Abstract
Aulacaspis yasumatsui Takagi invaded Guam in 2003 and caused the widespread mortality of the indigenous Cycas micronesica K.D. Hill population. The regeneration of the surviving tree population continues to be constrained 20 years later, and a look at the changes in megastrobili traits may inform future conservation management decisions concerning regeneration. We quantified megastrobilus reproductive effort and output from 2001 to 2022 to address this need. The reproductive effort of each megastrobilus was immediately reduced by the invasion, as the number of megasporophylls declined by 29%, and the number of ovules declined by 73% in 2006. Reproductive output was also damaged, as the percent seed set declined by 56% and the number of seeds per strobilus declined by 88%. These fecundity metrics have shown few signs of recovery through 2022. Our results reveal that chronic A. yasumatsui infestations, combined with other invasive herbivore threats, have damaged the host C. micronesica population through a sustained reduction in ovule production and the percent seed set for each megastrobilus, thereby impairing regeneration. This plant response to the biotic threats is distinct from the ongoing mortality of mature trees and emerging seedlings. Conservation interventions may be required to foster a return to adequate regeneration during future attempts to aid C. micronesica recovery.
1. Introduction
The island of Guam was invaded by Aulacaspis yasumatsui Takagi (Hemiptera, Diaspididae) in 2003 [1,2]. Mortality among the host tree Cycas micronesica K.D. Hill population was immediate [3,4]. The International Union for Conservation of Nature (IUCN) listed this insular cycad species as Endangered in 2006 [5], and it was added to the United States’ Endangered Species Act (ESA) in 2015 [6].
The infestation by this non-native specialist armored scale insect generated numerous negative outcomes for the host plant on Guam. Within three years, seedling mortality was 100%, juvenile mortality was 97%, and adult mortality was 63% [4]. Ongoing plant mortality due to A. yasumatsui herbivory and other biotic threats continued, and the mortality reached 96% by 2020 [4]. The fact that this cycad species was the most abundant tree in Guam’s forests in 2002 [7] underscores the compelling nature of this case study.
This example from Guam adds to a growing body of information on how invasive species threaten native organisms on islands. Indeed, invasive species are a major driver of biodiversity loss on islands, where their impacts are often more severe than on continents [8,9,10]. The many previously studied cases have accumulated knowledge about the control of invasive species specific to islands [11]. International conferences have been convened to assimilate what has been learned to conserve threatened native island species through the better management of island invasives [12,13]. Mammals [14], plants [15], and insects [16] have been studied independently, and in conjunction with each other, in the many case studies of island invasions. Herbivorous insect invasions, in particular, exert extreme consequences on their host populations, sometimes eliminating the host [17]. The costs incurred while responding to island invasions indicate that the greatest costs are associated with insect invasions, followed by plant invasions, and then mammal invasions [18].
Prior to this invasion of Guam, C. micronesica seedlings were the dominant component of the forest floor, with 1600 seedlings per ha reported within the areas of occupancy [4]. The gestalt appearance of the Guam forests drastically changed as a result of the immediate culling of the seedlings. We also noticed that all the newly emerging seedlings during the early years of the invasion were immediately killed by the ubiquitous A. yasumatsui pressures; therefore, we predicted long-lasting negative outcomes for the surviving cycad population, due to the sustained lack of regeneration and recruitment [19]. Following this prediction, we determined the influence of chronic herbivory on C. micronesica microstrobili traits, to begin to understand how this specialist scale herbivore damages regeneration [20]. During the years immediately after the invasion, the volume of the male cones declined to 24% of the pre-invasion volume, with some increase in volume during subsequent years. However, as of 2021, the male cone volume remained stunted at 57% of the pre-invasion size. The male cones of this cycad tree are crucial for sustaining the Anatrachyntis Meyrick (Lepidoptera, Cosmopterigidae) pollinator population, by serving as a venue for sexual encounters and ovipositioning, and then enabling the life history stages of larval development and recruitment to the pupal stage [1,21]. Following the invasion, the number of Anatrachyntis pupae counted from each cone declined to 64% of the pre-invasion numbers [20], with no recovery in the ensuing years.
Using height increment metrics, an estimated 70 y of demographic depth of the Guam C. micronesica population was culled from the surviving tree population between 2003 and 2020 [22]. Every added year of ongoing failed conservation actions delay a recovery of the reproductive output and the recruitment of long-lived seedlings, which will cause even more years of loss of demographic depth. Clearly, new knowledge about C. micronesica reproductive efforts and regeneration potential is urgently needed to inform ongoing conservation efforts to mitigate the lack of regeneration caused by the A. yasumatsui invasion.
A detailed look at how the female trees of this dioecious gymnosperm have responded to this consequential invasion is required to augment what we have learned about male tree behaviors. Our objectives were to address this need by quantifying the long-term post-invasion changes in the megastrobilus size metrics and seed set in the east-coast karst forests of northern Guam.
2. Materials and Methods
Our field work began in 2001 and continued through 2022. We employed the same habitats that were used for the microstrobili measurements, and the site characteristics have been previously described [20]. This east-coast terrain historically contained high density areas of occupancy, with the presence of C. micronesica individuals from the seedling stage to more than 5 m in height. The field measurements were performed during November–December of each year. The habitats were invaded by A. yasumatsui in 2005, and by early 2006 the standing crown of leaves had been killed on every stem. For years, each cohort of newly added leaves was immediately infested, and the trees rarely contained two sequential cohorts of living leaves.
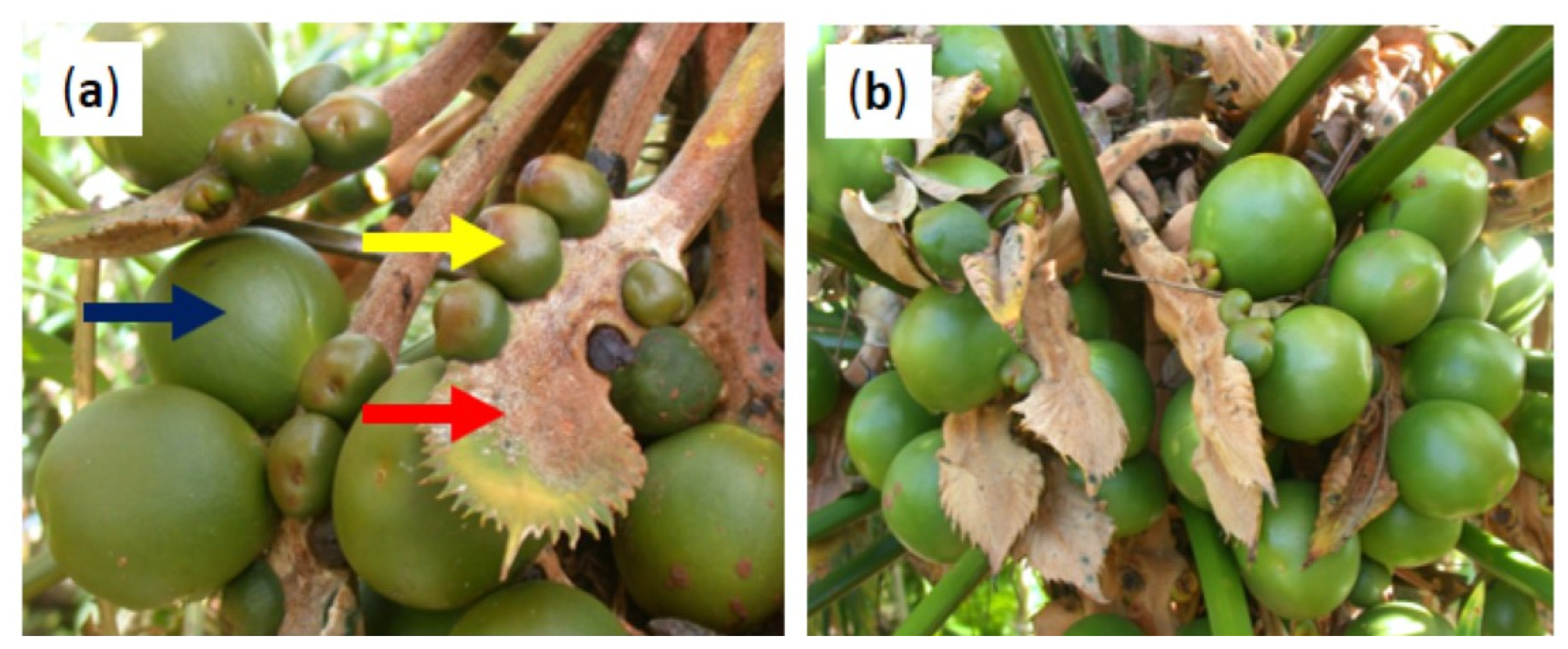
The peak season for C. micronesica megastrobili emergence before the invasion was April, with ovule receptivity occurring about one month later [23]. Determining the seed set in November─December was unambiguous for these naked gymnosperm ovules, due to the rapid increase in volume of the fertilized ovules [24], while the undeveloped ovules remained attached to the megasporophylls without a substantial increase in size (Figure 1a). The seed sarcotesta changes in color from green to bronze after about one year, so restricting our measurements to the trees with bright green seeds ensured our data were limited to the current year megastrobili. The megasporophylls were positively geotropic and relaxed at the time of our measurements (Figure 1b). A total of 50 megastrobili were measured each calendar year to quantify the various size metrics. The number of seeds and undeveloped ovules were counted and recorded for each individual sporophyll and the number of sporophylls was counted. The stem height was measured from the soil surface to the top of the cataphylls that terminate the stem apex.
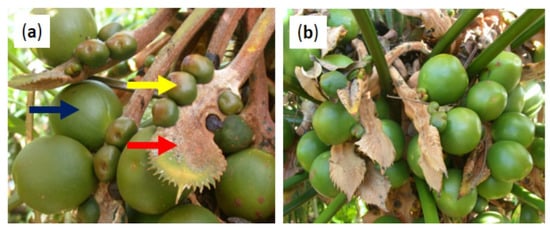
Figure 1.
Cycas micronesica megastrobilus traits. (a) Megasporophyll (red), undeveloped but with a retained ovule (yellow) and developing seed (blue). (b) Appearance of megastrobilus at the developmental stage used for the measurements.
For the measure of reproductive effort, we added undeveloped ovules and seeds to determine the total number of ovules that were produced within each strobilus. For the measure of reproductive output, we added the number of seeds for each strobilus. The seed set per strobilus was calculated as a percentage. The number of ovules per sporophyll was calculated for each strobilus. Megasporophylls are leaf homologs [25], and their function is to support ovules and seeds. However, some megasporophylls contain no ovules (Figure 2a). The number of sporophylls which contained no ovules was counted. Megasporophylls are typically isobilateral with two opposing ovules at each sporophyll node, but shortly after the invasion many sporophylls displayed an odd number of ovules (Figure 2b). The number of sporophylls in this category was counted. We then calculated the percentage of sporophylls within these two categories for each megastrobilus.

Figure 2.
Uncommon Cycas micronesica megastrobili traits. (a) Megastrobilus with numerous sporophylls that contain no ovules. (b) Examples of sporophylls that are not isobilateral.
Statistical analyses were performed using R (Version 3.6.3), to determine the differences between the pre-invasion years and the post-invasion years, or across years within each group. For all the variables, we first tested the data for normality and homogeneity of variances between the two groups, or within the groups. All the reproductive variables associated with the megastrobilus and stem height measurements indicated that the data were not normally distributed (Shapiro–Wilk test, W < 0.98, p < 0.0027), and the variances were not equal (folded f test, p < 0.013). To compare any two samples, the benchmark pre-invasion years with the post-invasion years, or the pre-invasion years with a subset of the post-invasion years, we used a Kruskal–Wallis rank-sum, two-tailed test. For the derived proportion variables, we used logistic regression (with a binomial distribution) to test for differences in the pre- versus post-invasion years’ differences. For example, for a seed set, the number of seeds was used as the dependent variable compared to total number of ovules for each year, and the pre- versus post-invasion years was the independent variable in the model. The reliability of the models was determined by the residual deviance as an indicator of overdispersion. If over-dispersed, we fitted the data to a quasibinomial distribution.
3. Results
3.1. Direct Metrics
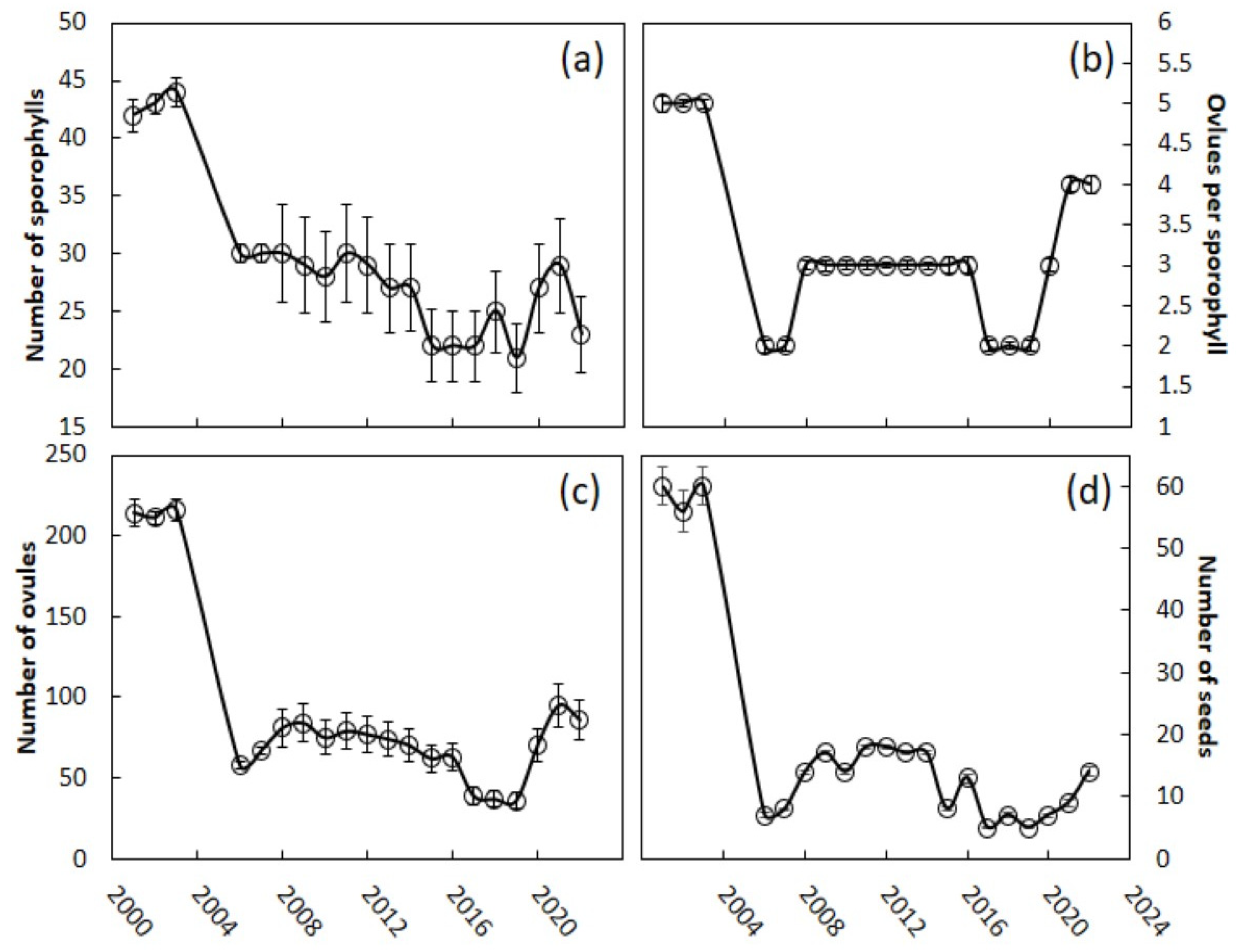
Every trait that we measured indicated that there were considerable declines in reproductive effort and output following the scale invasion. The megastrobili among the Guam C. micronesica trees contained a mean of 42 sporophylls during the pre-invasion years (Figure 3a). Finding the requisite 50 female trees with a young strobilus was difficult in 2006, as most of the trees were producing leaves to recover from the leaf loss in 2005. The 2006 strobili contained a mean of 30 sporophylls. The size of the megastrobili as defined by this metric has continued to be depressed, and all the comparisons of the pre-invasion years with the post-invasion years (or any subset of the post-invasion years) were significantly different (Kruskal–Wallis test = 330, χ2 approximation, p < 0.001). The years 2019–2022 were characterized by megastrobili with only 25 sporophylls on average. Megasporophylls contained a mean of five ovules prior to the scale invasion (Figure 3b), and many sporophylls contained six or eight ovules during these years. After the invasion, the mean number of ovules per sporophyll declined to two, and no sporophylls contained more than four ovules, with significant differences between the pre-invasion versus post-invasion years (Kruskal–Wallis test = 356, χ2 approximation, p < 0.001). Megasporophylls on Guam’s female trees contained only two to three ovules on average through 2020. The number of ovules per megasporophyll increased to four in the years 2021 and 2022, and some sporophylls contained six ovules for the first time since the scale invasion, but were still significantly different from the pre-invasion years (Kruskal–Wallis test = 102, χ2 approximation, p < 0.001). The megastrobili contained a mean of 214 ovules prior to the invasion (Figure 3c). The invasion caused an immediate 73% decline in this measure of reproductive effort. The mean number of ovules per megastrobilus was 68 for the years 2006–2022, significantly different from all the pre-invasion years (Kruskal–Wallis test = 380, χ2 approximation, p < 0.001). Each megastrobilus supported an average of 59 seeds prior to the scale invasion (Figure 3d). This metric also dramatically declined as a result of the invasion, declining 88% by 2006. The number of seeds contained in each megastrobilus has shown no obvious signs of recovery in recent years, and has averaged 12 for the years 2006–2022 (Kruskal–Wallis test = 381, χ2 approximation, p < 0.001, for the pre-invasion versus all post-invasion years).
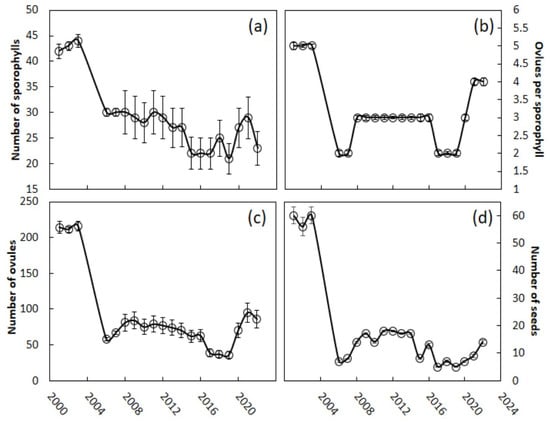
Figure 3.
Cycas micronesica megastrobili traits from 2001 to 2022. The scale Aulacaspis yasumatsui invaded the study sites in 2005. (a) Sporophylls per strobilus; (b) ovules per sporophyll; (c) ovules per strobilus; (d) seeds per strobilus. Mean ± SE, n = 50.
3.2. Derived Metrics and Tree Height
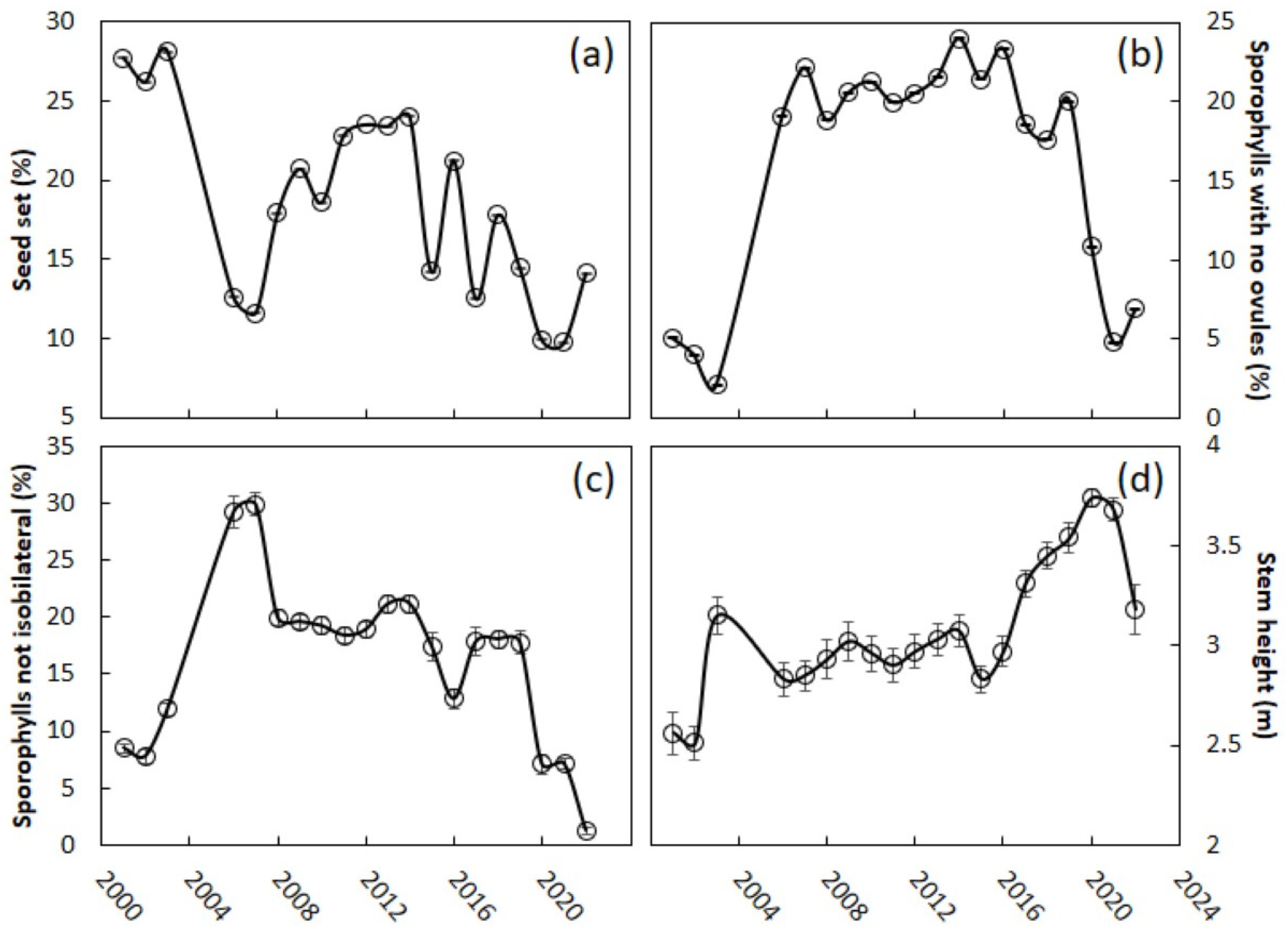
Guam’s female C. micronesica trees exhibited a 27% seed set in the years prior to the invasion (Figure 4a). This measure of reproductive output declined 56% in the first year after the invasion. The percentage seed set exhibited more year-to-year variability than the other megastrobilus traits during the post-invasion years. Overall, there was a significant difference between the pre- and post-invasion years (logistic regression, invasion variable, p < 0.001). A recovery in the seed set occurred from 2008 to 2014, and the highest percentage seed set years, 2011–2014 (23–24%), were not significantly different from the years 2001–2003 (26%–28%) (logistic regression, invasion variable, p = 0.23). Afterwards, the seed set began to decline again and in recent years has remained at 10%–14%, significantly different from the pre-invasion years (logistic regression, invasion variable, p < 0.001). Megasporophylls with no ovules can often be found within megastrobili, and prior to the invasion only 3.7% of the sporophylls were in this category (Figure 4b). This metric increased by 440% during the years after the invasion (logistic regression, post-invasion > pre-invasion years, p = 0.003). These seedless sporophylls accounted for about 20% of all the sporophylls through 2019. The past three years have shown signs that the production of these seedless sporophylls is returning to pre-invasion percentages (logistic regression, invasion variable, p = 0.049). Megasporophylls that are not isobilateral may also be found in some megastrobili, and prior to the invasion 9.4% of the sporophylls fell into this category (Figure 4c). Following the invasion, this metric increased by 69% (2006–2007 versus the pre-invasion years, logistic regression, invasion variable, p < 0.001), but began to decline again by 2008. About 15% to 20% of the sporophylls exhibited this behavior through 2018. The percentage of megasporophylls that were not isobilateral during the final three years of this study was not different from that during the pre-invasion years (logistic regression, invasion variable, p = 0.332). The mean height of the female trees that were randomly selected from the pre-invasion years ranged from 2.5 to 3.0 m (Figure 4d). This metric was relatively stable through 2016, but the pre-invasion years were significantly shorter than the 2006–2016 post-invasion years (Kruskal–Wallis test = 6.8, χ2 approximation, p = 0.009). Stem height was highest on average during 2019–2021.
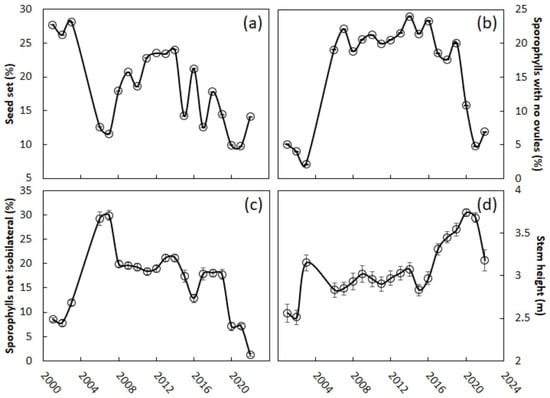
Figure 4.
Cycas micronesica megastrobili traits from 2001 to 2022. The scale Aulacaspis yasumatsui invaded the study sites in 2005. (a) Percent seed set; (b) sporophylls with no ovules; (c) sporophylls with odd number of ovules; (d) mean height of the study trees. Mean ± SE, n = 50 (SE values are very small and difficult to discern in (a,b)).
4. Discussion
The 2003 invasion of Guam by A. yasumatsui [1,2] has caused immeasurable negative ecosystem changes, partly because the naïve insular host tree species was the most abundant tree at the time, [7] and therefore served as a foundation species [26]. The influences of this invasion on C. micronesica mortality have been well-documented and reported [3,4]. By 2013, this species was no longer on the list of the 20 most abundant tree species in Guam [27], and by 2020 the population reached 96% mortality [4]. In order to foster effective species recovery, conservationists need to enumerate every distinct threat, then understand the driving mechanisms that underlie each threat, and how the disparate threats interact. We have shown that continued plant mortality is not the only negative outcome of this insect invasion, but the continued failure of C. micronesica to regenerate and recruit is also critical. Our historical predictions of sustained constraints on C. micronesica regeneration following this invasion [19] are now more fully understood. Our results reveal that a reduction in female reproductive effort (the number of ovules per megastrobilus) and reproductive output (the number of seeds per megastrobilus) occurred immediately after the invasion, and these important measures of plant fecundity have shown few signs of recovery among the surviving tree population of 2022. Considering the extent of population-level mortality and the unhealthy appearance of the surviving trees, the sustained production of megastrobili throughout the living population is surprising.
We recently reported similar negative outcomes for male C. micronesica trees, where the microstrobilus size metrics declined immediately after the invasion [20]. While both of the tree sexes had a large reduction in strobilus size traits (76% reduction in microstrobilus volume after the scale invasion, and an 88% reduction in megastrobilus seed number), microstrobilus volume has increased in recent years, but the megastrobilus size traits have not shown signs of recovery. Moreover, the number of Anatrachyntis pupae counted in C. micronesica microstrobili throughout the years since the invasion has revealed that some cones contained few to no pupae in recent years [20]. This development indicates that sustained observations of pollinator recruitment by conservationists are warranted to foresee a possible future decline in the pollinator population, as this moth relies on C. micronesica microstrobili for ovipositioning, larval development, and recruitment to the pupal stage [21,28]. Our collective observations of population-level behaviors indicate that the reduced reproductive effort of the female trees and the rapid mortality of seedlings immediately after emergence comprise the major reasons for the sustained reduction in C. micronesica regeneration following this consequential island invasion.
The construction of a sporophyll with no ovules (the blank sporophylls) or a reduced number of ovules (the sporophylls with odd numbers of ovules) may be one means of reducing the costs of reproduction. This plant behavior deserves further study, as it may be a universal response to all abiotic and biotic forms of female cycad plant stress. One approach to reduce the reproductive costs for these female trees may be to increase the window of time between megastrobilus initiation events [29]. This behavior would give the tree time to increase the stored pool of nonstructural resources required to support a new strobilus to maturity. However, this behavior would also mean the individual would remain uninvolved in population-level gamete exchange for extended periods of time. A second approach to reduce the costs of reproduction may be to initiate strobili more often, but limit the reproductive effort by constraining the sink activity of the most costly structures, in this case the number of mature seeds. The tree is not in full control of the percent seed set, as that is also under the control of pollinator behavior and population size. However, the maximum number of potential seeds within a strobilus is under the plant’s control. A reduction in the number of ovules per strobilus following the stress of the invasion could have been achieved by retaining the pre-invasion mean of five ovules per sporophyll, then reducing the number of sporophylls per megastrobilus. The female trees in Guam relied partly on this behavior (29% initial reduction), but these trees relied more heavily on reducing the number of ovules per sporophyll (60% initial reduction) as the means of reducing the total ovule count per strobilus. These findings may indicate a limited genetic control over the plasticity of sporophyll number per strobilus, and a greater genetic control over the plasticity of ovule number per sporophyll. These issues deserve further study.
These phenomena coalesce to indicate that even if conservation efforts or fortuitous events begin to partly mitigate the ongoing mortality of the surviving C. micronesica population, recovery of regeneration may not ensue without further research and intervention. Therefore, proactive conservation actions may be required to mitigate the sustained constraints on plant fecundity and the recruitment of long-lived seedlings. Our findings argue for an immediate focus on more research to inform these conservation efforts. We consider four avenues of immediate relevant research, which should be pursued in synchronicity to ensure that each agendum is cross-informed by the other agenda.
First, a lepidoptera taxonomist is urgently needed to clarify the identity of the pollinators. Cycad pollination mutualists are also typically consumers of male cone tissue. Cycas micronesica is the only cycad species known to rely exclusively on a micro-lepidopteran cone borer, as other cycad species rely on beetles and thrips as their mutualist. Our morphology observations indicate that the Anatrachyntis taxon found on Guam is the same as that found with the C. micronesica population on Rota Island, but distinct from that found on Yap Island. However, the taxon has not been unambiguously identified at the species level for any of these three insular populations. This must be corrected. The Guam moth is likely a two-island endemic insect, and is at risk of co-extinction [26]. The moth cannot be considered for listing under the Endangered Species Act if the binomial remains ambiguous, yet to date, no funds have been appropriated to correct this conservation emergency. Appropriating the services of a taxonomist to use every approach required to provide the binomial for the Guam, Rota, and Yap Anatrachyntis populations is urgent. If listed, this animal could be provided new conservation support, and this would synergize the conservation support being provided to C. micronesica.
Second, a source-sink physiologist is needed to better understand the nonstructural carbohydrate relations that define the timing and size of cycad growth events [30,31,32]. Traditional knowledge of the production of starch from Cycas revoluta Thunb. stem tissue exploited the fact that male tree stems contain more starch than female tree stems, and the greatest yield of starch can be obtained by harvesting immediately prior to a primary growth event [30]. New C. micronesica leaf and microstrobilus initiation and growth decreased the nonstructural carbohydrate content of subtending stems, confirming the reliance on stored stem resources for new growth [32]. The depletion of nonstructural carbohydrates preceded C. revoluta plant mortality during the A. yasumatsui infestations [33]. Guam’s chronic A. yasumatsui infestations have created a situation where the unrelenting hemipteran feeding behaviors, coupled with the resource-depleting primary growth events of the host trees, appear to have caused the surviving trees to be functioning at a constant deficit of nonstructural resources. Indeed, the post-invasion C. micronesica population on Guam has a reduced starch and sugar content of the stems [34] and seed gametophytes [35] as a result of the invasion. The deficit of resources stored in C. micronesica organs is the probable cause of the reduced reproductive effort for the male and female trees, and the deficit in stored resources in the megagametophytes may partly explain the reduced germination and early seedling survival of the seeds harvested from scale-infested trees [36]. The 4% of Guam’s pre-invasion population of C. micronesica that remains alive [4] is not thriving, but instead is in survival mode. A source-sink physiologist would be able to more fully understand how endogenous resource pools are involved in the ongoing constraints on plant survival, fecundity, regeneration, and recruitment.
Third, a plant anatomist will be required to understand the changes that have occurred to the stem apices. Pachycaulous cycad stems grow in diameter without the benefit of a secondary vascular cambium, and rely on a large primary thickening meristem (PTM) to construct most of the radial growth [30]. Healthy arborescent cycad stems exhibit minimal change in diameter for the full vertical span of the stem when compared to lignophyte trees (Figure 5a). A conspicuous decrease in the apical stem diameter of Guam’s surviving C. micronesica trees has occurred in recent years as the trees have responded to the resource-limited growing conditions since the A. yasumatsui invasion (Figure 5b). A plant anatomist is needed to understand how this behavior has influenced the size of the PTM. To our knowledge, this is the first case study of a population-level response of an apparent reduction in PTM size as a result of chronic biotic threats. If a reduction in PTM size is confirmed by an anatomist, the reasons behind the 44% decline in height increment [22], the reduced microstrobilus size [20], and the reduced megastrobilus size (reported herein) may become better understood. Moreover, we have observed a recent change of leaf morphology and allometry, such that petiole length has increased, rachis length has decreased, and leaflet number has declined. These overall smaller leaves mimic juvenile leaf traits. We predict these reductions in leaf size may benefit the C. micronesica population by reducing the drag coefficient of the canopies during future tropical cyclones. Clearly, a plant anatomist is urgently needed to interpret how this unusual apical stem behavior may influence the recovery of reproductive effort and fecundity, and how the resulting changes in leaf traits may influence tropical cyclone damage. What are the long-term consequences of this abnormal decrease in stem apex diameter? If the Guam population begins to recover in health due to conservation interventions, will the PTM grow to the size that characterized the trees prior to 2005? If so, will the stratum characterizing the 2005–2023 height growth remain constrained in diameter for the life of the tree, or will the tissues within this stratum be able to use diffuse mitotic activity to expand to the pre-invasion diameter?

Figure 5.
Apical 50 cm of a typical Guam Cycas micronesica stem stratum depicting before and after the Aulacaspis yasumatsui invasion. (a) A 214 cm tall Cycas micronesica tree in June 2003 with a robust crown of leaves and no narrowing of the stem. (b) A 230 cm tall C. micronesica tree in March 2023 with a stunted crown of leaves and narrowing of the stem apex. Aulacaspis yasumatsui invaded the habitat in 2005. The red arrows point to the top of the cataphylls, which protect the primary thickening meristem. The yellow arrow indicates the estimated stem height in 2005, based on published height increment data from the same habitat [22].
Fourth, a pollination biologist will be required to manage female plant fecundity. Prior to the invasion, we confirmed that in many Guam habitats the C. micronesica seed set was rarely greater than 30%. This limited seed set in Guam is in sharp contrast to the 80% seed set and the 90+ seeds per strobilus we have measured in Yap (T.E.M., unpublished). These observations indicate that the Guam Anatrachyntis taxon may be less efficient as a pollinator than the Yap Anatrachyntis taxon, or that other herbivore threats on Guam interact with the pollinators in a manner that is absent on Yap. They also indicate that the Guam trees could probably support many more seeds per strobilus than occurs under non-stressful conditions. The sustained reduction in the natural seed set of the surviving Guam trees may be reversed by a pollination biologist using controlled pollinations. If a source-sink physiologist confirms the recovery of the nonstructural carbohydrate content of the surviving stems, these management actions may improve species recovery by increasing tree fecundity without stressing the trees.
The list of invasive herbivore threats to Guam’s C. micronesica population is substantial [37]. In addition to the numerous invasive herbivores, the stem borer Acalolepta marianarum Aurivillius (Coleoptera: Cerambycidae) relies on C. micronesica stems for ovipositioning and larval development [1]. Irruptions of this native stem borer occurred after the A. yasumatsui invasion, due to reductions in tree health from the unprecedented alien insect feeding behaviors [38]. These developments exemplify how each herbivore possesses the potential to magnify the A. yasumatsui damage into an invasional meltdown [26]. Until adequate biological control organisms are successfully established on Guam, most alternative conservation actions, such as tree salvage and transplant projects, will probably not be successful [39]. The transplanted trees will likely die during the years after the project is completed and deemed a success. The long list of biological threats also illuminates the urgency of funding the four recommended research thrusts, as continued delays in mitigating the invasive pest damage indicate that an irruption of any one of the herbivore threats could occur at any time, leading to an unprecedented increase in C. micronesica mortality rates.
As discussed previously, both the male and female trees exhibited negative responses in reproductive behavior after the invasion. However, there are notable differences between the sexes in the recovery of some traits. These differential responses of the male versus female trees represent a distinct example of sexual dimorphism. Other examples of C. micronesica sexual dimorphism have been reported. More branching among the male trees occurred when compared to the female trees [40]. The frequency of strobilus production was greater for the male trees than for the female trees [23,28]. The height increment of the male trees exceeded that of the female trees [22]. Based on branching patterns, more female trees were killed following the A. yasumatsui invasion than male trees [40]. The chronic A. yasumatsui infestations reduced the height increment of the male trees to a greater extent than that of the female trees [22]. Understanding these and other behaviors that portray forms of sexual dimorphism may aid conservationists in developing a more effective C. micronesica recovery plan. Our results also indicate that cycads may be an ideal model species for studying how stressful conditions [41] and other global change factors [42] may influence diecious plant species in a manner that differs from hermaphrodite plant species.
We report herein an increase in female tree height throughout the years of this study, as we did for the male trees [20]. This finding does not indicate there were recruitment increments into large size categories due to population-level growth events. Instead, this metric captures the fact that more of the smaller individuals of the host tree have died in response to the chronic A. yasumatsui herbivory before the larger individuals have died [3,4]. Our annual search for appropriate megastrobili was random in this study, and as the insular population’s mean height increased as each year passed, the random sample of current-year megastrobili was conducted among the plant population that gradually increased in mean height.
This study adds to a growing body of literature documenting the multi-decadal changes to the C. micronesica population. Reaching an adequate level of understanding of the threats to the recovery of an endangered tree species requires long-term research by knowledgeable scientists. When germane research is conducted, conservationists benefit from an adaptive management approach, wherein every year of decision-making is supported by more of the accumulated new knowledge. In addition to this paper, our sustained research has resulted in several other publications based on long-term studies [4,20,22,43].
Benchmarking is an important endeavor for establishing knowledge of how global change factors influence biodiversity [44,45]. During an era of rapid change, if the research community fails to characterize various organism and population traits before substantial changes ensue, the opportunity to benchmark could be lost. Our pre-invasion megastrobili traits reported herein serve as one more benchmark for future conservationists to determine if the C. micronesica population will ever recover to a healthy pre-invasion status. We have previously reported that the pre-invasion height increment behaviors [22] and the pre-invasion microstrobili traits [20] may also serve as benchmarks for future C. micronesica conservation assessments of species recovery.
The threats causing tree extinctions are occurring on a global scale. The most common threat to tree conservation is habitat conversion for various uses, such as agriculture or urban development [46]. Our case study is an example of an alternative threat to trees, that of invasive herbivorous insects. As new invasions begin to threaten more tree species globally, our study may serve as an example to inform the mitigation efforts of future tree species extinction threats. Plant mortality during the initial three to four years after the invasion was so rapid [3,4], the conservation community was in triage mode without any local research to inform decisions. However, the research since those early years has been substantial, the greatest conservation intervention needs (biological control) are understood, and published recommendations from scientists with germane expertise have been numerous.
The constraints on the regeneration and recruitment of C. micronesica are consequential, but the direct threat of mature plant mortality remains the greatest threat to this gymnosperm tree. The implementation of a coalition of biological control organisms to reduce A. yasumatsui damage was the greatest C. micronesica conservation need as soon as the 2003 invasion occurred, was the greatest need when the host species was added to the IUCN Red List in 2006 [5], was the greatest need when it was listed under the ESA in 2015 [6], and remains the greatest conservation need today. The introduction and successful establishment of the armored scale predator Rhyzobius lophanthae Blaisdell (Coleoptera, Coccinellidae) in 2005 [47] is the probable reason for the surviving C. micronesica plants today. However, there are numerous limitations of this predator that do not allow the adequate control of A. yasumatsui (reviewed in [37,48]). We concur with previous recommendations [22,37,39,48] that the funding of a sustained, dedicated effort to establish a coalition of biological control organisms has been and continues to be the greatest conservation action priority needed to enable species recovery. The antecedent few years have been characterized by unprecedented declines in A. yasumatsui infestations, indicating that a fortuitous irruption of some biological control organism has occurred on Guam. Three years ago we recommended with urgency that the engaged conservation community should enact a dedicated effort to identify this organism [37]. To our knowledge, this has not occurred to date. This new knowledge is urgently needed for species recovery for two reasons. First, there is no reason for Guam conservationists to assume that this unknown biological control organism will sustain the future biological control of A. yasumatsui, and its precise identity will be required to monitor this in the future. Second, if a fortuitous invasion of Guam by an unprecedented biological control organism has indeed occurred, this organism needs to be transported and released on Rota to conserve the C. micronesica population there. The island of Rota was invaded by A. yasumatsui in 2007 [2], and the host C. micronesica population experienced devastation similar to that of Guam (T.E.M., unpublished data). A decline in the A. yasumatsui herbivory has also occurred recently on Rota Island (T.E.M., personal observations). There is no reason to believe the fortuitous biological control on Rota is due to the same organism exhibiting control on Guam. A survey of what is controlling A. yasumatsui on Rota is warranted, and if identified, that organism needs to be transported and released on Guam.
We conclude with three caveats. First, we used a fixed geographic range on the northeast coast of Guam for this study. We do not assume these same trends have occurred in other regions of Guam or in Rota, where all C. micronesica plants have been threatened by A. yasumatsui. Second, the recent fortuitous control of A. yasumatsui may be a turning point after which plant mortality will subside. If this does occur, then we predict that increases in stem apex, microstrobilus, and megastrobilus size will occur in the future in response to this turning point. The direct documentation of these changes by funded, competent scientists who understand cycad biology is warranted. Third, Guam is located within the most active tropical cyclone zone globally [49]. The island has not experienced direct landfall of an intense tropical cyclone since the 2003 A. yasumatsui invasion. Therefore, there are no historical observations that could inform how this form of large-scale abiotic disturbance will interact with the continuing demise of the island’s cycad population caused by insidious biotic threats. The ongoing changes that we have reported for male and female C. micronesica tree behaviors may be altered in unique ways when the next direct landfall of a tropical cyclone damages the vegetation of the island. Indeed, natural disturbances may profoundly influence the impact of invasive species [50]. The conservation community will need an embedded plant ecologist with knowledge of tropical cyclones to determine the consequences to species recovery when this occurs.
5. Conclusions
In this and previous studies, we have characterized specific declines in morphological traits and in the reproductive effort and output of both male and female trees since the invasion by A. yasumatsui. This, combined with the damage to regeneration and recruitment by the scale invasion and other threats, leads to sustained dire predictions for this species. We have suggested where essential research is needed to fully inform conservation efforts. We recommend that conservationists include this new knowledge in any species recovery agenda, along with the adaptive management research that we discussed in depth. We also recommend the critical evaluation of the results of any new conservation management schemes, and the continued monitoring of population recruitment and tree reproductive traits, as ways to evaluate the population health in light of the recent decline in A. yasumatsui incidence.
Author Contributions
Conceptualization, T.E.M. and L.I.T.; methodology, T.E.M. and L.I.T.; software, L.I.T.; formal analysis, L.I.T.; resources, T.E.M. and L.I.T.; data curation, T.E.M. and L.I.T.; writing—original draft preparation, T.E.M.; writing—review and editing, L.I.T.; funding acquisition, T.E.M. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the USDA CSREES grant number 2003-05495, and the United States Forest Service grant numbers 06-DG-11052021-206, 09-DG-11052021-173, 13-DG-11052021-210, and 17-DG-11052021-217.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data available upon request.
Acknowledgments
We thank Gil Cruz, Nirmala Dongol, Paris Marler, Frankie Matanane, and Robert Roemer for their aid during the field work, and stimulating discussions about Guam’s forest responses to the Aulacaspis yasumatsui invasion.
Conflicts of Interest
The authors declare there are no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
References
- Marler, T.; Muniappan, R. Pests of Cycas micronesica leaf, stem, and male reproductive tissues with notes on current threat status. Micronesica 2006, 39, 1–9. [Google Scholar]
- Marler, T.E. Cycad aulacaspis scale invades the Mariana Islands. Mem. N. Y. Bot. Gard. 2012, 106, 20–35. [Google Scholar]
- Marler, T.E.; Lawrence, J.H. Demography of Cycas micronesica on Guam following introduction of the armoured scale Aulacaspis yasumatsui. J. Trop. Ecol. 2012, 28, 233–242. [Google Scholar] [CrossRef]
- Marler, T.E.; Krishnapillai, M.V. Longitude, forest fragmentation, and plant size influence Cycas micronesica mortality following island insect invasions. Diversity 2020, 12, 194. [Google Scholar] [CrossRef]
- Bösenberg, J.D. Cycas micronesica. The IUCN Red List of Threatened Species 2022: E.T61316A68906033. 2022. Available online: https://dx.doi.org/10.2305/IUCN.UK.2022-1.RLTS.T61316A68906033.en (accessed on 1 June 2023).
- United States Fish & Wildlife Service. Endangered and threatened wildlife and plants; endangered status for 16 species and threatened status for 7 species in Micronesia. Fed. Regist. 2015, 80, 59424–59497. [Google Scholar]
- Donnegan, J.A.; Butler, S.L.; Grabowiecki, W.; Hiserote, B.A.; Limtiaco, D. Guam’s Forest Resources, 2002. Resource Bulletin PNW-RB-243; U.S. Department of Agriculture, Forest Service, Pacific Northwest Research Station: Portland, OR, USA, 2004.
- Russell, J.C.; Meyer, J.Y.; Holmes, N.D.; Pagad, S. Invasive alien species on islands: Impacts, distribution, interactions and management. Environ. Conserv. 2017, 44, 359–370. [Google Scholar] [CrossRef]
- Russell, J.C.; Kueffer, C. Island biodiversity in the Anthropocene. Annu. Rev. Environ. Resourc. 2019, 44, 31–60. [Google Scholar] [CrossRef]
- Spatz, D.R.; Holmes, N.D.; Will, D.J.; Hein, S.; Carter, Z.T.; Fewster, R.M.; Keitt, B.; Genovesi, P.; Samaniego, A.; Croll, D.A.; et al. The global contribution of invasive vertebrate eradication as a key island restoration tool. Sci. Rep. 2022, 12, 13391. [Google Scholar] [CrossRef]
- Simberloff, D.; Keitt, B.; Will, D.; Holmes, N.; Pickett, E.; Genovesi, P. Yes we can! Exciting progress and prospects for controlling invasives on islands and beyond. West. N. Am. Nat. 2018, 78, 942–958. [Google Scholar] [CrossRef]
- Veitch, C.R.; Clout, M.N. (Eds.) Turning the Tide: The Eradication of Invasive Species: Proceedings of the International Conference on Eradication of Island Invasives; Occasional Paper IUCN SSC No. 27; IUCN: Gland, Switzerland, 2002. [Google Scholar]
- Veitch, C.R.; Clout, M.N.; Martin, A.R.; Russell, J.C.; West, C.J. (Eds.) Island Invasives: Scaling up to Meet the Challenge; Occasional Paper IUCN SSC no. 62; IUCN: Gland, Switzerland, 2019. [Google Scholar]
- Wang, S.; Deng, T.; Zhang, J.; Li, Y. Global economic costs of mammal invasions. Sci. Total Environ. 2023, 857, 159479. [Google Scholar] [CrossRef]
- Brock, K.C.; Daehler, C.C. Island Plant Invasions. In Global Plant Invasions; Clements, D.R., Upadhyaya, M.K., Joshi, S., Shrestha, A., Eds.; Springer: Cham, Switzerland, 2022; pp. 253–278. [Google Scholar] [CrossRef]
- Kenis, M.; Auger-Rozenberg, M.A.; Roques, A.; Timms, L.; Péré, C.; Cock, M.J.W.; Settele, J.; Augustin, S.; Lopez-Vaamonde, C. Ecological effects of invasive alien insects. Biol. Invasions 2009, 11, 21–45. [Google Scholar] [CrossRef]
- Brockerhoff, E.G.; Liebhold, A.M. Ecology of forest insect invasions. Biol. Invasions 2017, 19, 3141–3159. [Google Scholar] [CrossRef]
- Bodey, T.W.; Angulo, E.; Bang, A.; Bellard, C.; Fantle-Lepczyk, J.; Lenzner, B.; Turbelin, A.; Watari, Y.; Courchamp, F. Economic costs of protecting islands from invasive alien species. Conserv. Biol. 2023, 37, e14034. [Google Scholar] [CrossRef] [PubMed]
- Marler, T.E.; Terry, L.I. Arthropod invasion disrupts Cycas micronesica seedling recruitment. Commun. Integr. Biol. 2011, 4, 778–780. [Google Scholar] [CrossRef] [PubMed]
- Marler, T.E.; Terry, L.I. Aulacaspis yasumatsui invasion reduced Cycas micronesica microstrobilus size and pollinator brood site competence. Insects 2021, 12, 1023. [Google Scholar] [CrossRef]
- Terry, I.; Roe, M.; Tang, W.; Marler, T.E. Cone insects and putative pollen vectors of the endangered cycad, Cycas micronesica. Micronesica 2009, 41, 83–99. [Google Scholar]
- Marler, T.E.; Griffith, M.P.; Krishnapillai, M.V. Height increment of Cycas micronesica informs conservation decisions. Plant Signal. Behav. 2020, 15, e1830237. [Google Scholar] [CrossRef]
- Dongol, N.; Marler, T.E. Season and frequency of Cycas micronesica leaf and reproductive events. Mem. N. Y. Bot. Gard. 2018, 117, 497–503. [Google Scholar]
- Marler, T.E.; Dongol, N. Models to describe Cycas micronesica leaf and strobili development. HortScience 2011, 46, 1333–1337. [Google Scholar] [CrossRef]
- Norstog, K.J.; Nicholls, T.J. The Biology of the Cycads; Cornell University Press: Ithaca, NY, USA, 1997. [Google Scholar]
- Marler, T.E.; Lindström, A.J. The value of research to selling the conservation of threatened species: The case of Cycas micronesica (Cycadopsida: Cycadales: Cycadaceae). J. Threat. Taxa 2014, 6, 6523–6528. [Google Scholar] [CrossRef]
- Lazaro, M.; Kuegler, O.; Stanton, S.; Lehman, A.; Mafnas, J.; Yatskov, M. Guam’s Forest Resources: Forest Inventory and Analysis, 2013; Resour. Bull. PNW-RB-270; U.S. Department of Agriculture, Forest Service, Pacific Northwest Research Station: Portland, OR, USA, 2020.
- Marler, T.E. Cycad mutualist offers more than pollen transport. Amer. J. Bot. 2010, 97, 841–845. [Google Scholar] [CrossRef] [PubMed]
- Marler, T.E. Time-size trade-offs in responses of cycads to male cone herbivory. Communic. Integr. Biol. 2010, 3, 602–603. [Google Scholar] [CrossRef] [PubMed]
- Thieret, J.W. Economic botany of the cycads. Econ. Bot. 1958, 12, 3–41. [Google Scholar] [CrossRef]
- Marler, T.E.; Lindström, A.J. Carbohydrates, pollinators, and cycads. Communic. Integr. Biol. 2015, 8, 2. [Google Scholar] [CrossRef] [PubMed]
- Marler, T.E.; Cruz, G.N. Cycas micronesica stem carbohydrates decline following leaf and male cone growth events. Plants 2020, 9, 517. [Google Scholar] [CrossRef]
- Marler, T.E.; Cascasan, A.N.J. Carbohydrate depletion during lethal infestation of Aulacaspis yasumatsui on Cycas revoluta. Int. J. Plant Sci. 2018, 179, 497–504. [Google Scholar] [CrossRef]
- Marler, T.E. Stem carbohydrates and adventitious root formation of Cycas micronesica following Aulacaspis yasumatsui infestation. HortScience 2018, 53, 1125–1128. [Google Scholar] [CrossRef]
- Marler, T.E.; Cruz, G.N. Source and sink relations mediate depletion of intrinsic cycad seed carbohydrates by Aulacaspis yasumatsui infestation. HortScience 2019, 54, 1712–1717. [Google Scholar] [CrossRef]
- Marler, T.E. Direct Aulacaspis yasumatsui infestation of pre-harvest Cycas seeds reduces germination and performance of seedlings. Horticulturae 2021, 7, 562. [Google Scholar] [CrossRef]
- Deloso, B.E.; Terry, L.I.; Yudin, L.S.; Marler, T.E. Biotic threats to Cycas micronesica continue to expand to complicate conservation decisions. Insects 2020, 11, 888. [Google Scholar] [CrossRef]
- Marler, T.E. Temporal variations in leaf miner, butterfly, and stem borer infestations of Cycas micronesica in relation to Aulacaspis yasumatsui incidence. HortScience 2013, 48, 1334–1338. [Google Scholar] [CrossRef]
- Marler, T.E.; Lindström, A.J. First, do no harm. Communic. Integr. Biol. 2017, 10, 5–6. [Google Scholar] [CrossRef] [PubMed]
- Marler, T.E.; Calonje, M. Stem branching of cycad plants informs horticulture and conservation decisions. Horticulturae 2020, 6, 65. [Google Scholar] [CrossRef]
- Liu, M.; Korpelainen, H.; Li, C. Sexual differences and sex ratios of dioecious plants under stressful environments. J. Plant Ecol. 2021, 14, 920–933. [Google Scholar] [CrossRef]
- Hultine, K.; Grady, K.; Wood, T.; Shuster, S.M.; Stella, J.C.; Whitham, T.G. Climate change perils for dioecious plant species. Nat. Plants 2016, 2, 16109. [Google Scholar] [CrossRef]
- Marler, T.E. Reciprocal garden study reveals acute spatial-edaphic adaptation for Cycas micronesica. Diversity 2021, 13, 237. [Google Scholar] [CrossRef]
- Sutherland, W.J.; Peel, M.J.S. Benchmarking as a means to improve conservation practice. Oryx 2010, 45, 56–59. [Google Scholar] [CrossRef]
- Robinson, W.D.; Peres, C.A. Benchmarking biodiversity in an era of rapid change. Front. Ecol. Evol. 2021, 9, 810287. [Google Scholar] [CrossRef]
- Rivers, M.; Newton, A.C.; Oldfield, S. Global Tree Assessment Contributors. Scientists’ warning to humanity on tree extinctions. Plants People Planet 2022, 4, 1–17. [Google Scholar] [CrossRef]
- Moore, A.; Marler, T.; Miller, R.H.; Muniappan, R. Biological control of cycad aulacaspis scale on Guam. Cycad Newsl. 2005, 28, 6–8. [Google Scholar]
- Marler, T.E.; Lindström, A.J.; Watson, G.W. Aulacaspis yasumatsui delivers a blow to international cycad horticulture. Horticulturae 2021, 7, 147. [Google Scholar] [CrossRef]
- Marler, T.E. Pacific island tropical cyclones are more frequent and globally relevant, yet less studied. Front. Environ. Sci. 2014, 2, 42. [Google Scholar] [CrossRef]
- Meyer, S.E.; Callaham, M.A.; Stewart, J.E.; Warren, S.D. Invasive Species Response to Natural and Anthropogenic Disturbance. In Invasive Species in Forests and Rangelands of the United States; Poland, T.M., Patel-Weynand, T., Finch, D.M., Miniat, C.F., Hayes, D.C., Lopez, V.M., Eds.; Springer: Cham, Switzerland, 2021. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).