Differential Effects of Hydrogen Peroxide and L-Lysine Treatments on the Growth of Freshwater Cyanophyta and Chlorophyta
Abstract
1. Introduction
2. Materials and Methods
2.1. Organisms and Cultured Conditions
2.2. Growth Measurements of Phytoplankton
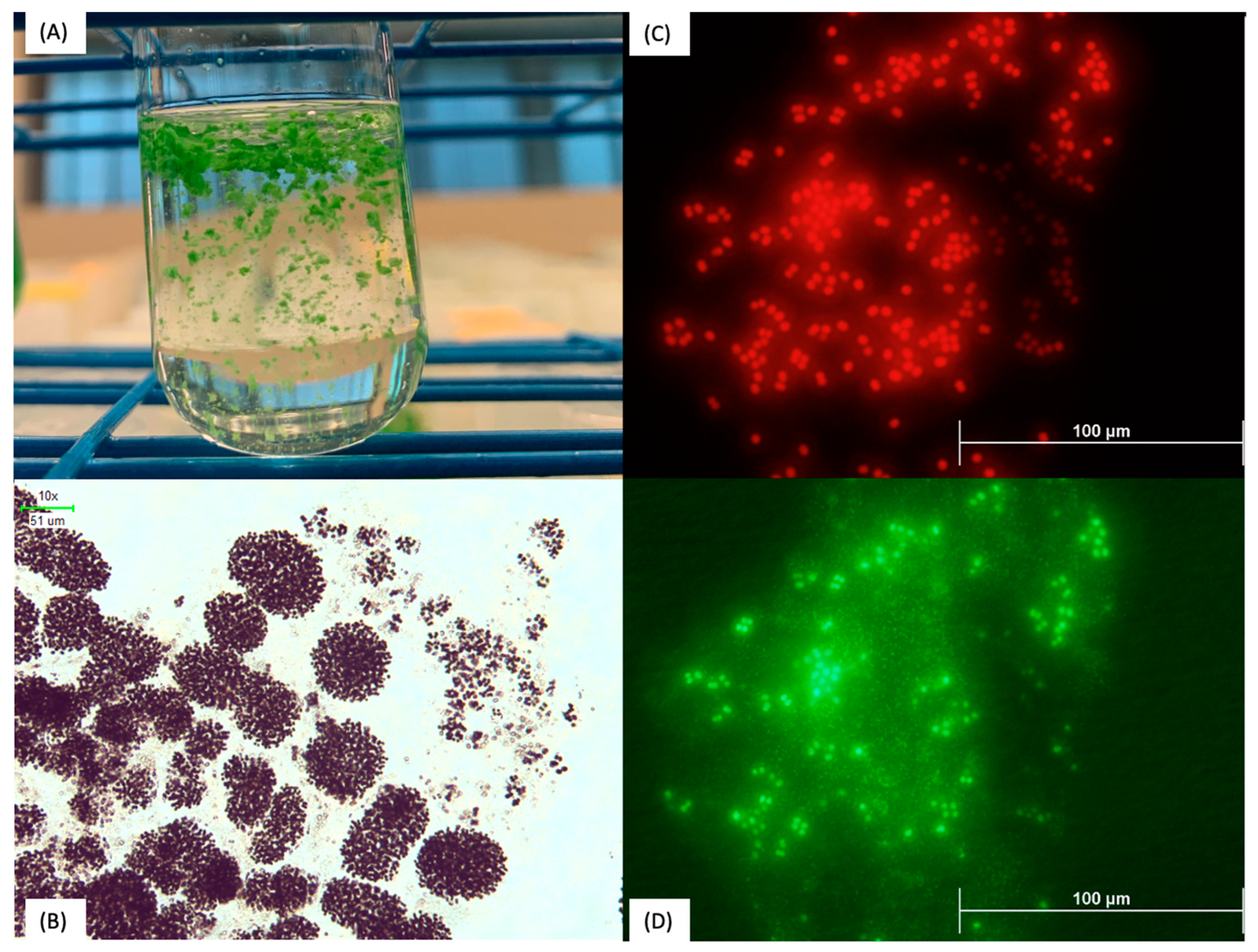
2.3. Fluorescence Microscopy
2.4. Statistical Analysis
3. Results and Discussion
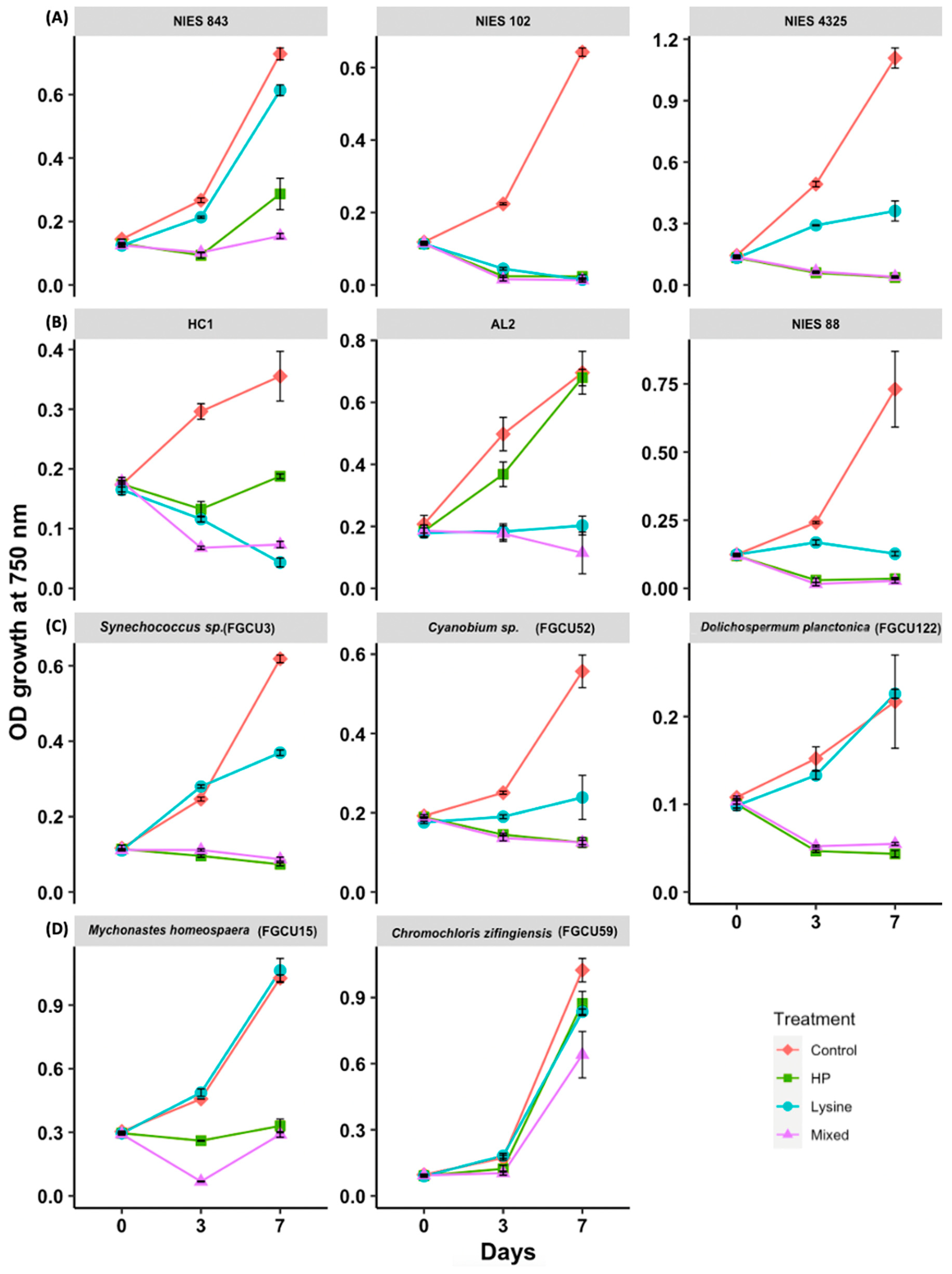
3.1. Dose Sensitivity of Microcystis aeruginosa and Cyanobium sp.
3.2. Differences in Sensitivity of Microcystis aeruginosa Strains to Hydrogen Peroxide and L-Lysine Treatments
3.3. Differences in Sensitivity of Other Phytoplankton to Hydrogen Peroxide and L-Lysine Treatments
3.4. Other Application Considerations
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Falconer, I.R. An overview of problems caused by toxic blue–green algae (cyanobacteria) in drinking and recreational water. Environ. Toxicol. 1999, 14, 5–12. [Google Scholar] [CrossRef]
- Guedes, I.A.; da Costa Leite, D.M.; Manhães, L.A.; Bisch, P.M.; Azevedo, S.M.; Pacheco, A.B.F. Fluctuations in microcystin concentrations, potentially toxic Microcystis and genotype diversity in a cyanobacterial community from a tropical reservoir. Harmful Algae 2014, 39, 303–309. [Google Scholar] [CrossRef]
- Oehrle, S.; Rodriguez-Matos, M.; Cartamil, M.; Zavala, C.; Rein, K.S. Toxin composition of the 2016 Microcystis aeruginosa bloom in the St. Lucie Estuary, Florida. Toxicon 2017, 138, 169–172. [Google Scholar] [CrossRef] [PubMed]
- Kramer, B.J.; Davis, T.W.; Meyer, K.A.; Rosen, B.H.; Goleski, J.A.; Dick, G.J.; Oh, G.; Gobler, C.J. Nitrogen limitation, toxin synthesis potential, and toxicity of cyanobacterial populations in Lake Okeechobee and the St. Lucie River Estuary, Florida, during the 2016 state of emergency event. PLoS ONE 2018, 13, e0196278. [Google Scholar] [CrossRef] [PubMed]
- Ndungu, L.K.; Steele, J.H.; Hancock, T.L.; Bartleson, R.D.; Milbrandt, E.C.; Parsons, M.L.; Urakawa, H. Hydrogen peroxide measurements in subtropical aquatic systems and their implications for cyanobacterial blooms. Ecol. Eng. 2019, 138, 444–453. [Google Scholar] [CrossRef]
- Urakawa, H.; Hancock, T.L.; Steele, J.H.; Dahedl, E.K.; Urakawa, H.E.; Ndungu, L.K.; Krausfeldt, L.E.; Rosen, B.H.; Lopez, J.V. Complete genome sequence of Microcystis aeruginosa FD4, isolated from a subtropical river in southwest Florida. Microbiol. Resour. Announc. 2020, 9, e00813-20. [Google Scholar] [CrossRef] [PubMed]
- Kinley-Baird, C.; Calomeni, A.; Berthold, D.E.; Lefler, F.W.; Barbosa, M.; Rodgers, J.H.; Laughinghouse IV, H.D. Laboratory-scale evaluation of algaecide effectiveness for control of microcystin-producing cyanobacteria from Lake Okeechobee, Florida (USA). Ecotoxicol. Environ. Saf. 2021, 207, 111233. [Google Scholar] [CrossRef] [PubMed]
- Lee, T.A.; Rollwagen-Bollens, G.; Bollens, S.M.; Faber-Hammond, J.J. Environmental influence on cyanobacteria abundance and microcystin toxin production in a shallow temperate lake. Ecotoxicol. Environ. Saf. 2015, 114, 318–325. [Google Scholar] [CrossRef]
- Havens, K.E.; Ji, G.; Beaver, J.R.; Fulton, R.S.; Teacher, C.E. Dynamics of cyanobacteria blooms are linked to the hydrology of shallow Florida lakes and provide insight into possible impacts of climate change. Hydrobiologia 2019, 829, 43–59. [Google Scholar] [CrossRef]
- Hanson, M.J.; Stefan, H.G. Side effects of 58 years of copper sulfate treatment of the Fairmont lakes, Minnesota 1. JAWRA J. Am. Water Resour. Assoc. 1984, 20, 889–900. [Google Scholar] [CrossRef]
- Weenink, E.F.; Luimstra, V.M.; Schuurmans, J.M.; Van Herk, M.J.; Visser, P.M.; Matthijs, H.C. Combatting cyanobacteria with hydrogen peroxide: A laboratory study on the consequences for phytoplankton community and diversity. Front. Microbiol. 2015, 6, 714. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Buley, R.P.; Fernandez-Figueroa, E.G.; Barros, M.U.; Rajendran, S.; Wilson, A.E. Hydrogen peroxide treatment promotes chlorophytes over toxic cyanobacteria in a hyper-eutrophic aquaculture pond. Environ. Pollut. 2018, 240, 590–598. [Google Scholar] [CrossRef] [PubMed]
- Pokrzywinski, K.L.; Bishop, W.M.; Grasso, C.R.; Fernando, B.M.; Sperry, B.P.; Berthold, D.E.; Laughinghouse, H.D., IV; Van Goethem, E.M.; Volk, K.; Heilman, M.; et al. Evaluation of a peroxide-based algaecide for cyanobacteria control: A mesocosm trial in Lake Okeechobee, FL, USA. Water 2022, 14, 169. [Google Scholar] [CrossRef]
- Hehmann, A.; Kaya, K.; Watanabe, M.M. Selective control of Microcystis using an amino acid–a laboratory assay. J. Appl. Phycol. 2002, 14, 85–89. [Google Scholar] [CrossRef]
- Takamura, Y.; Yamada, T.; Kimoto, A.; Kanehama, N.; Tanaka, T.; Nakadaira, S.; Yagi, O. Growth inhibition of Microcystis cyanobacteria by L-lysine and disappearance of natural Microcystis blooms with spraying. Microbes Environ. 2004, 19, 31–39. [Google Scholar] [CrossRef]
- Tian, L.; Chen, M.; Ren, C.; Wang, Y.; Li, L. Anticyanobacterial effect of l-lysine on Microcystis aeruginosa. RSC Adv. 2018, 8, 21606–21612. [Google Scholar] [CrossRef]
- Kaya, K.; Liu, Y.D.; Shen, Y.W.; Xiao, B.D.; Sano, T. Selective control of toxic Microcystis water blooms using lysine and malonic acid: An enclosure experiment. Environ. Toxicol. Int. J. 2005, 20, 170–178. [Google Scholar] [CrossRef]
- Kaneko, T.; Nakajima, N.; Okamoto, S.; Suzuki, I.; Tanabe, Y.; Tamaoki, M.; Nakamura, Y.; Kasai, F.; Watanabe, M.M. Complete genomic structure of the bloom-forming toxic cyanobacterium Microcystis aeruginosa NIES-843. DNA Res. 2007, 14, 247–256. [Google Scholar] [CrossRef]
- Parajuli, A.; Kwak, D.H.; Dalponte, L.; Leikoski, N.; Galica, T.; Umeobika, U.; Trembleau, L.; Bent, A.; Sivonen, K.; Wahlsten, M.; et al. A unique tryptophan C-prenyltransferase from the Kawaguchipeptin biosynthetic pathway. Angew. Chem. Int. Ed. 2016, 55, 3596–3599. [Google Scholar] [CrossRef]
- Tanabe, Y.; Yamaguchi, H.; Sano, T.; Kawachi, M. A novel salt-tolerant genotype illuminates the sucrose gene evolution in freshwater bloom-forming cyanobacterium Microcystis aeruginosa. FEMS Microbiol. Lett. 2019, 366, fnz190. [Google Scholar] [CrossRef]
- Yamaguchi, H.; Suzuki, S.; Osana, Y.; Kawachi, M. Genomic characteristics of the toxic bloom-forming cyanobacterium Microcystis aeruginosa NIES-102. J. Genom. 2020, 8, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, M. Isolation, cultivation and classification of bloom-forming Microcystis in Japan. In Toxic Microcystis; Watanabe, M.F., Harada, K., Carmichael, W.W., Fujiki, H., Eds.; CRC Press: Boca Raton, FL, USA, 1996; pp. 13–34. [Google Scholar]
- Otsuka, S.; Suda, S.; Li, R.; Matsumoto, S.; Watanabe, M.M. Morphological variability of colonies of Microcystis morphospecies in culture. J. Gen. Appl. Microbiol. 2000, 46, 39–50. [Google Scholar] [CrossRef] [PubMed]
- Kondo, R.; Yoshida, T.; Yuki, Y.; Hiroishi, S. DNA-DNA reassociation among a bloom-forming cyanobacterial genus, Microcystis. Int. J. Syst. Evol. Microbiol. 2000, 50, 767–770. [Google Scholar] [CrossRef] [PubMed]
- Cao, H.; Xu, D.; Zhang, T.; Ren, Q.; Xiang, L.; Ning, C.; Zhang, Y.; Gao, R. Comprehensive and functional analyses reveal the genomic diversity and potential toxicity of Microcystis. Harmful Algae 2022, 113, 102186. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2014. [Google Scholar]
- Hishinuma, S.; Yuki, M.; Fujimura, M.; Fukumori, F. OxyR regulated the expression of two major catalases, KatA and KatB, along with peroxiredoxin, AhpC in Pseudomonas putida. Environ. Microbiol. 2006, 8, 2115–2124. [Google Scholar] [CrossRef]
- Kim, M.; Shin, B.; Lee, J.; Park, H.Y.; Park, W. Culture-independent and culture-dependent analyses of the bacterial community in the phycosphere of cyanobloom-forming Microcystis aeruginosa. Sci. Rep. 2019, 9, 20416. [Google Scholar] [CrossRef]
- Kim, M.; Kim, W.; Lee, Y.; Park, W. Linkage between bacterial community-mediated hydrogen peroxide detoxification and the growth of Microcystis aeruginosa. Water Res. 2021, 207, 117784. [Google Scholar] [CrossRef]
- Lee, T.M.; Shiu, C.T. Implications of mycosporine-like amino acid and antioxidant defenses in UV-B radiation tolerance for the algae species Ptercladiella capillacea and Gelidium amansii. Mar. Environ. Res. 2009, 67, 8–16. [Google Scholar] [CrossRef]
- Reynolds, C.S. The Ecology of Phytoplankton; Cambridge University Press: Cambridge, UK, 2006. [Google Scholar]
- Beardall, J.; Allen, D.; Bragg, J.; Finkel, Z.V.; Flynn, K.J.; Quigg, A.; Rees, T.A.V.; Richardson, A.J.; Raven, J.A. Allometry and stoichiometry of unicellular, colonial and multicellular phytoplankton. New Phytologist. 2009, 181, 295–309. [Google Scholar] [CrossRef]
- Xiao, M.; Li, M.; Reynolds, C.S. Colony formation in the cyanobacterium Microcystis. Biol. Rev. 2018, 93, 1399–1420. [Google Scholar] [CrossRef]
- Van Le, V.; Srivastava, A.; Ko, S.R.; Ahn, C.Y.; Oh, H.M. Microcystis colony formation: Extracellular polymeric substance, associated microorganisms, and its application. Bioresour. Technol. 2022, 360, 127610. [Google Scholar]
- Gao, L.; Pan, X.; Zhang, D.; Mu, S.; Lee, D.J.; Halik, U. Extracellular polymeric substances buffer against the biocidal effect of H2O2 on the bloom-forming cyanobacterium Microcystis aeruginosa. Water Res. 2015, 69, 51–58. [Google Scholar] [CrossRef] [PubMed]
- Wu, Z.X.; Gan, N.Q.; Huang, Q.; Song, L.R. Response of Microcystis to copper stress–do phenotypes of Microcystis make a difference in stress tolerance? Environ. Pollut. 2007, 147, 324–330. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Shi, X.; Chen, C.; Yu, L.; Sun, C. Responses of Microcystis colonies of different sizes to hydrogen peroxide stress. Toxins 2017, 9, 306. [Google Scholar] [CrossRef]
- Zimba, P.V.; Dionigi, C.P.; Brashear, S.S. Selective toxicity of exogenous l-lysine to cyanobacteria, relative to a chlorophyte and a diatom. Phycologia 2001, 40, 483–486. [Google Scholar] [CrossRef]
- Kim, W.; Kim, M.; Park, W. Unlocking the mystery of lysine toxicity on Microcystis aeruginosa. J. Hazard. Mater. 2023, 448, 130932. [Google Scholar] [CrossRef]
- Yan, X.; Zhao, F.; Wang, G.; Wang, Z.; Zhou, M.; Zhang, L.; Wang, G.; Chen, Y. Metabolomic analysis of Microcystis aeruginosa after exposure to the algicide L-lysine. Bull. Environ. Contam. Toxicol. 2023, 110, 12. [Google Scholar] [CrossRef]
- Lürling, M.; Van Oosterhout, F. Effect of selected plant extracts and D-and L-lysine on the cyanobacterium Microcystis aeruginosa. Water 2014, 6, 1807–1825. [Google Scholar] [CrossRef]
- Jančula, D.; Maršálek, B. Seven years from the first application of polyaluminium chloride in the Czech Republic–effects on phytoplankton communities in three water bodies. Chem. Ecol. 2012, 28, 535–544. [Google Scholar] [CrossRef]
- Gensemer, R.W.; Playle, R.C. The bioavailability and toxicity of aluminum in aquatic environments. Crit. Rev. Environ. Sci. Technol. 1999, 29, 315–450. [Google Scholar] [CrossRef]
- Smeltzer, E. A successful alum/aluminate treatment of Lake Morey, Vermont. Lake Reserv. Manag. 1990, 6, 9–19. [Google Scholar] [CrossRef]
- Reitzel, K.; Jensen, H.S.; Egemose, S. pH dependent dissolution of sediment aluminum in six Danish lakes treated with aluminum. Water Res. 2013, 47, 1409–1420. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Song, Q.; Long, J.; Song, G.; Mi, W.; Bi, Y. Optimization method for Microcystis bloom mitigation by hydrogen peroxide and its stimulative effects on growth of chlorophytes. Chemosphere 2019, 228, 503–512. [Google Scholar] [CrossRef] [PubMed]
- Aragão, M.C.; Dos Reis, K.C.; Rocha, M.A.M.; de Oliveira Guedes, D.; Dos Santos, E.C.; Capelo-Neto, J. Removal of Dolichospermum circinale, Microcystis aeruginosa, and their metabolites using hydrogen peroxide and visible light. Aquat. Toxicol. 2021, 232, 105735. [Google Scholar] [CrossRef] [PubMed]
- Urakawa, H.; Steele, J.H.; Hancock, T.L.; Dahedl, E.K.; Schroeder, E.R.; Sereda, J.V.; Kratz, M.A.; García, P.E.; Armstrong, R.A. Interaction among spring phytoplankton succession, water discharge patterns, and hydrogen peroxide dynamics in the Caloosahatchee River in southwest Florida. Harmful Algae 2023, 126, 102434. [Google Scholar] [CrossRef]
- Lusty, M.W.; Gobler, C.J. The efficacy of hydrogen peroxide in mitigating cyanobacterial blooms and altering microbial communities across four lakes in NY, USA. Toxins 2020, 12, 428. [Google Scholar] [CrossRef]
- Shao, J.; Li, R.; Lepo, J.E.; Gu, J.D. Potential for control of harmful cyanobacterial blooms using biologically derived substances: Problems and prospects. J. Environ. Manag. 2013, 125, 149–155. [Google Scholar] [CrossRef]
- Murrell, M.C.; Lores, E.M. Phytoplankton and zooplankton seasonal dynamics in a subtropical estuary: Importance of cyanobacteria. J. Plankton Res. 2004, 26, 371–382. [Google Scholar] [CrossRef]
- Jardillier, L.; Zubkov, M.V.; Pearman, J.; Scanlan, D.J. Significant CO2 fixation by small prymnesiophytes in the subtropical and tropical northeast Atlantic Ocean. ISME J. 2010, 4, 1180–1192. [Google Scholar] [CrossRef]
- Matthijs, H.C.; Visser, P.M.; Reeze, B.; Meeuse, J.; Slot, P.C.; Wijn, G.; Talens, R.; Huisman, J. Selective suppression of harmful cyanobacteria in an entire lake with hydrogen peroxide. Water Res. 2012, 46, 1460–1472. [Google Scholar] [CrossRef]
- Weenink, E.F.; Matthijs, H.C.; Schuurmans, J.M.; Piel, T.; van Herk, M.J.; Sigon, C.A.; Visser, P.M.; Huisman, J. Interspecific protection against oxidative stress: Green algae protect harmful cyanobacteria against hydrogen peroxide. Environ. Microbiol. 2021, 23, 2404–2419. [Google Scholar] [CrossRef] [PubMed]
- Buley, R.P.; Adams, C.; Belfiore, A.P.; Fernandez-Figueroa, E.G.; Gladfelter, M.F.; Garner, B.; Wilson, A.E. Field evaluation of seven products to control cyanobacterial blooms in aquaculture. Environ. Sci. Pollut. Res. 2021, 28, 29971–29983. [Google Scholar] [CrossRef] [PubMed]
- Barrington, D.J.; Reichwaldt, E.S.; Ghadouani, A. The use of hydrogen peroxide to remove cyanobacteria and microcystins from waste stabilization ponds and hypereutrophic systems. Ecol. Eng. 2013, 50, 86–94. [Google Scholar] [CrossRef]
- Barrington, D.J.; Ghadouani, A.; Ivey, G.N. Environmental factors and the application of hydrogen peroxide for the removal of toxic cyanobacteria from waste stabilization ponds. J. Environ. Eng. 2011, 137, 952–960. [Google Scholar] [CrossRef]
- Dai, R.; Liu, H.; Qu, J.; Zhao, X.; Hou, Y. Effects of amino acids on microcystin production of the Microcystis aeruginosa. J. Hazard. Mater. 2009, 161, 730–736. [Google Scholar] [CrossRef]
- Chen, C.; Wang, Y.; Chen, K.; Shi, X.; Yang, G. Using hydrogen peroxide to control cyanobacterial blooms: A mesocosm study focused on the effects of algal density in Lake Chaohu, China. Environ. Pollut. 2021, 272, 115923. [Google Scholar] [CrossRef]
- Qian, H.; Yu, S.; Sun, Z.; Xie, X.; Liu, W.; Fu, Z. Effects of copper sulfate, hydrogen peroxide and N-phenyl-2-naphthylamine on oxidative stress and the expression of genes involved photosynthesis and microcystin disposition in Microcystis aeruginosa. Aquat. Toxicol. 2010, 99, 405–412. [Google Scholar] [CrossRef]
- Huo, X.; Chang, D.W.; Tseng, J.H.; Burch, M.D.; Lin, T.F. Exposure of Microcystis aeruginosa to hydrogen peroxide under light: Kinetic modeling of cell rupture and simultaneous microcystin degradation. Environ. Sci. Technol. 2015, 49, 5502–5510. [Google Scholar] [CrossRef]
- Matthijs, H.C.; Jančula, D.; Visser, P.M.; Maršálek, B. Existing and emerging cyanocidal compounds: New perspectives for cyanobacterial bloom mitigation. Aquat. Ecol. 2016, 50, 443–460. [Google Scholar] [CrossRef]
- Clearwater, S.J.; Jellyman, P.G.; Biggs, B.J.; Hickey, C.W.; Blair, N.; Clayton, J.S. Pulse-dose application of chelated copper to a river for Didymosphenia geminata control: Effects on macroinvertebrates and fish. Environ. Toxicol. Chem. 2011, 30, 181–195. [Google Scholar] [CrossRef]


| Strain | Microcystin Production | Axenic | Isolation Location |
|---|---|---|---|
| NIES-88 | X | Lake Kawaguchi, Japan | |
| NIES-102 | X | X | Lake Kasumigaura, Japan |
| NIES-843 | X | X | Lake Kasumigaura, Japan |
| NIES-4325 | X | Lake Abashiri, Japan | |
| FD4 | Caloosahatchee River, Fort Denaud, FL, USA | ||
| HC1 | X | Hickey Creek, Alva, FL, USA | |
| AL2 | X | Caloosahatchee River, Alva, FL, USA |
| Strain | Species | Taxon | Isolation Location |
|---|---|---|---|
| FGCU3 | Synechococcus sp. | Cyanophyta | Hickey Creek Alva, FL, USA |
| FGCU52 | Cyanobium sp. | Cyanophyta | Caloosahatchee River, Moore Haven, FL, USA |
| FGCU54 | Cyanobium sp. | Cyanophyta | Caloosahatchee River, Fort Denaud, FL, USA |
| FGCU122 | Dolichospermum planctonica | Cyanophyta | Lake Okeechobee, FL, USA |
| FGCU15 | Mychonastes homosphaera | Chlorophyta | Lake Okeechobee, FL, USA |
| FGCU59 | Chromochloris zofingiensis | Chlorophyta | Lake Okeechobee, FL, USA |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dahedl, E.K.; Urakawa, H. Differential Effects of Hydrogen Peroxide and L-Lysine Treatments on the Growth of Freshwater Cyanophyta and Chlorophyta. Ecologies 2023, 4, 355-370. https://doi.org/10.3390/ecologies4020023
Dahedl EK, Urakawa H. Differential Effects of Hydrogen Peroxide and L-Lysine Treatments on the Growth of Freshwater Cyanophyta and Chlorophyta. Ecologies. 2023; 4(2):355-370. https://doi.org/10.3390/ecologies4020023
Chicago/Turabian StyleDahedl, Elizabeth K., and Hidetoshi Urakawa. 2023. "Differential Effects of Hydrogen Peroxide and L-Lysine Treatments on the Growth of Freshwater Cyanophyta and Chlorophyta" Ecologies 4, no. 2: 355-370. https://doi.org/10.3390/ecologies4020023
APA StyleDahedl, E. K., & Urakawa, H. (2023). Differential Effects of Hydrogen Peroxide and L-Lysine Treatments on the Growth of Freshwater Cyanophyta and Chlorophyta. Ecologies, 4(2), 355-370. https://doi.org/10.3390/ecologies4020023






