± 0.18 a |
| P..VnV | 57.39 ± 0.47 b | 28.84 ± 0.97 f | 13.77 ± 0.51 d | 85.19 ± 0.97 a | 78.26 ± 0.24 b |
| PM.X. | 61.65 ± 0.95 a | 36.31 ± 0.10 c | 2.04 ± 0.19 h | 86.42 ± 0.05 a | 0.88 ± 0.09 e |
| M.G.X.nV | 56.25 ± 0.22 b | 34.17 ± 0.11 d | 9.58 ± 0.84 f | 84.86 ± 0.51 b | 74.42 ± 0.10 d |
| RPM.X.V | 45.39 ± 0.03 f | 42.41 ± 0.04 a | 12.20 ± 0.09 e | 81.72 ± 0.15 c | 81.65 ± 0.59 a |
| RM..nV | 44.31 ± 0.34 f | 39.46 ± 0.33 b | 16.23 ± 0.90 c | 81.41 ± 0.44 c | 74.14 ± 0.05 d |
| Zero | --- | --- | --- | --- | --- |
| RP.G. | 61.26 ± 0.64 a | 34.25 ± 0.25 d | 4.49 ± 0.33 g | 86.31 ± 0.30 a | 0.32 ± 0.96 e |
| Starch | Gums | Protein | ||||
|---|---|---|---|---|---|---|
| Sample | Mw (×108 g/mol) | Rz (nm) | Mw (×106 g/mol) | Rz (nm) | Mw (×106 g/mol) | Rz (nm) |
| RP.GX.nV | 2.37 ± 0.44 a | 188.60 ± 5.01 c | 2.31 ± 0.17 a | 22.01 ± 1.03 b | 1.11 ± 0.89 a | 12.06 ± 0.10 c |
| PM.G.V | 2.76 ± 0.89 a | 201.05 ± 4.06 b | 2.86 ± 0.07 a | 18.03 ± 1.05 c | 1.13 ± 0.75 a | 14.06 ± 0.04 b |
| RM.G.V | 2.24 ± 0.02 a | 175.58 ± 4.01 c | 2.87 ± 0.71 a | 18.08 ± 1.04 c | 1.32 ± 0.30 a | 16.07 ± 0.03 a |
| RPM.GX.VnV | 2.47 ± 0.99 a | 188.34 ± 7.09 c | 2.31 ± 0.83 a | 22.03 ± 0.96 b | 1.22 ± 0.28 a | 14.08 ± 0.09 b |
| R.X.VnV | 1.84 ± 0.93 a | 163.07 ± 3.08 d | 1.82 ± 0.05 b | 26.04 ± 1.07 a | 1.17 ± 0.44 a | 14.07 ± 0.02 b |
| .GX.V | --- | --- | 2.36 ± 0.41 a | 22.08 ± 0.99 b | 1.23 ± 0.28 a | 16.08 ± 0.07 a |
| P..VnV | 2.91 ± 0.68 a | 214.04 ± 3.01 a | --- | --- | 1.24 ± 0.94 a | 14.06 ± 0.05 b |
| PM.X. | 2.77 ± 0.41 a | 201.03 ± 3.05 b | 1.88 ± 0.80 a | 26.01 ± 1.05 a | --- | --- |
| M.G.X.nV | 2.64 ± 0.65 a | 188.08 ± 2.05 c | 2.33 ± 0.09 a | 22.10 ± 1.09 b | 1.12 ± 0.68 a | 12.01 ± 0.03 c |
| RPM.X.V | 2.45 ± 0.99 a | 188.39 ± 5.00 c | 1.86 ± 0.92 a | 26.01 ± 1.10 a | 1.33 ± 0.72 a | 16.03 ± 0.07 a |
| RM..nV | 2.24 ± 0.54 a | 175.55 ± 4.04 c | --- | --- | 1.14 ± 0.18 a | 12.09 ± 0.07 c |
| Zero | --- | --- | --- | --- | --- | --- |
| RP.G. | 2.37 ± 0.38 a | 188.54 ± 5.05 c | 2.8 ± 0.8 a | 18.07 ± 1.10 c | --- | --- |
| Samples | Total Starch (%) | Protein (%) | Lipids (%) | Ash (%) | Gums (%) |
|---|---|---|---|---|---|
| Control | 76.26 ± 0.54 a | 12.27 ± 0.17 e | 5.14 ± 0.68 a | 6.33 ± 0.84 f | --- |
| RP.GX.nV 5% | 74.34 ± 0.74 b | 12.66 ± 0.06 d | 4.43 ± 0.70 a | 8.57 ± 0.16 e | 2.02 ± 0.79 c |
| RP.GX.nV 10% | 72.41 ± 0.16 c | 13.05 ± 0.09 c | 4.10 ± 0.72 a | 10.44 ± 0.96 d | 4.05 ± 0.56 b |
| RP.GX.nV 15% | 70.49 ± 0.66 d | 13.44 ± 0.51 c | 3.96 ± 0.64 b | 12.11 ± 0.24 c | 6.07 ± 0.99 a |
| RPM.GX.VnV 5% | 72.45 ± 0.54 c | 13.20 ± 0.81 c | 4.31 ± 0.78 a | 10.04 ± 0.39 d | 3.30 ± 0.97 b |
| RPM.GX.VnV 10% | 68.63 ± 0.09 d | 14.13 ± 0.50 b | 3.91 ± 0.08 a | 13.33 ± 0.86 b | 5.70 ± 0.40 a |
| RPM.GX.VnV 15% | 64.82 ± 0.07 e | 15.06 ± 0.19 a | 3.65 ± 0.24 a | 16.47 ± 0.78 a | 6.50 ± 0.67 a |
| .GX.V 5% | 74.04 ± 0.32 b | 11.80 ± 0.79 f | 4.26 ± 0.19 a | 9.90 ± 0.24 d | 1.69 ± 0.17 c |
| .GX.V 10% | 71.81 ± 0.31 c | 11.33 ± 0.18 f | 3.67 ± 0.83 a | 13.18 ± 0.43 b | 3.39 ± 0.11 b |
| .GX.V 15% | 69.59 ± 0.04 d | 10.87 ± 0.33 f | 3.12 ± 0.52 b | 16.42 ± 0.32 a | 5.08 ± 0.21 a |
| RPM.X.V 5% | 75.30 ± 0.37 a | 11.78 ± 0.28 f | 3.54 ± 0.01 b | 9.38 ± 0.90 d | 2.04 ± 0.89 c |
| RPM.X.V 10% | 74.33 ± 0.52 b | 11.29 ± 0.03 f | 3.87 ± 0.96 a | 10.51 ± 0.18 d | 4.09 ± 0.88 b |
| RPM.X.V 15% | 73.37 ± 0.11 b | 10.80 ± 0.94 f | 3.78 ± 0.26 a | 12.05 ± 0.84 b | 6.13 ± 0.59 a |
| RDS (%) | SDS (%) | RS (%) | pGI | Protein Digestion (%) | |
|---|---|---|---|---|---|
| Control | 87.21 ± 0.20 a | 10.63 ± 0.13 g | 2.16 ± 0.40 i | 94.06 ± 0.21 a | 81.21 ± 0.73 d |
| RP.GX.nV 5% | 79.26 ± 0.27 c | 16.23 ± 0.52 d | 4.51 ± 0.37 h | 89.21 ± 0.21 c | 83.41 ± 0.93 c |
| RP.GX.nV 10% | 66.42 ± 0.08 g | 21.12 ± 0.54 b | 12.46 ± 0.74 d | 87.12 ± 0.65 d | 85.12 ± 0.44 b |
| RP.GX.nV 15% | 61.12 ± 0.06 h | 23.23 ± 0.85 a | 15.65 ± 0.53 c | 83.83 ± 0.33 f | 85.63 ± 0.67 b |
| .GX.V 5% | 80.14 ± 0.44 c | 13.43 ± 0.84 e | 6.43 ± 0.11 g | 89.75 ± 0.88 c | 83.11 ± 0.40 c |
| .GX.V 10% | 76.32 ± 0.10 d | 15.61 ± 0.73 d | 8.07 ± 0.37 f | 87.65 ± 0.41 d | 84.17 ± 0.40 b |
| .GX.V 15% | 70.1 ± 0.60 e | 19.1 ± 0.53 c | 10.78 ± 0.90 e | 85.97 ± 0.16 e | 83.56 ± 0.60 c |
| P..VnV 5% | 76.26 ± 0.13 d | 8.36 ± 0.43 h | 15.38 ± 0.60 c | 90.39 ± 0.48 c | 84.32 ± 0.72 b |
| P..VnV 10% | 55.14 ± 0.02 i | 9.12 ± 0.55 h | 35.74 ± 0.63 a | 87.70 ± 0.39 d | 85.61 ± 0.31 b |
| P..VnV 15% | 67.31 ± 0.41 f | 12.36 ± 0.94 f | 20.33 ± 0.79 b | 79.91 ± 0.53 g | 86.32 ± 0.36 a |
| RPM.X.V 5% | 83.36 ± 0.13 b | 7.24 ± 0.60 h | 9.40 ± 0.28 e | 92.17 ± 0.95 b | 83.21 ± 0.90 c |
| RPM.X.V 10% | 80.12 ± 0.19 c | 11.16 ± 0.36 g | 8.72 ± 0.91 e | 89.26 ± 0.60 c | 82.41 ± 0.77 c |
| RPM.X.V 15% | 76.31 ± 0.46 d | 14.16 ± 0.29 e | 9.53 ± 0.95 e | 87.00 ± 0.18 d | 84.31 ± 0.84 b |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
de la Rosa-Millan, J. The Role of Molecular and Structural Characteristics of Starch, Hydrocolloids, and Gluten in Bread In Vitro Digestibility. Polysaccharides 2025, 6, 46. https://doi.org/10.3390/polysaccharides6020046
de la Rosa-Millan J. The Role of Molecular and Structural Characteristics of Starch, Hydrocolloids, and Gluten in Bread In Vitro Digestibility. Polysaccharides. 2025; 6(2):46. https://doi.org/10.3390/polysaccharides6020046
Chicago/Turabian Stylede la Rosa-Millan, Julian. 2025. "The Role of Molecular and Structural Characteristics of Starch, Hydrocolloids, and Gluten in Bread In Vitro Digestibility" Polysaccharides 6, no. 2: 46. https://doi.org/10.3390/polysaccharides6020046
APA Stylede la Rosa-Millan, J. (2025). The Role of Molecular and Structural Characteristics of Starch, Hydrocolloids, and Gluten in Bread In Vitro Digestibility. Polysaccharides, 6(2), 46. https://doi.org/10.3390/polysaccharides6020046






