Abstract
Wild chimpanzees live in large and complex social communities, but their complexity is determined by the number of potential social partners as well as the frequent changes in group composition due to fission–fusion dynamics. Alternatively, captive housed chimpanzee groups are usually much smaller and less complex. However, studies have shown that groups can be housed in adjacent habitats, potentially increasing the number of social partners, i.e., granting additional relationship opportunities and enhanced social complexity. While most social network studies analyze social groups as closed systems, this study assessed the impact of social interactions between two neighboring groups of chimpanzees, based on two social network indices (Vertex strength centrality and Deviation from edge weight disparity). Furthermore, Linear Mixed Models (LMMs) were employed to assess whether gender, age, and intra-/intergroup directionality influenced these indices. We recorded occurrences of social behaviors, social proximity and whether chimpanzees observed individuals in the other group as a measure of interest. Most social behaviors were directed towards group members; however, 13% were directed towards the neighboring group. Although enclosure barriers constrained the chimpanzee’s capacity to physically interact, it does not necessarily impede social interactions with the outside world. Hence, the presence of neighboring chimpanzees potentially provides additional relationship opportunities, increasing social stimulation and complexity leading to an enriching social environment.
1. Introduction
Sociality is an adaptive biological trait that evolves when the benefits of group life outweigh the costs, such as intraspecific competition over resources, breeding success and the increased risk of disease transmission [1,2]. Chimpanzees (Pan troglodytes) are highly social animals, living in complex fission–fusion societies. The size of wild populations may range between 20 and 150 individuals, structured in hierarchical societies, consisting of multi-male, multi-female communities [3,4,5]. However, individuals from one community are rarely seen together in one place, because these communities break off into flexible, smaller interchangeable subgroups (fission). These smaller groups regularly meet up with other subgroups and may fuse or shuffle individuals frequently (fusion) [5]. This social system requires chimpanzees to have advanced social skills in order to navigate these flexible interchangeable social networks, but allows them to efficiently adapt to group size and composition (choice of group members), depending on resource availability and other environmental factors [6,7,8,9,10,11,12]. Several studies suggest that this dynamic of changing group size and composition may serve to minimize the costs of group life, i.e., reduce the competition for food as well as access to sexually receptive females [5,9,10,12,13,14,15,16,17,18,19,20,21]. Typically, only females, reaching sexual maturity, migrate from one community to another [5,22]. The majority of other chimpanzee encounters from different communities tend to result in intergroup violence [23]. Chimpanzees are highly territorial and there are many documented reports of chimpanzees aggressively defending core areas as well as patrolling these areas. These encounters often exceed nonphysical display behaviors and can result in serious wounding or even fatal outcomes [5,12,24,25]. Therefore, the social life of wild chimpanzee communities can be both highly stimulating and beneficial as well as dangerous and complicated to navigate. However, chimpanzees housed in captivity are not exposed to the stresses of their wild counterparts, including food shortages, dangers from predators, competition between communities or disease, as their caretakers take care of those needs. Indeed, environmental differences between captive and wild settings, such as the small size and limited complexity of captive habitats, may result in chimpanzees having fewer challenges and opportunities for stimulation because they do not spend the majority of their day on activities such as food acquisition and traveling. Furthermore, in most cases, captive chimpanzees may lack control and choice regarding their social environment, i.e., they cannot choose their group members or the size of their groups. Captive chimpanzee groups tend to be much smaller and are often unbalanced in terms of age divergence and sex ratio, as well as lacking the complexity of fission–fusion dynamics, i.e., they are generally stagnant rather than flexible without frequent changes in group size and composition. According to Webb et al. [26], captive chimpanzees kept in groups of seven or more individuals, where half or more of the group consisting of males and a wide age range, experience wellbeing benefits. Thus, this relatively flat and much less stimulating social environment in a captive setting is likely to restrain the chimpanzee’s natural potential, thereby impacting their behavior and potential wellbeing [26,27,28,29].
An adequate social environment can be considered social enrichment, as it provides constantly changing stimulation, challenging the animals socially and cognitively. Several studies demonstrate the importance and positive impact of social housing on chimpanzees’ health and behavior [26,29,30]. Considering the potential impact of their social environment, it is highly recommended to monitor the social dynamics of chimpanzees living in captive groups. Currently, a wide variety of assessment tools are available, including social network analysis (SNA), which has become the most popular over the last few decades [31,32,33]. SNA is an extremely useful tool, which allows researchers to describe, quantify and statistically compare the social relationships of individuals, thereby increasing our capacity to analyze and understand even highly complex social structures [34,35]. Regarding the analysis of social behavior patterns in animals, it has been used in a wide range of animal taxa, including insects [36], fish [37], cetaceans [38], and mammals [39] including a large number of studies of non-human primates [40,41,42,43,44,45,46,47]. It has been used in chimpanzees to advance our understanding regarding social learning [48], cooperation [49], effectiveness in the transmission of information [50], spread of diseases [51,52], the establishment of hierarchies [53] and to assess group cohesion and stability [11,54].
Although inter-community interactions in wild chimpanzee groups are well documented [55], to date, the vast majority of SNA studies have focused on social interactions in captive chimpanzees, and therefore consider only relationships and interactions between members of the same group, and hence only within closed social systems [46,56,57]. To the best of our knowledge there are only two studies of captive chimpanzees to analyze the impact of neighboring captive groups in relation to one another [58,59]. Baker and Aureli [58] examined the influence of vocalizations and noisy displays produced in neighboring chimpanzee groups that were physically and visually separated. They found that chimpanzees exhibited higher rates of hooting, displaying and agonistic behaviors against members of their own group when levels of neighbor vocalization were labeled as high. Videan et al. [59], using the same methods, found that neighboring chimpanzee group grooming vocalization increased the rate of focal group grooming as well. It was concluded that although chimpanzees of neighboring groups could neither physically interact nor see each other, they were stimulated and influenced by the vocalizations of close-by conspecifics (social contagion). Interestingly, Funkhouser et al. [60] suggested that even human caregivers should be considered potential social partners, and found caregivers can provide additional opportunities (beyond those with conspecifics) to establish and maintain meaningful social relationships. These studies successfully demonstrated that chimpanzee’s social networks are not necessarily limited to group members, nor were they fully restricted by the enclosures’ physical constraints. Further support of this was provided by Grand et al. [61], who investigated how the proximity of enclosures affected a bachelor and a breeding group of gorillas (Gorilla gorilla gorilla) in a zoo in the United States. Although the time spent on monitoring was low, they found that the bachelor group monitored the breeding group more than the other way around, and more non-contact agonistic events occurred when the members of the bachelor group could see the other group’s silverback. Furthermore, when the bachelors had the opportunity to see a female, vocalizations increased to get her attention.
Organizations housing large numbers of chimpanzees are often faced with limitations and issues such as housing facility designs, care management necessities, incompatibility between individuals, lack of social skills within the group, or incidences of excessive wounding. As a result, they may need to establish multiple smaller-sized social groups rather than a smaller number of larger groups. Thus, it may be challenging for zoos and sanctuaries to meet the social requirements to promote high levels of social and psychological wellbeing in a captive setting. Nevertheless, even if providing a stimulating social group composition may not always be possible, there may be other alternatives to increase social complexity and provide additional relationship opportunities. For example, having a close adjacent proximity of enclosures of different social groups may be beneficial, as it allows auditory and/or visual contact. As such, chimpanzees of different groups can potentially still interact with each other (intergroup interactions), although not physically and not to the same degree as with members of their own groups. These interactions should not be disregarded, since chimpanzees have an ample behavioral repertoire, allowing them to interact with each other without the need for direct physical contact [62,63,64,65,66,67,68,69,70,71].
Studies have also shown that chimpanzees housed in adjacent enclosures potentially have access to more social interaction partners and may be more socially stimulated as there are more conspecifics in view and as stated previously, might be influenced by social contagion [58,59]. An obvious disadvantage to physical barriers between groups is that allogrooming, which is one of the most important social tools to establish and maintain relationships, cannot be performed. However, it also prevents chimpanzees from wounding or physically harming chimpanzees from another group, while still enabling them to perform agonistic display behaviors, seek or provide support during agonistic events [72], and engage in affiliative behaviors such as social play, since these interactions do not necessarily require physical contact [62,63,64]. Interactions and communications between members of different groups might also be purely on a vocal–auditory level, including vocalizations such as alarm calling, pant-hooting, greeting, and panting [65,66,67,68,69,70,71]. Furthermore, even one-directional social behaviors, i.e., non-reciprocal behaviors that do not require or depend on the reaction of the receiver, might increase due to the presence of more conspecifics. For example, time spent on monitoring/observing the activity of group members might be extended by paying attention to chimpanzees of neighboring groups.
Nevertheless, the authors wish to highlight that we only discuss how intergroup interactions have the potential to increase social complexity, stimulation, and relationship opportunities, yet this is not necessarily equivalent to increasing the chimpanzee’s wellbeing itself. Although it has been shown that a more stimulating social environment potentially promotes higher levels of wellbeing, evaluations of these impacts must be discussed carefully.
For this study, we observed two groups of chimpanzees, housed in closely adjacent enclosures at the primate rescue center Fundació MONA. The groups were separated only by a steel mesh and an electric fence, keeping them from physically interacting while allowing all other types of interactions and potential social influences. Our primary objective was to determine the frequency of interactions between the two groups and if these interactions should be considered an extension of their social networks, thereby increasing the complexity of the social environment and potentially serving as additional social relationship opportunities.
2. Materials and Methods
2.1. Study Population
The study population consisted of a total of 12 former pet and entertainment chimpanzees housed at the primate rescue and rehabilitation center Fundació MONA, located in the north of Spain, Cataluña. The center is a member of the European Alliance of Rescue Centers and Sanctuaries (EARS) and has been providing life-long care to rescued primates since 2001. At the time of the data collection, all chimpanzees were adults (5 females and 7 males), ranging between 19 to 42 years of age (31.58 ± 8.40). Depending on their age, animals were labeled as adults or seniors, with seniors being 35 years of age or older.
Chimpanzees lived in two separated groups and had not undergone any group alterations during the previous year, i.e., no changes regarding group members including the birth or death of a chimpanzee. The “Mutamba” group consisted of 5 individuals, 2 females and 3 males (aged 26 ± 7.24), and the “Bilinga” group consisted of 7 individuals, 3 females and 4 males (aged 35.57 ± 7.06). Towards the end of the data collection, one female chimpanzee from the “Bilinga” group (Bea) died a natural death. The characteristics relevant to this study of each individual are shown in Table 1.

Table 1.
Characteristics of the study population.
Observations were conducted only while both chimpanzee groups were using the outdoor enclosure (5640 m2), which was divided into two habitats, separated by a 50 m long steel mesh and electrified fence (Figure 1). The smaller Mutamba group (N = 5) was located in a 2420 m2 enclosure and the Bilinga group (N = 7) was in the larger 3220 m2 enclosure. Both habitats consisted of a naturalistic terrain with Mediterranean vegetation (subject to seasonal changes) and were equipped with a multitude of artificial climbing structures, such as towers, ropes, wooden platforms and other enrichment devices. The chimpanzee facilities also included several indoor areas (140 m2), where they spend the nights and to which they were given access on rainy, extremely hot, or very cold days to take shelter. Chimpanzees had ad libitum access to water dispensers and were fed 4–5 times a day based on a diet plan consisting mostly of vegetables, fruits, pellets and dried fruits. The majority of their diet was provided, while the chimpanzees resided in the outdoor enclosures to stimulate foraging and other species typical behaviors. For more detailed information regarding their living conditions, see [73,74].

Figure 1.
The photograph depicts the two outdoor chimpanzee facilities, which are separated by an electrified fence (marked in red) [75].
2.2. Data Sampling
Observations were conducted from two observation towers providing maximum visibility of chimpanzee enclosures and the segment separating the enclosures. The chimpanzee’s social behaviors, including directionality (in other words, the information provided in our records, specifies which chimpanzee exhibited the behavior and to whom it was directed) and social proximity were recorded during the four months from March to June 2022. Data were collected using a multifocal (observing all members of one group simultaneously, including intergroup interactions) All Occurrence methodology [76], scoring all occurrences of behaviors described in the ethogram (Table S1). A behavior was scored again if the observed chimpanzee stopped said behavior and exhibited another behavior for more than 30 s. Each observation session lasted 20 min, and sessions were equally distributed throughout the day and week. Observers scored data on tablet devices, using the digital data collection software Zoomonitor [77]. Social interactions recorded (Table S1) and analyzed in this study (Table 2) were agonistic behaviors (agonistic dominance, agonistic submission, other agonistic), affiliative behaviors (grooming, following, feed together, embrace, social play, socio sexual) and social proximity (inter-social proximity, intra-social proximity). Additionally, observers scored “pay attention” when chimpanzees observed individuals from the other group (unilateral interaction), as an indicator of interest towards the neighboring group. Vigilance with respect to and monitoring of the members of our group were initially recorded, but later discarded, as observers could not accurately identify whether chimpanzees were gazing in the direction of group members due to the large enclosure size and limitations of the observation towers. Social proximity and pay attention were only recorded in the absence of another type of behavior or interaction mentioned above.

Table 2.
Simplified ethogram. Overview regarding the analyzed behaviors. (complete ethogram and definitions can be found in Table S1).
Observations were conducted daily between approximately 10:00 a.m. and 6:00 p.m., when chimpanzees had access to the outdoor enclosures. Both groups were observed for 55 h each, resulting in 55 h of intragroup interaction data for each group and 110 h of intergroup interaction data. In order to correct the difference in observation time and normalize the dataset, rates of each interaction type were calculated over the total observation time it could have potentially been scored. A total of two observers collected data for this study. Both observers were trained for three weeks—data from that training phase was checked but not used—and an inter-observer reliability test (agreement ≥ 85%) was performed before starting the data collection.
2.3. Social Network Analysis (SNA)
The social network graphs consist of nodes (vertices) representing chimpanzees and links (edges) representing the extent (normalized rates) to which chimpanzees were observed to interact with each other. Social networks in this study were based on weighted dyadic interactions of chimpanzees within the same group (intragroup) as well as chimpanzees belonging to different groups (intergroup). A total of 4 interaction matrices were created for each group and for the intergroup analysis, based on agonistic behaviors, affiliative behaviors, social proximity, and the combined Aggregate State. The Aggregate matrix reflects the combined interactions of the previous three matrices, without differentiating between the aggregated items. For the intergroup analysis, a fifth matrix was created based on pay attention data, which could only be observed between individuals belonging to different groups, due to its definition. Being considered a unilateral intergroup interaction without an equivalent on the intragroup level, it was excluded from the Aggregate State analysis.
We created our network graphs in UCINET (v 6.755), using the integrated Netdraw software for network visualization [78,79,80]. We adapted the layout setting, applying the “Edge-Weighted Spring Embedded” layout. This layout is based on a force-directed paradigm using algorithms to set the node positions in a way that minimizes the sum of forces in the network. Thus, nodes were distributed based on the sum of the attractive and repulsive forces acting on each node [81,82].
The interaction matrices were used to calculate two network measures for each of the previously mentioned interaction types produced within each group and between neighboring groups. The following network measures have previously been described and used by Kasper and Volker [83] and Kalcher-Sommersguter et al. [84]. Please consult these articles for more detailed explanations regarding the calculation and interpretation. Vertex strength centrality (VSC) is a measure designed to describe the standardized strength of an individual’s social activity, while taking the group size into account. Deviation from Edge Weight Disparity (DEWD) is a measure designed to reflect how evenly a chimpanzee distributes their interactions among potential social partners, allowing us to compare the interaction distribution between networks of different sizes. A value of zero indicates a perfectly even distribution, while higher values indicate an uneven distribution of interactions towards social partners.
2.4. Data Analysis
Based on the recorded occurrences, we calculated the relative frequencies (per hour) of each interaction type. This information was used to extract descriptive results regarding the interactions between individuals belonging to the same and different groups, in order to provide a more detailed understanding on intergroup interactions. Furthermore, the chimpanzee’s biographic information (Age and Sex) and information regarding previous social events as well as caregiver activities were taken into account in order to discuss their potential influence on the occurrence of certain interaction patterns.
To assess the potential impact of intra-/intergroup directionality (inter vs. intra), the chimpanzee’s age (adult vs. senior) and sex (male vs. female) on the occurrence of social interactions, we employed linear mixed models (LMMs). We ran a total of eight LMMs based on the same structural setup, only changing the dependent variable (i.e., social network indices for each interaction type): Agonistic VSC, Agonistic DEWD, Affiliative VSC, Affiliative DEWD, Proximity VSC, Proximity DEWD, Aggregate VSC, Aggregate DEWD. We did not employ any models for pay attention, as it could only be performed between individuals of different groups, and no equivalent behavior on an intragroup level was recorded that would have allowed any meaningful comparison. We included the individuals ID and the group to which they belonged as random factors to account for the repeated measures. QQ plots were visually checked to control the normal distribution of the residuals, which revealed no violations to the assumptions of our LMMs (Figure S1). All models were run using the “lmer” function of the lme4 package [85] in the R programming environment (version 4.2.0) [86]. We tested for multicollinearity between all fixed factors by calculating the variance inflation factor (VIF) using the “car” package [87]. Values remained low in all models (maximum VIF = 1.05).
To confirm the quality of the models, we tested whether the full models (containing all three fixed factors) were significant improvements over the null models (without fixed factors), using the “anova” function, ensuring that the fixed factors had a significant effect on the model outcome [88]. If the full model was a significant improvement over the corresponding null model, we applied the ANOVA function (Type III Analysis of Variance with Satterthwaite’s method) and a post hoc test based on the p-value obtained with the “glht” function (multiple comparison of means with Tukey Contrast, p-values adjusted by the Holm–Bonferroni method) to further explore the results of significant fixed factors.
3. Results
3.1. Occurrences of Social Interaction Types
During the data collection, a total of 261 agonistic behaviors, 1025 affiliative behaviors, 625 occurrences of social proximity and 297 occurrences of paying attention to the other group were recorded. Agonistic behaviors consisted of 30% submissive and 70% dominance-related behaviors, while allogrooming made up 46% of all affiliative behaviors. Thus, only considering behaviors, allogrooming was the most frequently observed behavior. When considering all four interaction categories, we found 12% to relate to agonistic behaviors, 46% to affiliative behaviors, 28% to social proximity and 13% to observing/paying attention to members of the other group.
When taking directionality into account, i.e., whether the behavior is directed toward a member of the group or toward a chimpanzee in the neighboring group (intra vs. inter), we found both groups to direct their social activity mostly to members of their own group. Only considering behaviors, 12.7% of affiliative and agonistic behaviors were directed towards individuals from the neighboring group, while 87.3% were directed at members of their own group. If we include the times chimpanzees were recorded to stay in social proximity, intergroup interactions decrease to 10.1%. However, if we also include “paying attention” (only recorded between individuals belonging to different groups) the intergroup interactions rise to 21.7% (Table 3). More detailed information regarding the summary and categorical breakdown of the previously described results can be found in Table S2.

Table 3.
Distribution of interaction types among group members (intra) and chimpanzees from the neighboring group (inter).
Figure 2 is a graphical representation of how each interaction type was distributed among either members of the other group (inter) or members of the same group (intra). A total of 61.9% of all agonistic and 2.2% of all affiliative behaviors were directed towards the other group. Social proximity occurred in 95.2% of cases with members of their own group.
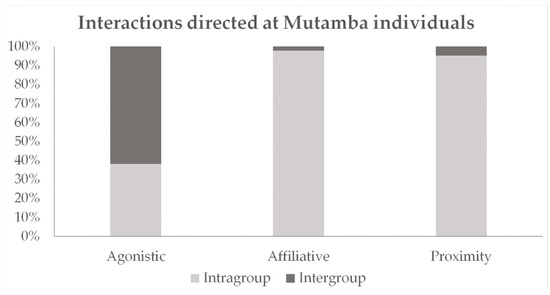
Figure 2.
Overview of the distribution of social interactions, proximity, and interest of the individuals of a group towards their own group or towards their neighbors.
We found differences regarding social activity and directionality between the two groups. Although Mutamba was the smaller group (Mutamba N = 5 vs. Bilinga N = 7), 62% of all interactions recorded were produced by members of this group. Thus, the Mutamba group can be considered more socially active compared to the Bilinga group. Conversely, members from the Bilinga group directed 17.6%, while Mutamba directed only 10.2% of their agonistic and affiliative behaviors towards members of the other group.
Regarding agonistic behaviors, we further explored the sub-categories submissive and dominance-related behaviors. As expected, we found males (of both groups) to perform most of the dominance-related behaviors, while agonistic submission was performed more frequently by female chimpanzees. More detailed information regarding directionality, taking both the group and the individual’s sex into account, can be found in Table S3.
Observers recorded potential factors that may have contributed to the onset of aggressive behaviors in each group before or at the time of the first recorded aggressive event. We found that only 5.8% of all intragroup agonistic events happened during or shortly after an aggression occurred in or with the neighboring group. Furthermore, in 15.2% of all intragroup agonistic events, caregivers were close by, conducting care or management activities, such as maneuvering animal doors or providing food or enrichment. Thus, in 79% of cases, intragroup aggressions occurred without a detectable external onset factor.
3.2. Graphical Representation of Social Networks
In order to demonstrate the complexity of the chimpanzees’ social networks and to highlight the potential impact of interactions between members from the neighboring groups, we created several graphical representations of undirected weighted networks.
3.2.1. Intragroup Social Networks
Figure 3 represents the classical approach of only considering individuals from one group, i.e., only taking interactions between members of the same group into account, while ignoring interactions that occurred on an intergroup level. Here, several networks were drawn, based on the different interaction types (agonistic, affiliative, social proximity), as well as a combined version in which all three interaction types were aggregated into a single network (Aggregate State).
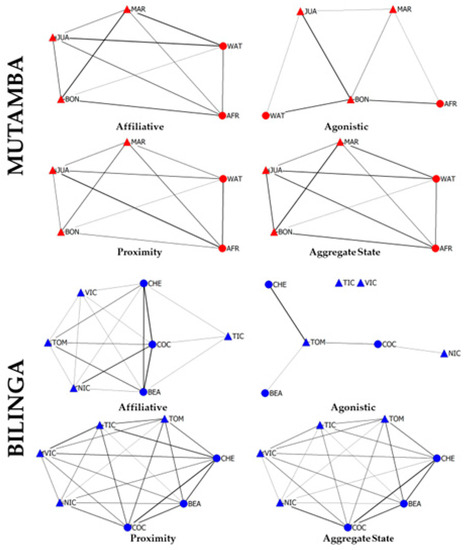
Figure 3.
Classical social network representation of both groups. Each network is based on one social inter-action type (affiliative, agonistic and social proximity), plus a fourth network based on the sum of the three previous interactions (Aggregate State). Edge weight is proportional to the weighted index value of the interaction pairs. The node layout is based on the force-directed algorithm “Spring-Embedded distribution”. Node shape represents the sex of the individual (triangle for males and circle for females) and the color represents the group (Mutamba is red and Bilinga is blue). The node labels correspond to the individuals listed in Table 1.
Although we previously stated that the Mutamba group was socially more active, due to the small group size, the graphical representation indicates low levels of complexity. Each chimpanzee can only establish a maximum of four relationships. The agonistic network is the only Mutamba network that lacks edges between some nodes. Conversely, the Bilinga group, consisting of seven individuals, is far more complex, as each individual may establish interactions with six group members. Nevertheless, as previously stated, chimpanzees from this group were socially less active, which is further highlighted by the missing edges in both the affiliative and agonistic networks.
3.2.2. Intragroup Social Networks
Figure 4 includes the data of both intragroup representations, as well as the interactions that were recorded between individuals of neighboring groups. Here, an additional network was drawn based on the “pay attention” interaction type. However, it is important to understand that the “pay attention” network has to be evaluated with care, as it is the only interaction type that can only occur between members from different groups.
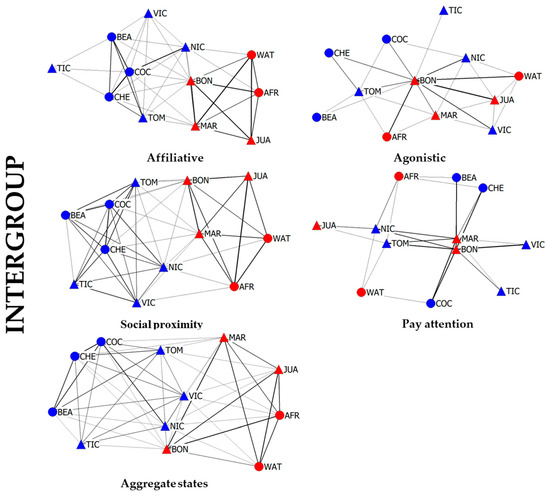
Figure 4.
Social networks including intra- and intergroup interactions. Each network is based on one social interaction type (affiliative, agonistic, social proximity and pay attention) plus a fifth network based on the sum of affiliative, agonistic, social proximity interactions (Aggregate State). Edge weight is proportional to the weighted index value of the interaction pairs. The node layout is based on the force-directed algorithm “Spring-Embedded distribution”. Node shape represents the sex of the individual (triangle for males and circle for females), and the color represents the group (Mutamba is red and Bilinga is blue). The node labels correspond to the individuals listed in Table 1.
Looking at the graphical representations of the combined inter- and intragroup networks, we can clearly see a drastic increment in complexity. Here, each chimpanzee can potentially establish relationships with 11 conspecifics. Networks based on affiliative behaviors and social proximity still show a clear separation between groups, although several individuals do interact with members from the neighboring group. However, the partition between the groups is far less obvious in the agonistic network indicating similar levels of agonistic interactions between members of the same as well as members of the neighboring group. As a result, the aggregated network shows similar tendencies of a blurred group partition as well, although not as extreme as observed in the agonistic network. Regarding the “pay attention” network, we observe that all chimpanzees at some point observed members from the neighboring group, yet two chimpanzees from each group (all males) were especially active (NIC, TOM, MAR, BON). All five graphical representations depict male chimpanzees as occupying central positions with regard to the intergroup separation. Furthermore, all chimpanzees are connected to at least one other individual in all of the networks; hence, no isolated chimpanzees are detected.
Figure 5 depicts the social network based on all four types of interaction (agonistic, affiliative, social proximity and pay attention). Although, as we have previously mentioned, we cannot objectively compare this network with intragroup Aggregate State networks, we believe that it may be relevant to present the aggregated network state including information regarding “pay attention” behaviors, as it is one of the few interactions that can occur on an intergroup level. While there are several behaviors that cannot be exhibited on an intergroup level, i.e., any social behaviors that require physical contact, such as grooming or hugging, pay attention is the only behavior recorded in this study that can only be exhibited on an intergroup level. Adding the information of pay attention, reduces the distance between individuals belonging to different groups and further blurs the partition line between neighboring groups.
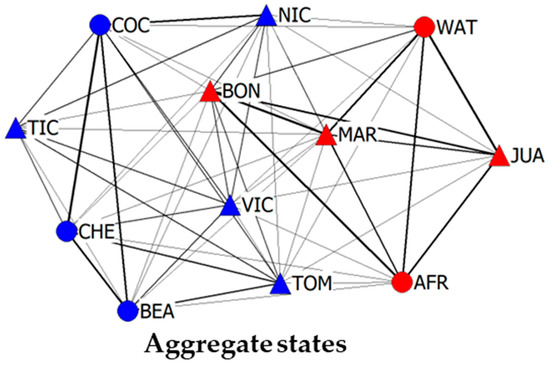
Figure 5.
Social networks including intra- and intergroup interactions. Network based on the sum of affiliative, agonistic, social proximity and pay attention (Aggregate State). Edge weight is proportional to the weighted index value of the interaction pairs. The node layout is based on the force-directed algorithm “Spring-Embedded distribution”. Node shape represents the sex of the individual (triangle for males and circle for females), and the color represents the group (Mutamba is red and Bilinga is blue). The node labels correspond to the individuals listed in Table 1.
3.3. Linear Mixed Models
We ran a total of eight LMMs to investigate the potential impact of intra-/intergroup directionality (inter vs. intra), the chimpanzee’s age (adult vs. senior) and sex (male vs. female) on the occurrence of social interactions observed between chimpanzees of the same and neighboring groups. Three out of the eight models were not improved by including the fixed factors, and thus none of the predictors had any impact on the chimpanzees’ interaction pattern (Table S4).
3.3.1. Agonistic Interactions
The comparison between the full and null model was not significant. Thus, agonistic VSC and DEWD were not improved in comparison to the respective null models. Hence, none of the fixed factors produced a significant impact on the chimpanzee’s agonistic activity or distribution, i.e., no significant differences were found between chimpanzees of different age or sex, or when considering the inter/intra directionality.
3.3.2. Affiliative Interactions
Affiliative activity (VSC) was significantly influenced by the inter/intra directionality (F1,22 = 24.4, p < 0.001) and the sex (F1,22 = 4.46, p < 0.05) of the chimpanzee (Table S4). With respect to sex, we found that females engaged significantly more often in affiliative interactions than males (females > males, z = −2.113, p < 0.05; Table S5). Regarding inter/intra directionality, we found chimpanzees to direct their affiliative interactions significantly more often towards members of their own group (intra > inter, z = 4.944, p < 0.001; Table S5). Inter/intra directionality also significantly influenced the distribution of affiliative interactions (DEWD; F1,12 = 52.24, p < 0.001; Table S4, Figure 6). Specifically, our results indicate that the chimpanzees distributed their affiliative attention more evenly within their group, while being much more selective towards members of the other group (inter > intra, z = −7.228, p < 0.001; Table S5). Confidence interval plots regarding the significant fixed factors are shown in Figure 6.
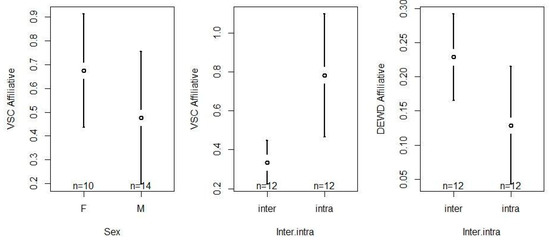
Figure 6.
Confidence interval plots of affiliative behaviors (VSC and DEWD) and the significant fixed effects sex and inter/intra directionality.
3.3.3. Social Proximity
Social proximity (VSC) was significantly influenced by the inter/intra directionality (F1,12 = 78.3, p < 0.001) and the sex (F1,10 = 5.21, p < 0.05) of the chimpanzee (Table S4). With respect to sex, we observed that females spent more time/scans in social proximity than males (females > males, z = −2.282, p < 0.05; Table S5). Regarding inter/intra directionality, we found that chimpanzees stayed significantly more often in social proximity with members of their own group (intra > inter, z = 8.848, p < 0.001; Table S5). Inter/intra directionality also significantly influenced the distribution of social proximity (DEWD; F1,22 = 102.7, p < 0.001; Table S4, Figure 7). Specifically, our results indicate that the chimpanzees distributed their time spent in social proximity with group members more evenly, while being much more selective towards members of the other group (inter > intra, z = −10.13, p < 0.001; Table S5). Confidence interval plots regarding the significant fixed factors are shown in Figure 7.
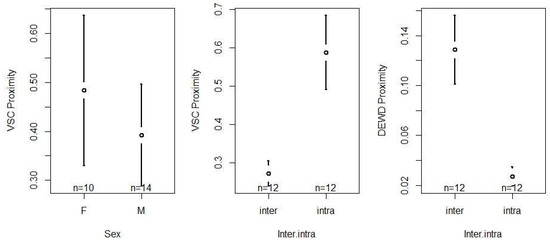
Figure 7.
Confidence interval plots of social proximity (VSC and DEWD) and the significant fixed effects sex and inter/intra directionality.
3.3.4. Aggregated Interactions
Aggregated social activity (VSC), based on agonistic behaviors, affiliative behaviors and social proximity, was significantly influenced by the inter/intra directionality (F1,22 = 40.6511, p < 0.001; Table S4). Specifically, we found that, when considering all three interaction types, chimpanzees interacted significantly more with members of their own group and less with members of the neighboring group (intra > inter, z = 6.376, p < 0.001; Table S5). Confidence interval plots regarding the significant fixed factor are shown in Figure 8.
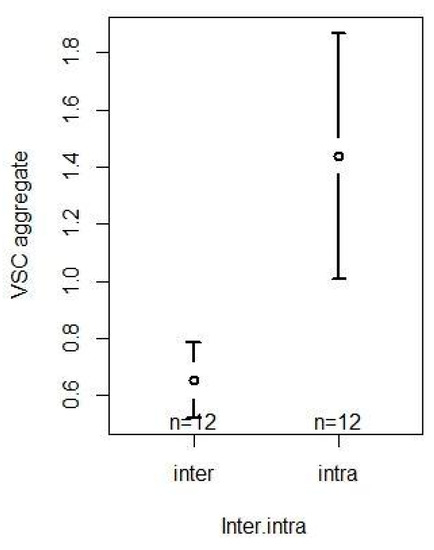
Figure 8.
Confidence interval plots of the aggregated interactions (VSC) and the significant fixed effect Inter/intra directionality.
4. Discussion
In this study, we demonstrated that although enclosure barriers directly constrain chimpanzees’ capacity to physically interact, it does not necessarily impede the possibility to socially interact with the outside world. Hence, the social networks of captive chimpanzee populations may potentially extend further than traditionally assumed, including interactions with conspecifics that are not part of their own group. To this end, we observed two groups of sanctuary chimpanzees, housed in adjacent enclosures, separated by an electric fence that restricted physical interactions, but allowed animals to see, hear and interact in any other non-physical way.
Indeed, we found that chimpanzees direct 13% of their social behaviors (affiliative and agonistic behaviors) towards chimpanzees of the neighboring group as well as frequently paying attention to individuals and/or occurrences happening in the neighboring enclosure. As expected, the significant impact of the inter-/intragroup factor in all LMMs regarding the strengths of social interactions (VSC), except for agonistic interactions, indicated that the majority of social interactions were directed at members of their own group. Thus, interactions indicating socio-positive intentions on an intergroup level should be regarded as an additional opportunity to socialize, rather than a substitution of intragroup social activity. Specifically, we found that interactions based on social proximity and affiliative behaviors were mostly directed and more evenly distributed among members of their own group, while agonistic interactions were evenly distributed between chimpanzees from either group. Agonistic behaviors towards the neighboring group consisted mostly of display behaviors, yet also included submissive behaviors. Display behaviors are commonly seen in males as a means of showing strength, superiority and dominance over other individuals, as well as of impressing females (courtship display) [89,90,91,92]. This behavior serves as a non-contact signal to impress and/or intimidate others and may reduce the probability of a physical confrontation that may carry the risk of wounding [93]. These behaviors are often directed at a specific individual, yet chimpanzees have frequently been reported to exhibit undirected bluff displays, allowing them to show their strength, while further lowering the risk of a physical response from an otherwise targeted individual [94]. Although there is no clear evidence, it may be that the chimpanzees in our study exhibited displays towards the neighboring group with similar intentions as seen in individuals exhibiting an undirected bluff display, as the separation fence made a physical response impossible. Thus, chimpanzees could direct their display behaviors towards members of the neighboring group without the risk of receiving any injuries from targeted individuals, while still demonstrating their strength to their own group members. Furthermore, we found that most occurrences of submissive behaviors were directed towards members of their own group, while intergroup displays were either ignored or were more likely to be answered by a counter display, yet seldom received a submissive response from members of the neighboring group (Table S3). The fact that only 5.8% of all intragroup aggressions occurred after an intergroup or intragroup aggression in the neighboring group, as well as the infrequent submissive response between individuals from neighboring groups, suggests that agonistic interactions between neighboring groups are unlikely to have produced an important negative impact on an intragroup level in our study population. Nevertheless, more detailed data, including behavioral indicators relating to stress and tension are necessary to confirm this assumption. Conversely, we suspect that intergroup displays my provide individuals with the opportunity to get rid of pent-up energy or discontent, to function as a risk reduced way to demonstrate strength, as well as serving to increase group cohesion by uniting individuals from the same group [5,95].
Our results indicate that females were more active in regard to social proximity and affiliative behaviors in comparison to males, yet were less frequently observed to interact with chimpanzees from the neighboring group in general. Although studies on wild populations report social interactions to be more frequent among males [96], several studies in captivity suggest that the females’ social potential becomes apparent due to the absence of factors such as resource competition and dispersal patterns in captivity [97]. Furthermore, reported interactions between different chimpanzee populations in the wild, i.e., boundary patrolling and territory defense, are mostly performed by adult males, while females and younger chimpanzees often remain in more central areas of the population’s territory [98].
Both social proximity and affiliative behaviors occurred significantly more often between group members compared to directed towards the neighboring group. This was further highlighted by the respective DEWD LMMs, which indicated that chimpanzees of both sexes distributed their social attention much more evenly between members of their own group, while being much more selective regarding interaction partners from the other group. We predicted that intergroup interactions would be much lower than intragroup interactions, as we considered them to be an additional opportunity to socially interact, rather than it being necessary to dedicate energy to establishing or maintaining relationships with members from the other group. Nevertheless, we still expected these values to be slightly higher than observed, as several of the chimpanzees had been transferred from one group to the other in the past (more than 5 years ago), i.e., they knew each other from before, and previous studies conducted on the same populations have reported higher interaction rates between certain individuals while still being housed in the same group [57]. Although we did not expect to find the same level of interactions after transferring them to another group, we assumed some familiarity and closeness to remain, due to the proximity between enclosures. Nevertheless, one explanation for the low frequency of affiliative behaviors and social proximity could be that few direct benefits may be gained by establishing and maintaining relationships with chimpanzees from the other group, such as improved access to resources [99], direct support during aggressive events [100,101], or reduced likelihood of becoming the victim of aggression [102,103]. Hence, it might be more beneficial and efficient for individuals to dedicate effort regarding relationship care towards members of their own group.
Age was the only factor that did not produce any significant results in any of our models. A study conducted by Baker [104] on captive housed chimpanzees indicated that older chimpanzees engaged less in agonistic interactions, but would score similar levels of affiliative interactions compared to younger chimpanzees. The results regarding affiliative behaviors are in line with Baker’s finding yet we could not confirm a lower agonistic rate for senior chimpanzees. However, the chimpanzees in this study were ranging between 19 to 42 years of age and no adolescent chimpanzees were present, resulting in an average age of 31.58 ± 8.40. Thus, the age variation in our population might not have been diverse enough to significantly capture a difference of agonistic activity between older and younger chimpanzees.
Our data suggest that chimpanzees housed in adjacent enclosures provide additional social stimulation and complexity, as the chimpanzees have more potential socialization partners to interact with. However, another stimulating aspect of close proximity conspecifics from another group could be simply produced by their presence itself, without the need for direct or reciprocal social interactions. Therefore, with this in mind, observers recorded when chimpanzees would dedicate their attention/gaze towards members of the other group, i.e., as spectators. This behavior, called “paying attention” in this study, functions as an indicator of interest/attention regarding individuals and events occurring in the neighboring group. “Paying attention” was only recorded if a chimpanzee was occupying a location close to the separation fence, visibly directing and maintaining their gaze towards one or several individuals of the neighboring group, while not showing any clear signs of affiliative or agonistic intentions. Sadly, due to the distance and angle, observers were not able to objectively identify when chimpanzees were observing members of their own group in the same manner. Thus, based on our data, we can only comment on and discuss findings regarding “pay attention” directed to the neighboring group. Nevertheless, chimpanzees were observed to exhibit twice as many times “pay attention” behaviors as they did agonistic behaviors, making “pay attention” the most frequently recorded behavior directed towards the neighboring group. However, we did not include “pay attention” scores in the previous analysis, and decided to discuss this behavior separately, as (a) it was only recorded on an intergroup level, and (b) it is strictly speaking not a social interaction, considering that the receiving individual does not even need to realize that he/she is being targeted by this behavior. Nevertheless, we believe that this behavior, if occurring frequently, indicates increased social stimulation, which could not be gained while living in a small-sized population without other conspecifics close by.
Our data show that the presence of an adjacent chimpanzee group may produce an increase in social stimulation and complexity, as seen in the graphical representations of the chimpanzee’s social networks presented in this study (Figure 3, Figure 4 and Figure 5). The amount of potential interaction partners typically depends on the population’s group size [26]. Previous studies have shown that, overall, captive chimpanzee groups may be relatively small, stagnant and lack flexibility and diversity, which potentially leads to a diminished social complexity and social stimulation [26,28,30], when compared to wild populations. However, in captive settings, smaller group sizes are commonplace due to facility limitations, individuals lacking social skills or the occurrence of excessive aggressions that might lead to lethal or severe injuries. Yet, our results indicate that adjacent housing may be an alternative to increase complexity and social stimulations without risking the chimpanzees’ physical health, i.e., through the occurrence of lethal or severe injuries.
Prior studies have shown that monitoring and evaluating sociality in captive chimpanzees objectively can be a challenging task. While most social network studies simplify the data collection and interpretation by focusing on the most significant and most exhibited social behavior [105], typically allogrooming, they tend to detect tendencies quickly, yet potentially lose important information [106]. The use of multiplex networks increases our capacity to objectively evaluate complex social structures [107,108]. In this study, we used a simple aggregation of varying social interactions, which led to a slight increase in the perception of complexity, as some missing links between chimpanzees were detected and the strength of interactions between some dyads became more prominent (Figure 3, Figure 4 and Figure 5). Nevertheless, when treating each group as a closed network, limited by the enclosure constraints, even in the aggregated state, we can still perceive a certain simplicity in the social structure. However, when taking interactions with other conspecific of the neighboring group into account the complexity increases exponentially. Again, this is simply achieved by augmenting the amount of potential interaction partners and/or considering interactions with conspecifics from a neighboring group. However, chimpanzees from different groups might opt to not interact with each other, which would result in two clearly separated subgroups, either connected by one or a few bridge individuals or totally lacking any links. Chimpanzees observed in this study appeared to take advantage of this additional source of interaction partners, as all chimpanzees were observed to interact in some way with one or several individuals from the neighboring group. However, as expected, both the strength as well as the distribution of interactions among group members and neighbors differed greatly. In the two groups of chimpanzees studied, we found no significant difference regarding the target of their agonistic behaviors, i.e., chimpanzees directed and distributed their agonistic behaviors evenly among group members and neighbors. Conversely, social proximity and affiliative behaviors were more evenly distributed among group members and occurred significantly more frequently on an intragroup level. Although chimpanzees in our study population appeared to take advantage of these additional interaction partners, they made a clear differentiation between group members and neighbors, most likely as they could not physically interact with neighboring chimpanzees, i.e., interactions resulted in limited benefits and consequences.
5. Conclusions
A recent study indicated that caregivers may provide additional opportunities for captive chimpanzees to establish and maintain meaningful social relationships [60]. To the best of our knowledge, this was the first study to suggest ignoring the physical barrier of the enclosure when evaluating the chimpanzees’ social networks. Indeed, our findings support this work regarding additional relationship opportunities even among conspecifics. In this study, we demonstrated that by housing chimpanzees in neighboring enclosures, it is possible to provide additional social stimulation and increase the complexity of their social environment. Our findings highlight the importance of considering relationships and social interactions that may extend towards beyond the enclosure barriers when studying or evaluating sociability and/or social networks in captive chimpanzees. Our data also provide evidence that chimpanzee social networks may not be as closed as traditionally assumed.
Furthermore, our findings may be of interest in future discussions and decisions regarding captive health, care, welfare, and husbandry protocols for captive chimpanzees. Our data suggest that housing chimpanzees in close proximity to other social groups provides significant social opportunities and stimulation, leading to an enriching social environment. However, it is crucial to monitor intergroup activity and assess its potential impact on primate welfare. While increased social stimulation and complexity may promote positive wellbeing, it could also lead to negative consequences such as excessive social pressure or incompatibility between individuals or agonistic negative interactions among chimpanzees. Frequent occurrence of aggression in captive groups tends to be labeled as undesired, especially if wounding or serious injuries are involved, i.e., potentially leading to a reduce state of wellbeing. Furthermore, intragroup aggressions are typically answered or followed by submissive or agonistic behaviors from targeted chimpanzees. Yet, this might not be the case for intergroup interactions, as our results indicate that most intergroup displays were ignored by the targeted individuals. This is likely due to the fact that no physical danger was perceived by the targeted individuals. Nevertheless, even unanswered agonistic displays may produce a negative impact on the receiving chimpanzees’ wellbeing, and needs to be considered. Thus, in the future, it would be interesting to expand the ethogram including non-social behaviors, such as self-directed or abnormal behaviors, yawning or scratching, as potential indicators of stress, i.e., measuring the response to agonistic intergroup events. Although this study was focused on demonstrating the frequency of social interactions between neighboring chimpanzee groups and how this affects intergroup interactions, we are aware of the small sample size of our study. Hence, we plan to include other populations in the future to increase the sample size as well as adapting the data collection methodology in order to evaluate the impact on the chimpanzee’s wellbeing in more detail. Another option would be to also include physiological measures and taking their vocalizations into account.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/ecologies4020025/s1. Table S1: Ethogram used for multifocal All Occurrence data collection; Table S2: Descriptive results regarding the distribution of interaction types among group members (intra) and chimpanzees from the neighboring group (inter); Table S3: Summary of observed interactions (%) describing the distribution among target individuals while considering the target’s sex and group; Table S4: Linear mixed model comparison between Null and Full models and Anova Type III Analysis of variance of all three fixed factors; Table S5: Post hoc test results of the linear mixed models. Multiple comparison of means with Tukey contrasts (adjusted p values with Holm–Bonferroni method); Figure S1: Plot of the residual normality distribution of the four significant LMM models.
Author Contributions
Conceptualization, J.G.-D. and D.C.; methodology, J.G.-D., D.R. and D.C.; software, J.G.-D. and D.C.; validation, J.G.-D., D.R. and D.C.; formal analysis, J.G.-D. and D.C.; investigation, J.G.-D.; resources, D.C.; data curation, J.G.-D.; writing—original draft preparation, J.G.-D.; writing—review and editing, J.G.-D., D.R. and D.C.; visualization, J.G.-D.; supervision, D.C. and D.R.; project administration, D.C. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
This research was conducted in accordance with all national and institutional guidelines for the care and management of primates as established by the Fundació MONA, the Association for the Study of Animal Behavior Society, and the Spanish Government (RD 53/2013). This study is based purely on behavioral observations, respecting the safety and wellbeing of the observed animals.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data is contained within the supplementary material.
Acknowledgments
The authors wish to thank the student Carolina Oliveras for her participation in data collection during this study, as well as to our dear colleague Lorraine Docherty for her input and her assistance with the English editing and proofreading. The authors are also extremely grateful to the caregivers and the volunteer staff involved in the care of the chimpanzees at Fundació MONA for their support during the duration of this study.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Silk, J.B. The Adaptive Value of Sociality in Mammalian Groups. Philos. Trans. Royal Soc. B Biol. Sci. 2007, 362, 539–559. [Google Scholar] [CrossRef]
- Silk, J. Evolutionary Perspectives on the Links between Close Social Bonds, Health, and Fitness. In Sociality, Hierarchy, Health: Comparative Biodemography; Weinstein, M.L.M., Ed.; Academies Press: Washington, DC, USA, 2014; pp. 121–144. [Google Scholar]
- Takahata, Y. Social Relationships among Adult Males. In Chimpanzees of the Mahale Mountains; Nishida, T., Ed.; The University of Tokyo Press: Tokyo, Japan, 1990; pp. 148–170. [Google Scholar]
- Nishida, T. A Quarter Century of Research in the Mahale Mountains: An Overview. The Chimpanzees of the Mahale Mountains: Sexual and Life History Strategies; The University of Tokyo Press: Tokyo, Japan, 1990; pp. 3–35. [Google Scholar]
- Chivers, D.J. Goodall 1986. The chimpanzees of Gombe: Patterns of behavior. Harvard University Press, Cambridge (Massachusetts). 673 pages. ISBN 0-674-11649-6. Price: £19.95 (hardback). J. Trop. Ecol. 1987, 3, 190–191. [Google Scholar] [CrossRef]
- Nishida, T. The Social Group of Wild Chimpanzees in the Mahali Mountains. Primates 1968, 9, 167–224. [Google Scholar] [CrossRef]
- Sugiyama, Y. Social Organization of Chimpanzees in the Budongo Forest, Uganda. Primates 1968, 9, 225–258. [Google Scholar] [CrossRef]
- Halperin, S. Temporary Association Patterns in Free Ranging Chimpanzees: An Assessment of Individual Grouping Preferences. In The Great Apes; Hamburg, D.A., Mccown, E.R., Eds.; Benjamin/Cummings: San Francisco, CA, USA, 1979; pp. 491–499. [Google Scholar]
- Boesch, C. Social Grouping in Taï Chimpanzees. In Great Ape Societies; McGrew, W., Marchant, L., Nishihada, T., Eds.; Cambridge University Press: Cambridge, UK, 1996; pp. 101–113. [Google Scholar]
- Ghiglieri, M. The Chimpanzees of the Kibale Forest; Columbia University Press: New York, NY, USA, 1984. [Google Scholar]
- Lehmann, J.; Boesch, C. To Fission or to Fusion: Effect of Community Size on Wild Chimpanzee (Pan Troglodytes versus) Social Organisation. Behav. Ecol. Sociobiol. 2004, 56, 207–216. [Google Scholar] [CrossRef]
- Boesch, C.; Boesch, H. The Chimpanzees of the Taï Forest; Oxford University Press: Oxford, UK, 2000. [Google Scholar]
- Matsumoto-Oda, A.; Hosaka, K.; Huffman, M.A.; Kawanaka, K. Factors Affecting Party Size in Chimpanzees of the Mahale Mountains. Int. J. Primatol. 1998, 19, 999–1011. [Google Scholar] [CrossRef]
- Wrangham, R. Why Are Male Chimpanzees More Gregarious than Mothers? A Scramble Competition Hypothesis. In Primate Males; Kappeler, P., Ed.; Cambridge University Press: Cambridge, UK, 2000; pp. 248–258. [Google Scholar]
- Riss, D.C.; Busse, C.D. Fifty-Day Observation of a Free-Ranging Adult Male Chimpanzee. Folia Primatol. 1977, 28, 283–297. [Google Scholar] [CrossRef]
- Wrangham, R.W.; Smuts, B.B. Sex Differences in the Behavioural Ecology of Chimpanzees in the Gombe National Park, Tanzania. J. Reprod. Fertil. Suppl. 1980, 28, 13–31. [Google Scholar]
- Isabirye-Basuta, G. Food Competition Among Individuals in a Free-Ranging Chimpanzee Community in Kibale Forest, Uganda. Behaviour 1988, 105, 135–147. [Google Scholar] [CrossRef]
- Sakura, O. Factors Affecting Party Size and Composition of Chimpanzees (Pan Troglodytes Verus) Bossou, Guinea. Int. J. Primatol. 1994, 15, 167–183. [Google Scholar] [CrossRef]
- Stanford, C.B.; Goodall, J.; Wallis, J.; Mpongo, E.; Wallis, J.; Goodall, J. Hunting Decisions in Wild Chimpanzees. Behaviour 1994, 131, 1–18. [Google Scholar] [CrossRef]
- Chapman, C.A.; Chapman, L.J.; Wrangham, R.W. Ecological Constraints on Group Size: An Analysis of Spider Monkey and Chimpanzee Subgroups. Behav. Ecol. Sociobiol. 1995, 36, 59–70. [Google Scholar] [CrossRef]
- Doran, D. Influence of Seasonality on Activity Patterns, Feeding Behavior, Ranging, and Grouping Patterns in Taï Chimpanzees. Int. J. Primatol. 1997, 18, 183–206. [Google Scholar] [CrossRef]
- Wrangham, R.W. Female Social Relationships and Social Organization of Kibale Forest Chimpanzees. Human Orig. 1992, 1, 81–98. [Google Scholar]
- Mitani, J.C.; Watts, D.P.; Amsler, S.J. Lethal Intergroup Aggression Leads to Territorial Expansion in Wild Chimpanzees. Curr. Biol. 2010, 20, R507–R508. [Google Scholar] [CrossRef]
- Kawanaka, K.; Nishida, T. Recent Advances in the Study of Inter-Unit-Group Relationships and Social Structure of Wild Chimpanzees of the Mahali Mountains. In Proceedings of the Symposium 5th Congress International Primatological Society, Nagoya, Japan, 21–24 August 1974; pp. 173–186. [Google Scholar]
- Nishida, T.; Hiraiwa-Hasegawa, M.; Hasegawa, T.; Takahata, Y. Group Extinction and Female Transfer in Wild Chimpanzees in the Mahale National Park, Tanzania. Z. Tierpsychol. 1985, 67, 284–301. [Google Scholar] [CrossRef]
- Neal Webb, S.J.; Hau, J.; Schapiro, S.J. Does Group Size Matter? Captive Chimpanzee (Pan Troglodytes) Behavior as a Function of Group Size and Composition. Am. J. Primatol. 2019, 81, e22947. [Google Scholar] [CrossRef] [PubMed]
- Llorente, M.; Riba, D.; Ballesta, S.; Feliu, O.; Rostán, C. Rehabilitation and Socialization of Chimpanzees (Pan Troglodytes) Used for Entertainment and as Pets: An 8-Year Study at Fundació Mona. Int. J. Primatol. 2015, 36, 605–624. [Google Scholar] [CrossRef]
- Lehmann, J.; Korstjens, A.H.; Dunbar, R.I.M. Group Size, Grooming and Social Cohesion in Primates. Anim. Behav. 2007, 74, 1617–1629. [Google Scholar] [CrossRef]
- Pascual, A.; Kalcher-Sommersguter, E.; Riba, D.; Crailsheim, D. Long-Term Assessment of Captive Chimpanzees: Influence of Social Group Composition, Seasonality and Biographic Background. Animals 2023, 13, 424. [Google Scholar] [CrossRef] [PubMed]
- Brent, L.; Lee, D.; Eichberg, J. The Effects of Single Caging on Chimpanzee Behavior; Laboratory Animal Science: Philadelphia, PA, USA, 1989. [Google Scholar]
- Farine, D.R.; Whitehead, H. Constructing, Conducting and Interpreting Animal Social Network Analysis. J. Anim. Ecol. 2015, 84, 1144–1163. [Google Scholar] [CrossRef]
- Sueur, C.; Jacobs, A.; Amblard, F.; Petit, O.; King, A.J. How Can Social Network Analysis Improve the Study of Primate Behavior? Am. J. Primatol. 2011, 73, 703–719. [Google Scholar] [CrossRef]
- McCowan, B.; Anderson, K.; Heagarty, A.; Cameron, A. Utility of Social Network Analysis for Primate Behavioral Management and Well-Being. Appl. Anim. Behav. Sci. 2008, 109, 396–405. [Google Scholar] [CrossRef]
- Freeman, L.C. The Development of Social Network Analysis; BookSurge, LLC: North Charleston, SC, USA, 2004. [Google Scholar]
- Wasserman, S.; Faust, K. Social Network Analysis: Methods and Applications. In Structural Analysis in the Social Sciences; Granovetter, M., Ed.; Cambridge University Press: Cambridge, UK, 1994; p. 825. [Google Scholar]
- Alwash, N.; Levine, J.D. Network Analyses Reveal Structure in Insect Social Groups. Curr. Opin. Insect. Sci. 2019, 35, 54–59. [Google Scholar] [CrossRef] [PubMed]
- Wilson, A.D.M.; Croft, D.P.; Krause, J. Social Networks in Elasmobranchs and Teleost Fishes. Fish Fish. 2014, 15, 676–689. [Google Scholar] [CrossRef]
- Garg, S.; Gandhi, T.K.; Panigrahi, B.K. Social Network Measures Association with Social and Intelligent Behaviors in Dolphin Network. In Proceedings of the Confluence 2021: 11th International Conference on Cloud Computing, Data Science and Engineering, Noida, Uttar Pradesh, India, 28–31 January 2021; pp. 655–659. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, H.; Johnston, L.J.; Martin, W. Do Pigs Form Social Structures: An Application of Social Network Analysis? J. Anim. Sci. 2017, 95, 7. [Google Scholar] [CrossRef]
- Flack, J.C.; Girvan, M.; De Waal, F.B.M.; Krakauer, D.C. Policing Stabilizes Construction of Social Niches in Primates. Nature 2006, 439, 426–429. [Google Scholar] [CrossRef] [PubMed]
- Sueur, C.; Petit, O. Organization of Group Members at Departure Is Driven by Social Structure in Macaca. Int. J. Primatol. 2008, 29, 1085–1098. [Google Scholar] [CrossRef]
- Lehmann, J.; Boesch, C. Sociality of the Dispersing Sex: The Nature of Social Bonds in West African Female Chimpanzees, Pan Troglodytes. Anim. Behav. 2009, 77, 377–387. [Google Scholar] [CrossRef]
- Henzi, S.P.; Lusseau, D.; Weingrill, T.; Van Schaik, C.P.; Barrett, L. Cyclicity in the Structure of Female Baboon Social Networks. Behav. Ecol. Sociobiol. 2009, 63, 1015–1021. [Google Scholar] [CrossRef]
- Ramos-Fernández, G.; Boyer, D.; Aureli, F.; Vick, L.G. Association Networks in Spider Monkeys (Ateles Geoffroyi). Behav. Ecol. Sociobiol. 2009, 63, 999–1013. [Google Scholar] [CrossRef]
- Brent, L.J.N.; Lehmann, J.; Ramos-Fernández, G. Social Network Analysis in the Study of Nonhuman Primates: A Historical Perspective. Am. J. Primatol. 2011, 73, 720–730. [Google Scholar] [CrossRef]
- Crailsheim, D.; Romani, T.; Llorente, M.; Kalcher-Sommersguter, E. Assessing the Sociability of Former Pet and Entertainment Chimpanzees by Using Multiplex Networks. Sci. Rep. 2020, 10, 20969. [Google Scholar] [CrossRef] [PubMed]
- Radosevich, L.M.; Jaffe, K.E.; Minier, D.E. The Utility of Social Network Analysis for Informing Zoo Management: Changing Network Dynamics of a Group of Captive Hamadryas Baboons (Papio Hamadryas) Following an Introduction of Two Young Males. Zoo Biol. 2021, 40, 503–516. [Google Scholar] [CrossRef] [PubMed]
- Hobaiter, C.; Poisot, T.; Zuberbühler, K.; Hoppitt, W.; Gruber, T. Social Network Analysis Shows Direct Evidence for Social Transmission of Tool Use in Wild Chimpanzees. PLoS Biol. 2014, 12, e1001960. [Google Scholar] [CrossRef]
- Croft, D.P.; Krause, J.; Darden, S.K.; Ramnarine, I.W.; Faria, J.J.; James, R. Behavioural Trait Assortment in a Social Network: Patterns and Implications. Behav. Ecol. Sociobiol. 2009, 63, 1495–1503. [Google Scholar] [CrossRef]
- Pasquaretta, C.; Levé, M.; Claidiere, N.; Van De Waal, E.; Whiten, A.; MacIntosh, A.J.J.; Pelé, M.; Bergstrom, M.L.; Borgeaud, C.; Brosnan, S.F.; et al. Social Networks in Primates: Smart and Tolerant Species Have More Efficient Networks. Sci. Rep. 2014, 4, 7600. [Google Scholar] [CrossRef]
- Rushmore, J.; Caillaud, D.; Matamba, L.; Stumpf, R.M.; Borgatti, S.P.; Altizer, S. Social Network Analysis of Wild Chimpanzees Provides Insights for Predicting Infectious Disease Risk. J. Animal Ecol. 2013, 82, 976–986. [Google Scholar] [CrossRef]
- Carne, C.; Semple, S.; Morrogh-Bernard, H.; Zuberbühler, K.; Lehmann, J. The Risk of Disease to Great Apes: Simulating Disease Spread in Orang-Utan (Pongo Pygmaeus Wurmbii) and Chimpanzee (Pan Troglodytes Schweinfurthii) Association Networks. PLoS ONE 2014, 9, e95039. [Google Scholar] [CrossRef]
- Hobson, E.A. Differences in Social Information Are Critical to Understanding Aggressive Behavior in Animal Dominance Hierarchies. Curr. Opin. Psychol. 2020, 33, 209–215. [Google Scholar] [CrossRef]
- Beisner, B.A.; Jin, J.; Fushing, H.; McCowan, B. Detection of Social Group Instability among Captive Rhesus Macaques Using Joint Network Modeling. Curr. Zool. 2015, 61, 70–84. [Google Scholar] [CrossRef] [PubMed]
- Goodall, J. Social Rejection, Exclusion, and Shunning among the Gombe Chimpanzees. Ethol. Sociobiol. 1986, 7, 227–236. [Google Scholar] [CrossRef]
- Sosa, S.; Zhang, P.; Cabanes, G. Social Networks Dynamics Revealed by Temporal Analysis: An Example in a Non-Human Primate (Macaca Sylvanus) in “La Forêt Des Singes. ” Am. J. Primatol. 2017, 79, e22662. [Google Scholar] [CrossRef] [PubMed]
- Crailsheim, D.; Stüger, H.P.; Kalcher-Sommersguter, E.; Llorente, M. Early Life Experience and Alterations of Group Composition Shape the Social Grooming Networks of Former Pet and Entertainment Chimpanzees (Pan Troglodytes). PLoS ONE 2020, 15, e0226947. [Google Scholar] [CrossRef]
- Baker, K.C.; Filif’, A.; Aureli, P.O. BRIEF REPORT The Neighbor Effect: Other Groups Influence Lntragroup Agonistic Behavior in Captive Chimpanzees. Am. J. Primatol. 1996, 40, 28–291. [Google Scholar] [CrossRef]
- Videan, E.N.; Fritz, J.; Schwandt, M.; Howell, S. Neighbor Effect: Evidence of Affiliative and Agonistic Social Contagion in Captive Chimpanzees (Pan Troglodytes). Am. J. Primatol. 2005, 66, 131–144. [Google Scholar] [CrossRef] [PubMed]
- Funkhouser, J.A.; Mayhew, J.A.; Mulcahy, J.B.B.; Sheeran, L.K. Human Caregivers Are Integrated Social Partners for Captive Chimpanzees. Primates 2021, 62, 297–309. [Google Scholar] [CrossRef] [PubMed]
- Grand, A.P.; Leighty, K.A.; Cory, L.J.; Maloney, M.A.; Phillips, R.S.; Bettinger, T.L. The Neighbor Effect in Bachelor and Breeding Groups of Western Lowland Gorillas (Gorilla Gorilla Gorilla). Int. J. Comp. Psychol. 2013, 26. [Google Scholar] [CrossRef]
- Goodall, J. The Behaviour of Free-Living Chimpanzees in the Gombe Stream Reserve. Anim. Behav. Monographs 1968, 1, 161–311. [Google Scholar] [CrossRef]
- Van Hooff, J. A Structural Analysis of the Social Behavior of a Semi-Captive Group of Chimpanzees. In Social Communication and Movement: Studies of Interaction and Expression in Man and Chimpanzee; von Cranach M, V.I., Ed.; Academic Press: London, UK, 1973; pp. 75–162. [Google Scholar]
- De Waal, F.M. The Communicative Repertoire of Captive Bonobos (Pan Paniscus), Compared to That of Chimpanzees. Behaviour 1988, 106, 183–251. [Google Scholar] [CrossRef]
- Clark, A.P. Rank Differences in the Production of Vocalizations by Wild Chimpanzees as a Function of Social Context. Am. J. Primatol. 1993, 31, 159–179. [Google Scholar] [CrossRef]
- Clark, A.P.; Wrangham, R.W. Acoustic Analysis of Wild Chimpanzee Pant Hoots: Do Kibale Forest Chimpanzees Have an Acoustically Distinct Food Arrival Pant Hoot? Am. J. Primatol. 1993, 31, 99–109. [Google Scholar] [CrossRef]
- Clark, A.P.; Wrangham, R.W. Chimpanzee Arrival Pant-Hoots: Do They Signify Food or Status? Int. J. Primatol. 1994, 15, 185–205. [Google Scholar] [CrossRef]
- Hauser, M.D.; Teixidor, P.; Fields, L.; Flaherty, R. Food-Elicited Calls in Chimpanzees: Effects of Food Quantity and Divisibility. Anim. Behav. 1993, 45, 817–819. [Google Scholar] [CrossRef]
- Mitani, J.C.; Nishida, T. Contexts and Social Correlates of Long-Distance Calling by Male Chimpanzees. Anim. Behav. 1993, 45, 735–746. [Google Scholar] [CrossRef]
- Mitani, J.C.; Brandt, K.L. Social Factors Influence the Acoustic Variability in the Long-distance Calls of Male Chimpanzees. Ethology 1994, 96, 233–252. [Google Scholar] [CrossRef]
- Arcadi, A.C. Phrase Structure of Wild Chimpanzee Pant Hoots: Patterns of Production and Interpopulation Variability. Am. J. Primatol. 1996, 39, 159–178. [Google Scholar] [CrossRef]
- Coe, C.L.; Levin, R. Dominance Assertion in Male Chimpanzees (Pan Troglodytes). Aggres. Behav. 1980, 6, 161–174. [Google Scholar] [CrossRef]
- Llorente, M.; Riba, D.; Mosquera, M.; Ventura, M.; Feliu, O. Hunting Activity Among Naturalistically Housed Chimpanzees (Pan Troglodytes) at the Fundació Mona (Girona, Spain). Predation, Occasional Consumption and Strategies in Rehabilitated Animals. Animals 2012, 2, 363–376. [Google Scholar] [CrossRef]
- Cano, R. Use of Natural Resources by Sanctuary Chimpanzees (Pan Troglodytes): Understanding the Significance of Naturalistic Environments; Hedmark University College: Hedmark, Norway, 2014. [Google Scholar]
- The Aerial View of Fundació Mona: Centre de Recuperació de Primats [Screenshot from Google Earth]. Available online: https://earth.google.com/web/@41.90231829,2.81685567,91.8588093a,146.98586859d,35y,29.52203631h,53.4998055t,360r (accessed on 18 May 2023).
- Altmann, J. Observational Study of Behavior: Sampling Methods. Behaviour 1974, 49, 227–266. [Google Scholar] [CrossRef]
- Wark, J.D.; Cronin, K.A.; Niemann, T.; Shender, M.A.; Horrigan, A.; Kao, A.; Ross, M.R. Monitoring the Behavior and Habitat Use of Animals to Enhance Welfare Using the ZooMonitor App. Anim. Behav. Cogn. 2019, 6, 158–167. [Google Scholar] [CrossRef]
- Johnson, J.D. UCINET: A Software Tool for Network Analysis. Commun. Educ. 1987, 36, 92–94. [Google Scholar] [CrossRef]
- Borgatti, S. NetDraw: Graph Visualization Software. In Harvard: Analytic Technologies; University of Kentucky: Lexington, KY, USA, 2002. [Google Scholar]
- Apostolato, I.-A. An Overview of Software Applications for Social Network Analysis. Int. Rev. Soc. Res. 2015, 3, 71–77. [Google Scholar] [CrossRef]
- Fruchterman, T.M.J.; Reingold, E.M. Graph Drawing by Force-Directed Placement. Softw. Pract. Exp. 1991, 21, 1129–1164. [Google Scholar] [CrossRef]
- Golbeck, J.; Mutton, P. Spring-Embedded Graphs for Semantic Visualization. In Visualizing the Semantic Web: XML-Based Internet and Information Visualization; Geroimenko, V., Chen, C., Eds.; Springer Verlag: London, UK, 2005; pp. 172–182. [Google Scholar]
- Kasper, C.; Voelkl, B. A Social Network Analysis of Primate Groups. Primates 2009, 50, 343–356. [Google Scholar] [CrossRef] [PubMed]
- Kalcher-Sommersguter, E.; Preuschoft, S.; Franz-Schaider, C.; Hemelrijk, C.K.; Crailsheim, K.; Massen, J.J.M. Early Maternal Loss Affects Social Integration of Chimpanzees throughout Their Lifetime. Sci. Rep. 2015, 5, 16439. [Google Scholar] [CrossRef]
- Bates, D.; Mächler, M.; Bolker, B.M.; Walker, S.C. Fitting Linear Mixed-Effects Models Using Lme4. J. Stat. Softw. 2015, 67, 1–48. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Core Team: Vienna, Austria, 2018. [Google Scholar]
- Field, A. Discovering Statistics Using SPSS; SAGE: London, UK, 2009. [Google Scholar]
- Dobson, A. An Introduction to Generalized Linear Models; University of Bath: Bath, UK, 2002. [Google Scholar]
- Nakamura, M.; Hosaka, K.; Itoh, N.; Zamma, K. Mahale Chimpanzees: 50 Years of Research; Cambridge University Press: Cambridge, UK, 2015. [Google Scholar]
- Wrangham, R.W. Evolution of Coalitionary Killing. Yrbk. Phys. Anthropol. 1999, 42, 1–30. [Google Scholar] [CrossRef]
- Muller, M. Agonistic Relations among Kanyawara Chimpanzees. In Behavioural Diversity in Chimpanzees and Bonobos; Boesch, C., Hohmann, G., Marchant, L., Eds.; Cambridge University Press: Cambridge, UK, 2002; pp. 112–124. [Google Scholar]
- Newton-Fisher, N.; Thompson, M. Comparative Evolutionary Perspectives on Violence. In The Oxford Handbook of Evolutionary Perspectives on Violence, Homicide, and War; Shackelford, T., Weekes-Shackelford, V., Eds.; Oxford University Press: Oxford, UK, 2012; pp. 41–60. [Google Scholar]
- Kret, M.E.; Prochazkova, E.; Sterck, E.H.M.; Clay, Z. Emotional Expressions in Human and Non-Human Great Apes. Neurosci. Biobehav. Rev. 2020, 115, 378–395. [Google Scholar] [CrossRef]
- De Waal, F.B.; Van Hooff, J.A. Side-Directed Communication and Agonistic Interactions in Chimpanzees. Behaviour 1981, 77, 164–198. [Google Scholar] [CrossRef]
- Samuni, L.; Mielke, A.; Preis, A.; Crockford, C.; Wittig, R.M. Intergroup Competition Enhances Chimpanzee (Pan Troglodytes Verus) In-Group Cohesion. Int. J. Primatol. 2020, 41, 342–362. [Google Scholar] [CrossRef]
- Lehmann, J.; Boesch, C. Sexual Differences in Chimpanzee Sociality. Int. J. Primatol. 2008, 29, 65–81. [Google Scholar] [CrossRef]
- De Waal, F.B.M. Chimpanzee Cultures; Wrangham, R.W., McGrew, W.C., De Waal, F.B., Heltne, B.G., Eds.; Harvard University Press: Cambridge, UK, 1996; pp. 243–260. [Google Scholar]
- Massaro, A.P.; Gilby, I.C.; Desai, N.; Weiss, A.; Feldblum, J.T.; Pusey, A.E.; Wilson, M.L. Correlates of Individual Participation in Boundary Patrols by Male Chimpanzees. Philos. Trans. Royal Soc. B 2022, 377, 20210151. [Google Scholar] [CrossRef] [PubMed]
- Samuni, L.; Preis, A.; Mielke, A.; Deschner, T.; Wittig, R.M.; Crockford, C. Social Bonds Facilitate Cooperative Resource Sharing in Wild Chimpanzees. Proc. Royal Soc. B 2018, 285, 20181643. [Google Scholar] [CrossRef]
- Koyama, N.F.; Caws, C.; Aureli, F. Interchange of Grooming and Agonistic Support in Chimpanzees. Int. J. Primatol. 2006, 27, 1293–1309. [Google Scholar] [CrossRef]
- Newton-Fisher, N.E. Female Coalitions against Male Aggression in Wild Chimpanzees of the Budongo Forest. Int. J. Primatol. 2006, 27, 1589–1599. [Google Scholar] [CrossRef]
- Nurmi, N.O.; Hohmann, G.; Goldstone, L.G.; Deschner, T.; Schülke, O. The “Tolerant Chimpanzee”—Towards the Costs and Benefits of Sociality in Female Bonobos. Behav. Ecol. 2018, 29, 1325–1339. [Google Scholar] [CrossRef]
- Palagi, E.; Paoli, T.; Tarli, S.B. Short-Term Benefits of Play Behavior and Conflict Prevention in Pan Paniscus. Int. J. Primatol. 2006, 27, 1257–1270. [Google Scholar] [CrossRef]
- Baker, K.C. Advanced Age Influences Chimpanzee Behavior in Small Social Groups. Zoo Biol. 2000, 19, 111–119. [Google Scholar] [CrossRef]
- Whitehead, H. Analysing Animal Social Structure. Anim. Behav. 1997, 53, 1053–1067. [Google Scholar] [CrossRef]
- Hobson, E.A.; Ferdinand, V.; Kolchinsky, A.; Garland, J. Rethinking Animal Social Complexity Measures with the Help of Complex Systems Concepts. Anim. Behav. 2019, 155, 287–296. [Google Scholar] [CrossRef]
- De Domenico, M.; Porter, M.A.; Arenas, A. MuxViz: A Tool for Multilayer Analysis and Visualization of Networks. J. Complex. Netw. 2015, 3, 159–176. [Google Scholar] [CrossRef]
- Finn, K.R.; Silk, M.J.; Porter, M.A.; Pinter-Wollman, N. The Use of Multilayer Network Analysis in Animal Behaviour. Anim. Behav. 2019, 149, 7–22. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).