Abstract
Tebuthiuron is a selective herbicide for woody species and is commonly manufactured and sold as a granular formulation. This project investigated the use of infrared spectroscopy for the quality analysis of tebuthiuron granules, specifically the prediction of moisture content and tebuthiuron content. A comparison of different methods showed that near-infrared spectroscopy showed better results than mid-infrared spectroscopy, while a handheld NIR instrument (MicroNIR) showed slightly improved results over a benchtop NIR instrument (Antaris II FT-NIR Analyzer). The best-performing models gave an R2CV of 0.92 and RMSECV of 0.83% w/w for moisture content, and R2CV of 0.50 and RMSECV of 7.5 mg/g for tebuthiuron content. This analytical technique could be used to optimise the manufacturing process and reduce the costs of post-manufacturing quality assurance.
1. Introduction
Tebuthiuron is a thiadiazolyl urea herbicide (Figure 1) primarily used for the control of woody plants. Application is typically via pellet-type (granular) formulations containing tebuthiuron (200–400 mg/g), which may be applied from the ground (either by hand or mechanically), or aerially dispersed if a large area is to be treated. As tebuthiuron is highly water-soluble [1], it leaches from the granules into the soil [2], where it is subsequently absorbed by the roots and translocated to the leaf tissue [3]. Its mode of action is through inhibition of Photosystem II, thus preventing photosynthesis in the affected plant [4]. The degradation and persistence of tebuthiuron is still an area under investigation, with studies reporting half-lives between 20 days [5] and 16–22 days [6], to as high as one year [7], 12.9 months [8], ‘considerably greater’ than 15 months [9] and even 2–7 years [10]. du Toit and Sekwadi [11] reported that tebuthiuron residue remained active in soil for 8 years after application.
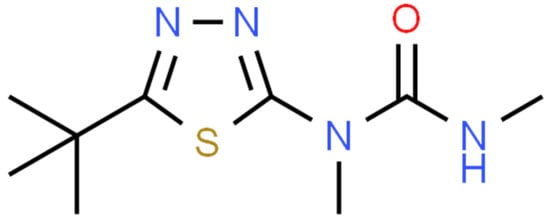
Figure 1.
The chemical structure of tebuthuiron. Retrieved from http://www.chemspider.com/ (accessed on 28 September 2022) under Creative Commons 4.0 license.
The structure of tebuthiuron includes two amide bonds (sharing the one carbonyl group) and a unique aromatic thiadiazolyl group (Figure 1), which should make it well-suited to detection by non-destructive analytical techniques such as infrared spectroscopy. Both of these chemical moieties would be expected to show distinct spectral characteristics in the mid-infrared (MIR) and near-infrared (NIR) regions. Additionally, the high concentration of tebuthiuron in most commercial tebuthiuron products (200–400 mg/g), should make it relatively simple to detect using MIR/NIR spectroscopy. The benefits of infrared spectroscopy over traditional analytical techniques include its speed (real-time), no ongoing costs related to traditional analysis costs, non-destructiveness and versatility (many units are portable; some are even handheld).
The previous literature has documented the use of NIR spectroscopy for the prediction of pesticide concentrations in liquid solutions [12], although this particular study did not analyse pesticides present in granular/powder form. Other authors have used MIR spectroscopy for the production quality control of numerous pesticides in liquid and solid forms [13]. Consequently, there is increasing interest in the use of infrared spectroscopy as a process analytical technology (PAT) tool [14]. However, no previous studies were found using infrared spectroscopy for the analysis of tebuthiuron content in any sample matrices.
Consequently, the aim of this work was to investigate the prospect of using infrared spectroscopy for a rapid, non-invasive method for the assessment of tebuthiuron and moisture, another important analyte in the manufacturing quality assurance process. This included a comparison of the performance of different infrared spectrophotometers for this purpose.
2. Materials and Methods
2.1. Sample Description
Sixty-eight (68) granular tebuthiuron samples (Regain™ brand) were manufactured by Cirrus Ag (North Rockhampton, Australia) over a period of approximately 10 months (April 2021–February 2022). These were each collected at specific time points as the final product came off the manufacturing line. The samples were stored in an air-conditioned room (approx. 20 °C) after receipt at the laboratory. Additionally, five samples of tebuthiuron powder (>95% purity) were included for comparative purposes, although they were not included in the quantitative modelling.
Two types of Regain formulation were produced by Cirrus Ag: one containing 200 mg/g of the active ingredient (i.e., tebuthiuron) and the other containing 400 mg/g. Throughout the manuscript, these are abbreviated at Regain200 and Regain400, respectively. The exact composition of the Regain granules is a trade secret, hence cannot be disclosed in this paper.
2.2. Sample Preparation
To ensure representative sampling of the granules upon receipt at the laboratory, each sample bag was thoroughly shaken and pellets were subsampled from at least 6 different locations throughout the bag. Approximately half of the sample (20–30 g) was subsampled, with the pellets then ground to a fine powder (Breville Coffee & Spice Grinder; Botany, NSW, Australia). When weighing out the required mass of powder for each extraction, care was taken to ensure that powder from at least 3 different locations within the sample subset was included.
2.3. Analysis of Moisture Content
The moisture content was measured on the intact (unground) Regain samples. Approximately 3 g of each sample was weighed into a pre-weighed aluminium foil tray, before being dried in a laboratory oven (Memmert 400; Buechenbach, Germany) at 110 °C overnight (16 h) until reaching a constant mass. After cooling to room temperature, the samples were reweighed, with the moisture content was determined as the loss in mass upon drying. Only one replicate was performed for each sample; however, triplicate analyses were performed on one sample to assess the reproducibility of the method.
To provide an extra data point for the NIR prediction of moisture content, the dried granules from all 68 samples were combined and mixed thoroughly. This aggregate granule sample was taken to have a moisture content of 0%, as it had been already dried at 110 °C for 16 hrs and did not lose any mass upon further drying.
2.4. Tebuthiuron Extraction Protocol
The extraction method was adapted from Lydon et al. [15] and validated by our laboratory. Approximately, 30 mg of the finely ground Regain powder was weighed into a 50 mL centrifuge tube. The total mass of the tube + sample was then recorded, before 20 mL of 90% v/v methanol was added using a calibrated bottle-top dispenser. The tube + sample + methanol was then re-weighed, with the determined mass of methanol converted to volume using the density of 90% methanol (0.823 ± 0.001 g/mL; n = 6 independent measurements). This allowed for the added volume of methanol to be determined with a much higher level of accuracy than that which could be obtained using a calibrated pipette.
The powder was extracted using an end-over-end shaker (Ratek RM-4; Boronia, VIC, Australia) operating at 50 rpm for 30 min. The extract was then centrifuged (1000 rcf for 5 min) and the supernatant collected for direct HPLC analysis (no dilution required). All samples were extracted and analysed in triplicate.
2.5. Tebuthiuron Analysis by HPLC
The tebuthiuron content of the methanol extracts was determined by high-performance liquid chromatography (HPLC). The HPLC analysis method used was adapted from Ferracini et al. [16]. Tebuthiuron was quantified on an Agilent 1100 HPLC system, comprising a G1313A autosampler, G1322A vacuum degasser, G1311A quaternary pump and G1315B diode array detector. A reversed-phase C18 column was used (Agilent Eclipse XDB-C18; 250 × 4.6 mm; 5 µm pore size; Agilent Technologies, Santa Clara, USA) along with a C18 guard column (Agilent Eclipse XDB-C18; 12.5 × 4.6 mm; 5 µm pore size). The injection volume was 5 µL and the detection wavelength was 254 nm. The elution method was an isocratic mixture of 50% methanol/50% water, at a flow rate of 1 mL min−1. The total run time was 15 min.
The tebuthiuron concentration of the samples was determined using an external calibration of analytical-grade tebuthiuron standard (Sigma-Aldrich Australia; North Ryde. NSW, Australia), ranging between 100–1000 mg L−1. Results were expressed as mg/g, on an as-is basis.
Figure 2 shows a typical chromatogram obtained from the extract of a Regain400 sample, demonstrating the absence of any interfering compounds at the selected wavelength.
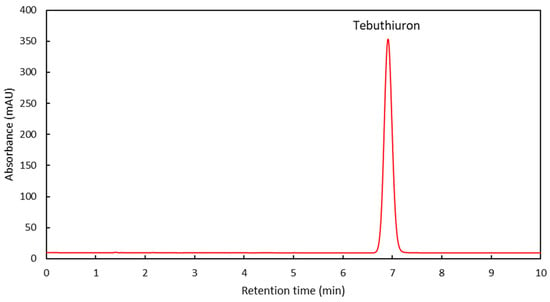
Figure 2.
A typical HPLC chromatogram of a Regain extract, with the location of the tebuthiuron peak indicated.
There was no clear consistency in the literature for the λmax or detection wavelength for tebuthiuron. Weber [17] reported λmax values of 252 nm in neutral solution and 261 nm in acidic conditions for tebuthiuron. Lourencetti et al. [18] found a λmax of 255 nm, while more recently, Ferreira et al. [19] reported the λmax of tebuthiuron to be 253 nm. Similarly, Lydon et al. [15] used a wavelength of 254 nm for the quantification of tebuthiuron via HPLC, while other authors have used 245 nm [20] and 247 nm [16,18].
Consequently, preliminary investigations were conducted to determine the optimum detection wavelength to use for HPLC analysis, through HPLC-DAD analysis and scanning tebuthiuron solutions using a Thermo Scientific Genesys 10S UV-Vis spectrophotometer (Sydney, Australia).
2.6. Validation of the HPLC Method
To assess the linearity of the HPLC method, tebuthiuron standards were prepared between 0.1–1000 mg L−1 and analysed using HPLC.
To assess the intra-day precision of the method, six replicate injections of 100 mg L−1 tebuthiuron standard were analysed on the same day. Similarly, the inter-day precision was assessed by injecting a sample of 100 mg L−1 tebuthiuron standard over six different days and comparing the peak areas.
To assess the stability of the methanolic tebuthiuron extracts, one Regain extract was analysed immediately following extraction and re-analysed after it had been stored for 8 days at room temperature.
The reproducibility of the finalised extraction method was determined by extracting and analysing 20 Regain samples each in triplicate, with the %CV calculated for each sample. In addition, the reproducibility of the extraction and HPLC method was assessed by performing 7 replicate extractions on one sample of homogenised Regain400 powder.
2.7. Assessment of Sample Variation
Based on preliminary results and observations during the manufacturing process, it was thought that there could be a high level of variation in tebuthiuron content between different portions of each Regain batch. To test this hypothesis, ten samples were randomly selected from different parts of one Regain400 sample (see Figure 3). These were then ground, extracted and analysed separately (each in triplicate).

Figure 3.
Sampling of the ten spatial replicates of the Regain400 sample. The red circles show the sampled areas.
2.8. Collection of FTIR Spectra
Mid-infrared (MIR) spectra were collected from the powdered samples using a Bruker Alpha FTIR (Fourier transform infrared) spectrophotometer (Bruker Optics Gmbh, Ettlingen, Germany) fitted with a platinum diamond attenuated total reflectance (ATR) single reflection module. FTIR spectroscopy requires the samples to be in a powdered form, as it necessitates firm contact between the sample and the ATR platform used to collect the spectra. Consequently, the FTIR spectra could only be collected from the powdered Regain samples, not the whole granular product.
The reflection module was covered with powder (approximately 100–200 mg) and pressure was applied to achieve uniform contact between the ATR interface and powder. Air was used as a reference background; the background measurement was performed every 15 min. Cross-contamination of samples was minimised by cleaning and drying the platform with isopropyl alcohol and laboratory Kimwipes® between samples. Using the OPUS software version 7.5 (Bruker Optics Gmbh, Ettlingen, Germany), the FTIR spectra were recorded between 4000 and 400 cm−1 as the average of 24 scans at a resolution of 4 cm−1. Three spectra were collected from each sample, repacking the instrument with fresh powder each time.
2.9. Collection of NIR Spectra—Benchtop Instrument
Near-infrared (NIR) spectra were collected from both the granular and powdered Regain samples using Antaris II FT-NIR Analyzer (Thermo Scientific; Madison, WI, USA). This instrument provides a high level of accuracy and reproducibility, making it highly suitable for method development purposes. Throughout the report, this is referred to as the “benchtop” NIR method.
The instrument was operated in reflectance mode, using the integrating sphere with a rotating sample cup (30 mm diameter). Spectra were collected between 1000–2500 nm (10,000–4000 cm−1), as the mean of 32 scans (resolution of 8 cm−1). The optimised gain was found to be 2×, with an empty attenuator screen. Background (dark) reference measurements were collected every hour. Spectra were collected in triplicate, repacking the sample cup with fresh granules or powder each time. The spectra were exported in *.csv format, with the mean of the triplicate spectra for each sample used in subsequent data analysis.
2.10. Collection of NIR Spectra—Handheld Instrument
NIR spectra were also collected from the granular Regain samples using a handheld NIR instrument, in order to determine the typical accuracy that could be obtained using portable NIR instrumentation. Handheld instrumentation would be greatly beneficial in an industrial setting due to its portability and lower cost (less than 1/3 of the benchtop Antaris instrument). Furthermore, instruments such as the MicroNIR may be suitable for installation as in-line sensors in an industrial setting.
A MicroNIR OnSite handheld spectrometer (Viavi; Santa Rosa, CA, USA) was used for this work. Spectra were collected across the full wavelength range of this instrument (908–1676 nm); the integration time was set to 100 ms. Reference dark and light spectra were collected every 10 min. Again, spectra were collected in triplicate and exported in *.csv format. The mean of the triplicate spectra for each sample was used in subsequent data analysis.
2.11. Independent Test Set—Using Handheld NIR
The best-performing infrared spectrophotometer was applied to an independent test set (i.e., samples not used in the model calibration). For this, thirteen additional samples (12 of Regain400 and 1 of Regain200) were sourced from Cirrus Ag (Rockhampton, Australia). NIR spectra were collected from the granules using the MicroNIR instrument and predictions made using the optimum model for each analyte.
2.12. Data Analysis
Chemometric analysis of the infrared spectra was conducted in the Unscrambler X 10.5 software (Camo ASA, Oslo, Norway). A variety of pre-processing methods were trialled, including the use of the standard normal variate (SNV) algorithm and the 1st and 2nd derivatives were calculated using a Savitzky–Golay algorithm with varying numbers of smoothing points. These are abbreviated as number–letter combinations showing the derivative number and number of smoothing points, e.g., 1d5 indicates 1st derivative with 5 smoothing points.
Partial least squares regression (PLSR) was used as the regression method. The maximum number of components considered in each model was set to 7, to reduce the possibility of overfitting. Full cross-validation of the PLSR models was conducted using the leave-one-out method.
To avoid creating a ‘two-point’ model, only Regain400 samples were included in the models for tebuthiuron content. The spectra and loadings were plotted using R Studio running R 4.2.2 (R Foundation for Statistical Computing, Vienna, Austria) [21].
3. Results and Discussion
3.1. Validation of the HPLC Method
Analysis of the tebuthiuron peak using HPLC-DAD showed that the maximum absorbance was located at 254 nm (Figure 4). This was confirmed by subsequent UV spectral scans of pure tebuthiuron standard in 100% methanol, which revealed a λmax of 253.5 nm. Hence, a detection wavelength of 254 nm was chosen for this work, agreeing with Lydon et al. [15].
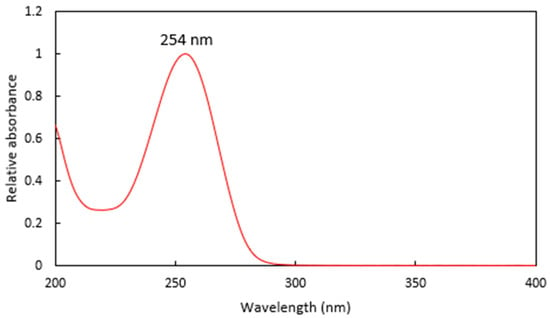
Figure 4.
The UV spectra of a Regain extract, showing the λmax at 254 nm.
For the analysis of linearity, the tebuthiuron standards were found to be linear over the range of 0.1–1000 mg L−1, with an R2 value of 0.9999 (Figure 5).
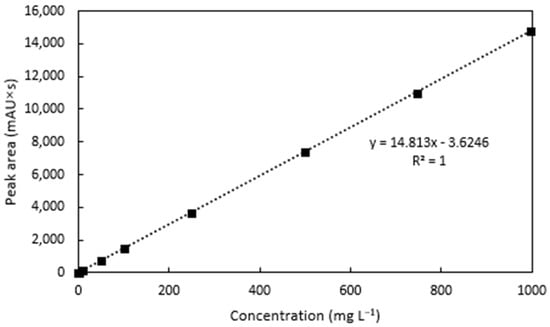
Figure 5.
Linearity of the tebuthiuron standards.
As can be seen in Table 1, the intra-day precision of the HPLC method was quite high, with a mean relative error (coefficient of variation) of 0.35%. The inter-day precision was slightly poorer than the intra-day precision (Table 1), with a mean coefficient of variation of 0.95%.

Table 1.
Intra-day and inter-day precision of replicate injections of 100 mg L−1 tebuthiuron standards analyzed using the HPLC method.
For the assessment of extract stability at room temperature, there was virtually no change in the tebuthiuron concentration (CV = 0.07%), indicating high stability of the tebuthiuron content over the 8 day storage period (Table 2).

Table 2.
Stability of the Regain methanolic extract after 8 days of storage at room temperature.
Reproducibility of the Extraction and HPLC Method
In order to create accurate prediction models using infrared spectroscopy, it is essential to have accurate analytical protocols for quantifying the analyte in question. In other words, the “reference” values used to calibrate the IR models must be accurate; otherwise, the IR models will be of no use.
For the reproducibility of the finalised extraction method, the mean %CV of the 20 samples extracted and analysed in triplicate was 2.1 ± 1.8% (n = 20), indicating an acceptable level of reproducibility.
Additionally, for the seven extractions performed on a single homogenised, powdered sample of Regain400, most of the replicate measurements showed good agreement with one another. One result (Replicate 2) was identified as an outlier and removed from subsequent calculations (Table 3). The mean content of the remaining samples was 401.3 ± 7.0 mg/g tebuthiuron, corresponding to a coefficient of variation of 1.74%.

Table 3.
Replicate tebuthiuron content measurements performed on replicate extracts from one powdered, homogenised Regain400 sample.
Furthermore, the mean coefficient of variation across 104 samples—each analysed in triplicate—was 1.46%, corresponding to an error ±5.8 mg/g for a sample with a nominal tebuthiuron content of 400 mg/g. This supported the reproducibility of the HPLC method for measuring tebuthiuron content in the Regain samples. In addition, the routine use of triplicate analyses for the assessment of tebuthiuron content allows for the detection and removal of any outlier results.
3.2. Observations on Moisture Content
The standard deviation (i.e., laboratory error) of the triplicate moisture analysis was found to be 0.42% w/w (Table 1), with a coefficient of variation of 3.9%, indicating acceptable analytical error.
In the samples of Regain400, a moderate negative correlation between the tebuthiuron and moisture content was found (Figure 6); however, this relationship was very weak (R2 = 0.05).
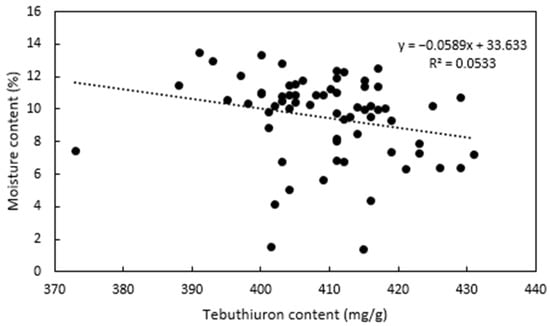
Figure 6.
Relationship between the tebuthiuron content and the moisture content of the Regain400 samples.
3.3. Assessment of Spatial Variation in the Sample
The results from the sample variation experiment indicated a moderate amount of spatial variation in the tebuthiuron content, with the mean content of each sampling replicate ranging from 412.3–421.0 mg/g (Table 4). The mean content was 416.1 ± 3.3 mg/g, giving a coefficient of variation of 0.79%. The results are also shown visually in Figure 7.

Table 4.
Assessment of the variation in tebuthiuron content between different portions of one Regain400 sample.

Figure 7.
The mean tebuthiuron contents for the ten spatial replicates analysed. The numbers over each sample location provide the mean tebuthiuron content in mg/g.
3.4. Analysis of FTIR Spectra
Figure 8 shows the FTIR spectra of one of the Regain samples, overlaid with the FTIR spectra of a sample of pure tebuthiuron powder. The tebuthiuron spectrum appeared similar to literature records on SpectraBase (https://spectrabase.com/spectrum/Gwi6TOkmgNy; accessed on 22 June 2022), with a complex pattern of peaks. The major peaks and their aetiological bonds are summarised in Table 5. Not all of the peaks between 1500–900 cm−1 were able to be confidently assigned identities, due to the complexity in this region. The Regain400 sample showed additional peaks at 1026, 1002, 910 and <600 cm−1, due to absorptions from the other matrix constituents. In contrast, the pure tebuthiuron powder samples showed minimal absorbance in this region (1026–910 cm−1).
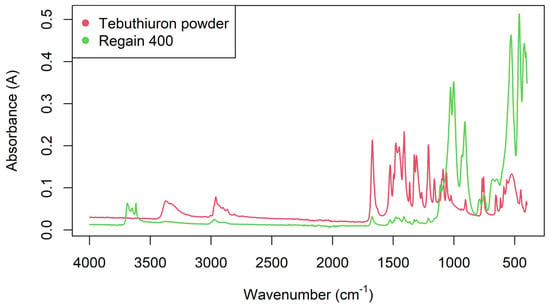
Figure 8.
FTIR spectra of a typical powdered 400 mg/g Regain sample and a sample of pure tebuthiuron powder (99.6% purity).

Table 5.
The major peaks observed in the FTIR spectrum of pure tebuthiuron powder and their assigned bonds.
Partial least squares regression performed on the FTIR spectra indicated that this technique could be used to predict the moisture content of the samples with reasonable accuracy (R2cv = 0.85; RMSECV = 1.12%; Table 6). The RPD (ratio of performance to deviation; equal to the standard deviation of the entire calibration set divided by the RMSECV) was 2.55, indicating good predictive ability for this analyte [28].

Table 6.
Optimum pre-processing methods for the prediction of moisture and tebuthiuron content in powdered Regain samples using FTIR spectroscopy. The best-performing model for each analyte is highlighted in bold.
However, FTIR spectroscopy was unable to predict the tebuthiuron content of the Regain400 samples with a high level of accuracy (R2cv = 0.43; RMSECV = 8.0 mg/g; RPD = 1.32). The best-performing results for both moisture and tebuthiuron content were found using standard normal variate (SNV) pre-processing of the FTIR spectra (Figure 9 and Figure 10).
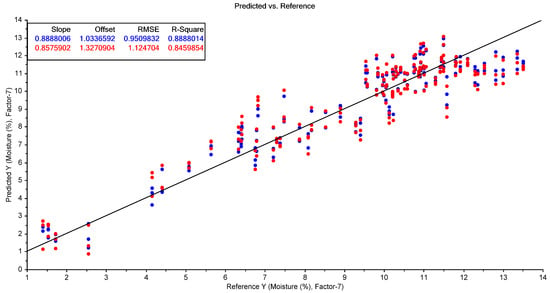
Figure 9.
The results of the best-performing PLSR model for the prediction of moisture content using the FTIR spectra of the Regain samples (using SNV pre-processing). The blue points show the model calibration, while the red points show the model cross-validation.
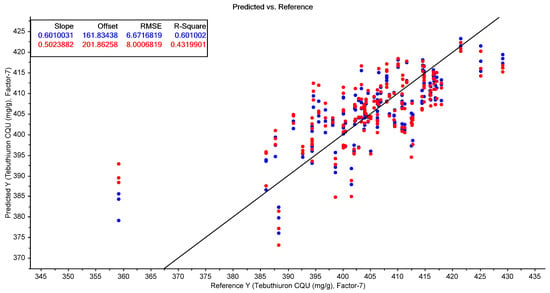
Figure 10.
The results of the best-performing PLSR model for the prediction of tebuthiuron content using the FTIR spectra of the powdered Regain400 samples (using SNV pre-processing). The blue points show the model calibration, while the red points show the model cross-validation.
3.5. Analysis of Benchtop NIR Spectra—Granules
The NIR spectra collected from the whole Regain granules is shown in Figure 11. Four of the oldest samples (shown in blue) had much lower absorbances across the NIR spectrum; possibly due to a different matrix composition for the non-active ingredients. These samples also contained the lowest moisture contents. However, the NIR spectra of the remaining samples were more consistent.
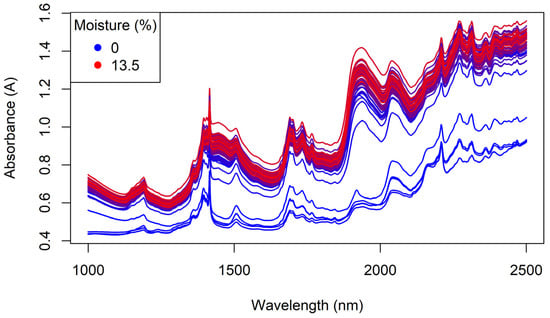
Figure 11.
The benchtop NIR spectra of the Regain granules, coloured by moisture content.
The main peaks observed in a pure (99.6% assay) sample of tebuthiuron powder (Figure 12) were at 1190, 1380, 1506, 1733 (shoulder), 2042 and 2269 nm. These were attributed to CH3 (second overtone), CH3 (second overtone), amide bond (second overtone), S-H (first overtone), C-S-C stretch and amide bond (amide I and III region), respectively [29].
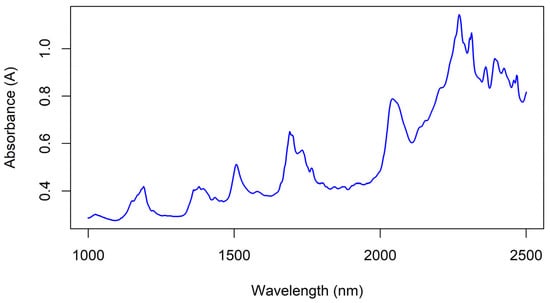
Figure 12.
The NIR spectra of a sample of pure tebuthiuron powder (99.6% assay), measured using the benchtop Antaris instrument.
The Regain400 samples showed additional peaks at 1396 (weak), 1415 (strong), and 2210 nm (strong) (cf. Figure 11). These are most likely attributable to CH3 (second overtone), H2O (second overtone) and CHO or CH3 combination bands, arising from non-active ingredients of the formulation (e.g., from organic matter in clay components or from polymer-like ingredients) [30].
The best-performing PLSR models for the prediction of moisture and tebuthiuron content using the benchtop Antaris NIR instrument are shown in Table 7. The highest accuracy for the prediction of moisture content was found using no pre-processing (Figure 13); however, the best model for tebuthiuron content used 2d11 pre-processing (Figure 14).

Table 7.
Optimum pre-processing methods for the prediction of moisture and tebuthiuron content in Regain granules using the benchtop Antaris NIR instrument. The best-performing model for each analyte is highlighted in bold.
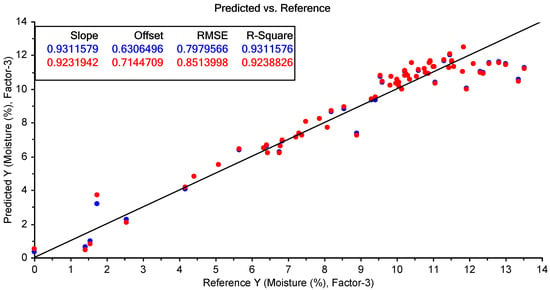
Figure 13.
The results of the best-performing PLSR model for the prediction of moisture content using the benchtop NIR spectra collected from the Regain granules (using no pre-processing). The blue points show the model calibration, while the red points show the model cross-validation.
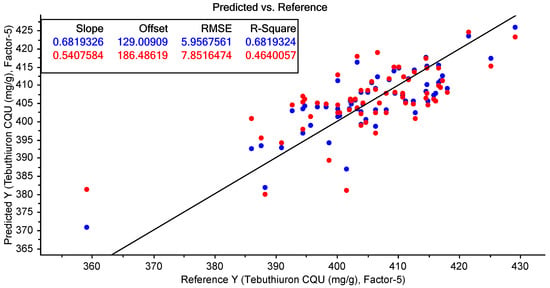
Figure 14.
The results of the best-performing PLSR model for the prediction of tebuthiuron content using the benchtop NIR spectra collected from the Regain400 samples (using 2d11 pre-processing). The blue points show the model calibration, while the red points show the model cross-validation.
The model accuracy was quite high for moisture content (R2cv of 0.93 and RMSECV of 0.85%); however, the prediction results for tebuthiuron content were somewhat poorer (R2cv of 0.46 and RMSECV of 7.9 mg/g). However, the RMSECV of the tebuthiuron calibration was only slightly lower than the standard deviation of the analytical (HPLC) reference method (7.0 mg/g). Consequently, this indicates that the accuracy of the NIR method was approaching the maximum accuracy expected using this reference method.
The model loadings for the moisture content PLSR model showed positive contributions from the peaks at 1936, 1450 and 1190 nm (Figure 15). These regions correspond to the H2O 1st overtone, amide second overtone and C-H second overtone, respectively. The greatest contribution was observed from the H2O 1st overtone, confirming that the model was principally measuring moisture in the samples. The smaller contribution from the amide region should be considered in future investigations, as it appears that the tebuthiuron content may be moderately influencing the model.
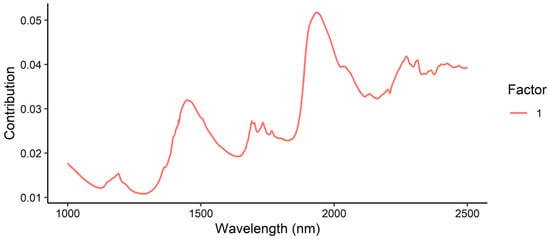
Figure 15.
Loadings plot for the prediction of moisture content in Regain granules using the benchtop Antaris NIR instrument.
The loading plot for the tebuthiuron content model (Figure 16) showed major contributions in the 2200–2400 nm region, corresponding to the CH3 combination band and amide I and III regions. Contributions were also observed around 1420 nm (H2O second overtone) and 1700 nm (S-H first overtone). Again, this confirmed that the model was principally looking at tebuthiuron in the sample, with a possible minor influence of moisture content.
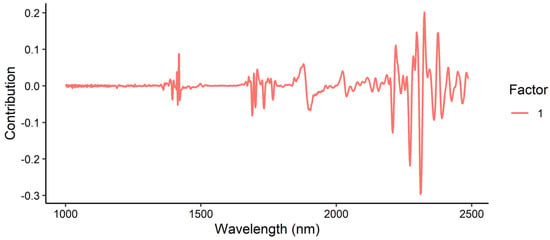
Figure 16.
Loadings plot for the prediction of tebuthiuron content in Regain granules, using the benchtop Antaris NIR instrument.
3.6. Analysis of Benchtop NIR Spectra—Powder
Generally, the accuracy of results obtained by NIR spectroscopy is improved by having a more homogenous sample matrix. Large particle sizes can lead to light scattering effects, which in turn biases the resultant NIR spectra obtained [31,32]. Using finely powdered matrices reduces this scattering effect and may consequently provide improved insight into the true sample composition. Consequently, the granule samples were ground to a fine powder and the NIR spectra collected from the powdered samples using the benchtop Antaris instrument. Figure 17 shows the NIR spectra of the powdered Regain samples, which were quite similar to the spectra collected from the granules (cf. Figure 11).
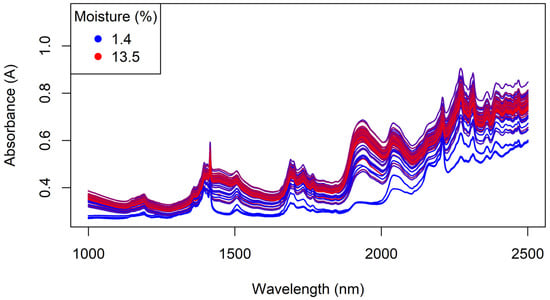
Figure 17.
The benchtop NIR spectra of the powdered Regain samples, coloured by moisture content.
The results for the prediction of moisture and tebuthiuron content from the powdered samples are shown in Table 8. Interestingly, the moisture prediction was less accurate when using the powdered samples compared to the whole granules, with an RMSECV of 1.02% for the best-performing model, compared to an RMSECV of 0.85% for the granules. This is an important observation from a rapid quality control viewpoint, as it indicates that NIR spectra obtained from the intact/whole Regain granules will perform just as well—if not better—than spectra obtained from the powdered samples, thus removing the need for the time-consuming grinding process.

Table 8.
Optimum pre-processing methods for the prediction of moisture and tebuthiuron content in powdered Regain samples using the benchtop Antaris NIR instrument. The best-performing model for each analyte is highlighted in bold.
In contrast, the prediction accuracy for tebuthiuron content was slightly increased compared to NIR spectra collected from the whole granules (RMSECV of 7.7 mg/g, compared to 7.9 mg/g). However, this slight increase in accuracy would not be significant enough to justify the additional sample preparation time in most practical applications.
3.7. Analysis of Handheld NIR Spectra—Granules
Given the promising results observed for the benchtop NIR spectra collected from the granules, the MicroNIR spectra were only collected from the granular Regain samples, not the powdered Regain samples. Additionally, the analysis of powder samples using this handheld instrument would require the instrument to be cleaned after every sample to prevent cross-contamination, adding to the time taken to collect the NIR spectra from each sample.
The NIR spectra of the Regain granules collected with the MicroNIR instrument are shown in Figure 18. The wavelength range of this instrument (908–1676 nm) is narrower than that of the benchtop Antaris instrument (1000–2500 nm); however, it still contains important information in the regions of 1185 nm (CH3 second overtone), 1360 nm (shoulder; CH3 second overtone), 1408 nm (H2O second overtone) and 1509 nm (amide bon d second overtone). Consequently, it was expected that this wavelength range could still be used for the prediction of moisture and tebuthiuron content.
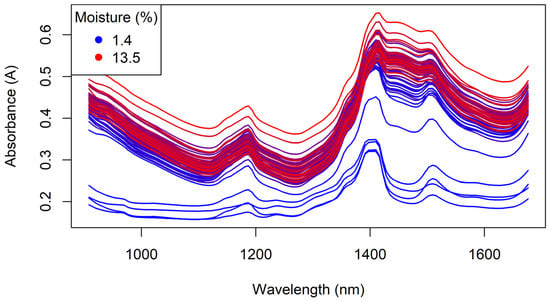
Figure 18.
The handheld MicroNIR spectra of the Regain granules, coloured by moisture content.
The performance statistics for the models developed for the prediction of moisture and tebuthiuron content using the MicroNIR spectra are shown in Table 9. Notably, the best models for moisture and tebuthiuron content were both slightly better (lower mean error) than those previously found using the benchtop NIR instrument. This supports the proposition of using handheld NIR instrumentation for real-time quality assessment of Regain products.

Table 9.
Optimum pre-processing methods for the prediction of moisture and tebuthiuron content in Regain granules using the handheld MicroNIR instrument. The best-performing model for each analyte is highlighted in bold.
As seen from the calibration graph in Figure 19, the MicroNIR instrument was able to predict the moisture content of the Regain samples with reasonably high accuracy across most of the range tested. Towards the higher range of moisture contents (>12%), the accuracy flattened off, with the model under-predicting the moisture content of all of these samples.
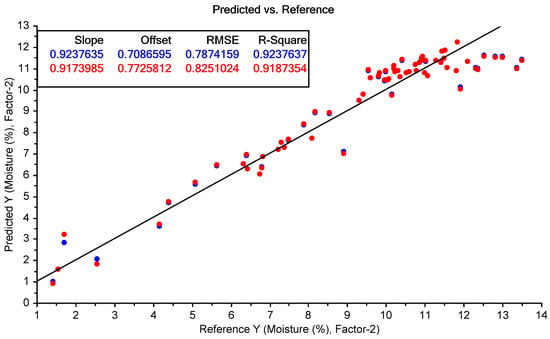
Figure 19.
MicroNIR prediction of moisture content. The blue points show the model calibration, while the red points show the model cross-validation.
The loading plot of the PLSR model for moisture content (Figure 20) shows the greatest contribution at 1410 nm, corresponding to the second overtone of H2O. A minor positive contribution was also observed at 1156 nm (CH3 second overtone) as well as negative contributions around 1497 nm (likely corresponding to the second overtone of the amide region).
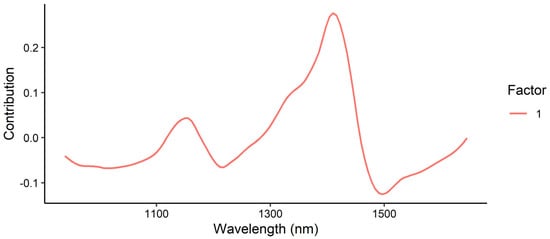
Figure 20.
Loadings plot for the prediction of moisture content using the MicroNIR instrument.
The calibration graph for tebuthiuron content showed increased variability compared to the moisture content prediction (Figure 21), with a few samples which could potentially be outliers. The R2 for cross-validation was not particularly high (0.53), but the RMSECV was quite good (7.5 mg/g), particularly compared to the mean laboratory error of 7.0 mg/g for tebuthiuron analysis using HPLC. Inclusion of a larger number of samples, particularly covering a wider calibration range, could potentially improve the prediction accuracy.
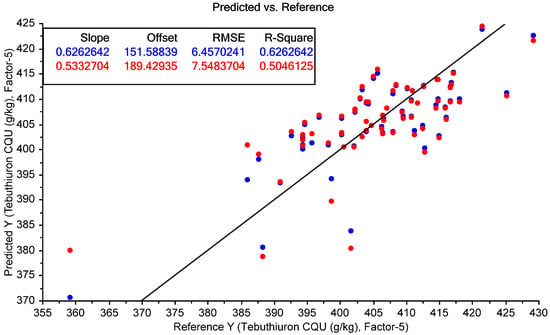
Figure 21.
MicroNIR prediction of tebuthiuron content. The blue points show the model calibration, while the red points show the model cross-validation.
The loadings plot for tebuthiuron prediction (Figure 22) showed the largest contributions at 1379 and 1453 nm. These likely correspond to the CH3 second overtone and amide band overtone. Both of these bonds are found in the structure of tebuthiuron (Figure 1).
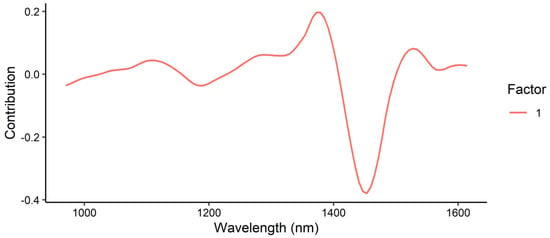
Figure 22.
Loadings plot for the prediction of tebuthiuron content using the MicroNIR instrument.
3.8. Comparison of Different Instruments and Sample Matrices
Table 10 provides a succinct summary of the best-performing models from all the different methods of infrared spectroscopy trialled, as well as the different matrix types (powder vs. granules).

Table 10.
Optimum pre-processing methods for the prediction of moisture and tebuthiuron content in powdered and granular Regain samples, using three different IR instruments. The best-performing model for each analyte is highlighted in bold.
The best-performing models were found using the handheld MicroNIR instrument, applied to the whole Regain granule samples. This was true for both moisture and tebuthiuron content. Consequently, this demonstrates that the handheld MicroNIR instrument should be highly suitable for the rapid, in-situ quality assessment of Regain granules throughout the manufacturing process.
3.9. Independent Test Set—Handheld NIR
The final stage of this work was to test the performance of the handheld NIR models using an independent set of samples (i.e., ones not used in the model calibrations). NIR spectra were collected from the granules using the MicroNIR instrument and predictions made using the optimum model for each analyte.
All of the PLS scores of the test set lay within the bounds of the scores of the calibration set (Figure 23), indicating that the spectra of the test set samples were similar enough to the spectra of the calibration set to allow accurate predictions to be made.
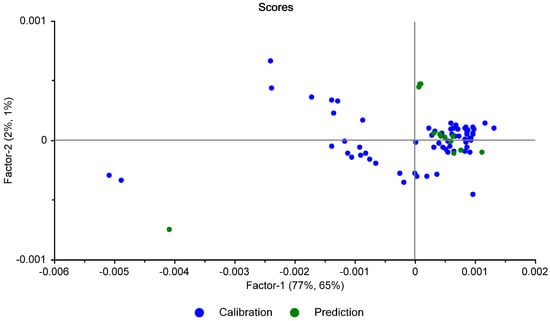
Figure 23.
Scores plot of the calibration and test set spectra.
The prediction results are shown in Table 11, alongside the reference measurements. Most of the moisture predictions were relatively close, with a mean prediction error of +0.36% w/w. The RMSEP (root mean square error of prediction) was 0.93% w/w, with a R2pred of 0.52.

Table 11.
Prediction and reference results for the independent test set.
Similarly, the prediction of tebuthiuron was quite accurate, with a mean prediction error of just +0.3 mg/g. The range of prediction errors varied between −7 and +5 mg/g. As shown in Figure 24, the prediction results were relatively linear (R2pred = 0.63), with an RMSEP of 3.8 mg/g. Again, this was well within the expected range of error associated with the HPLC reference method (7 mg/g).
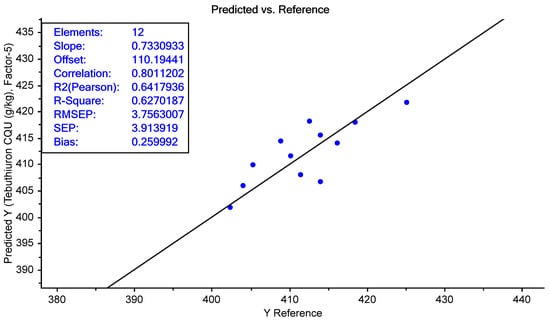
Figure 24.
The predicted vs. measured tebuthiuron content of the Regain 400 samples in the independent test set (n = 12).
It should be noted that the single Regain200 sample was excluded from the tebuthiuron prediction results, as the model had only previously been trained on the Regain400 samples; therefore, we could not predict the tebuthiuron content of the Regain200 sample with acceptable accuracy.
4. Conclusions
The results from this study suggest that NIRS is quite accurate for the rapid prediction of moisture content and moderately accurate for the prediction of tebuthiuron content. Handheld and even benchtop NIR devices could not only allow for rapid quality control, but also for improvement of the manufacturing process. This form of rapid, on-site testing with sufficient accuracy could allow for isolation of sources of process variation and guide targeted efforts to minimize their effects. This is particularly important as unwanted variations in the processes can be costly, cause manufacturing downtime, or be an indication of phenomena that reduce the plant reliability and performance. Overall, the results found here support the use of the handheld MicroNIR instrument for future studies and potential real-time implementation. Furthermore, the use of a larger calibration set is likely to moderately improve the prediction accuracy of the tebuthiuron model.
Author Contributions
Conceptualization, J.B.J., M.I. and M.N.; methodology, J.B.J.; software, J.B.J.; validation, J.B.J.; formal analysis, J.B.J.; investigation, J.B.J.; resources, J.B.J., M.N. and H.F.; data curation, J.B.J.; writing—original draft preparation, J.B.J.; writing—review and editing, J.B.J., H.F., M.I. and M.N.; visualization, J.B.J.; supervision, M.N.; project administration, J.B.J.; funding acquisition, M.N. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available on request from the corresponding author.
Acknowledgments
Thanks to Kerry Walsh for providing access to the NIR instrumentation.
Conflicts of Interest
Hugh Farquhar is a current employee and Mansel Ismay is a past employee of Cirrus Ag, the manufacturer of Regain™. Aside from supplying the samples, Cirrus Ag had no role in the collection, analysis or interpretation of data.
References
- Bovey, R.W.; Burnett, E.; Meyer, R.E.; Richardson, C.; Loh, A. Persistence of Tebuthiuron in Surface Runoff Water, Soil, and Vegetation in the Texas Blacklands Prairie. J. Environ. Qual. 1978, 7, 233–236. [Google Scholar] [CrossRef]
- Faria, A.T.; Souza, M.F.; Rocha de Jesus Passos, A.B.; da Silva, A.A.; Silva, D.V.; Zanuncio, J.C.; Rocha, P.R.R. Tebuthiuron leaching in three Brazilian soils as affected by soil pH. Environ. Earth Sci. 2018, 77, 214. [Google Scholar] [CrossRef]
- McNeil, W.K.; Stritzke, J.F.; Basler, E. Absorption, Translocation, and Degradation of Tebuthiuron and Hexazinone in Woody Species. Weed Sci. 1984, 32, 739–743. [Google Scholar] [CrossRef]
- Hatzios, K.K.; Penner, D.; Bell, D. Inhibition of Photosynthetic Electron Transport in Isolated Spinach Chloroplasts by Two 1,3,4-Thiadiazolyl Derivatives 1. Plant Physiol. 1980, 65, 319–321. [Google Scholar] [CrossRef]
- Cerdeira, A.L.; Desouza, M.D.; Queiroz, S.C.N.; Ferracini, V.L.; Bolonhezi, D.; Gomes, M.A.F.; Rosa, M.A.; Balderrama, O.; Rampazzo, P.; Queiroz, R.H.C.; et al. Leaching and half-life of the herbicide tebuthiuron on a recharge area of Guarany aquifer in sugarcane fields in Brazil. J. Environ. Sci. Health Part B 2007, 42, 635–639. [Google Scholar] [CrossRef]
- Qian, Y.; Matsumoto, H.; Liu, X.; Li, S.; Liang, X.; Liu, Y.; Zhu, G.; Wang, M. Dissipation, occurrence and risk assessment of a phenylurea herbicide tebuthiuron in sugarcane and aquatic ecosystems in South China. Environ. Pollut. 2017, 227, 389–396. [Google Scholar] [CrossRef]
- Helling, C.S. The science of soil residual herbicides. In Soil Residual Herbicides: Science and Management. Topics in Canadian Weed Science; van Acker, R., Ed.; Canadian Weed Science Society: Saint-Anne-de-Bellevue, QC, Canada, 2005; Volume 3, pp. 3–22. [Google Scholar]
- Elanco. Environmental Fate of Graslan in Rangeland Ecosystems and Safety to Non-Target Organisms; Elanco: Greenfield, FL, USA, 1988. [Google Scholar]
- Chang, S.S.; Stritzke, J.F. Sorption, Movement, and Dissipation of Tebuthiuron in Soils. Weed Sci. 1977, 25, 184–187. [Google Scholar] [CrossRef]
- Johnsen, T.N., Jr.; Morton, H.L. Tebuthiuron Persistence and Distribution in Some Semiarid Soils. J. Environ. Qual. 1989, 18, 433–438. [Google Scholar] [CrossRef]
- du Toit, J.C.O.; Sekwadi, K.P. Tebuthiuron residues remain active in soil for at least eight years in a semi-arid grassland, South Africa. Afr. J. Range Forage Sci. 2012, 29, 85–90. [Google Scholar] [CrossRef]
- Metrohm. Quantification of Five Effective Components in Pesticides by Visible Near-Infrared Spectroscopy; Version 1, NIR Application Note NIR–056; Metrohm: Herisau, Switzerland, 2017. [Google Scholar]
- Armenta, S.; Quintás, G.; Garrigues, S.; de la Guardia, M. Mid-infrared and Raman spectrometry for quality control of pesticide formulations. TrAC Trends Anal. Chem. 2005, 24, 772–781. [Google Scholar] [CrossRef]
- Wu, Z.; Peng, Y.; Chen, W.; Xu, B.; Ma, Q.; Shi, X.; Qiao, Y. NIR spectroscopy as a process analytical technology (PAT) tool for monitoring and understanding of a hydrolysis process. Bioresour. Technol. 2013, 137, 394–399. [Google Scholar] [CrossRef] [PubMed]
- Lydon, J.; Engelke, B.F.; Helling, C.S. Simplified high-performance liquid chromatography method for the simultaneous analysis of tebuthiuron and hexazinone. J. Chromatogr. A 1991, 536, 223–228. [Google Scholar] [CrossRef]
- Ferracini, V.L.; Queiroz, S.C.; Gomes, M.A.; Santos, G.L. Método para a determinação de hexazinone e tebutiuron em água (Method for determination of hexazinone and tebuthiuron in water). Química Nova 2005, 28, 380–382. [Google Scholar] [CrossRef]
- Weber, J.B. Ionization of Buthidazole, VEL 3510, Tebuthiuron, Fluridone, Metribuzin, and Prometryn. Weed Sci. 1980, 28, 467–474. [Google Scholar] [CrossRef]
- Lourencetti, C.; de Marchi, M.R.R.; Ribeiro, M.L. Determination of sugar cane herbicides in soil and soil treated with sugar cane vinasse by solid-phase extraction and HPLC-UV. Talanta 2008, 77, 701–709. [Google Scholar] [CrossRef]
- Figueiredo Ferreira, A.V.D.T.P.; Barbosa, L.V.; de Souza, S.D.; Ciuffi, K.J.; Vicente, M.A.; Trujillano, R.; Korili, S.A.; Gil, A.; de Faria, E.H. Titania-triethanolamine-kaolinite nanocomposites as adsorbents and photocatalysts of herbicides. J. Photochem. Photobiol. Chem. 2021, 419, 113483. [Google Scholar] [CrossRef]
- Su, M.; Jia, L.; Wu, X.; Sun, H. Residue investigation of some phenylureas and tebuthiuron herbicides in vegetables by ultra-performance liquid chromatography coupled with integrated selective accelerated solvent extraction–clean up in situ. J. Sci. Food Agric. 2018, 98, 4845–4853. [Google Scholar] [CrossRef]
- Team, R.C. A Language and Environment for Statistical Computing; Version 4.0.2; R Foundation for Statistical Computing: Vienna, Austria, 2020. [Google Scholar]
- Sigma Aldrich. IR Spectrum Table & Chart. Available online: https://www.sigmaaldrich.com/technical-documents/articles/biology/ir-spectrum-table.html (accessed on 31 March 2022).
- Jabs, A. Determination of Secondary Structure in Proteins by Fourier Transform Infrared Spectroscopy (FTIR). Available online: http://jenalib.leibniz-fli.de/ImgLibDoc/ftir/IMAGE_FTIR.html (accessed on 28 March 2022).
- Riaz, T.; Zeeshan, R.; Zarif, F.; Ilyas, K.; Muhammad, N.; Safi, S.Z.; Rahim, A.; Rizvi, S.A.A.; Rehman, I.U. FTIR analysis of natural and synthetic collagen. Appl. Spectrosc. Rev. 2018, 53, 703–746. [Google Scholar] [CrossRef]
- Long, F.; Chen, Z.; Han, K.; Zhang, L.; Zhuang, W. Differentiation between Enamines and Tautomerizable Imines Oxidation Reaction Mechanism using Electron-Vibration-Vibration Two Dimensional Infrared Spectroscopy. Molecules 2019, 24, 869. [Google Scholar] [CrossRef]
- Norimasa, N.; Hiromu, S.; Tatsuo, M. Vibrational Spectra and Molecular Structure of Ethyl Methyl Sulfide. Bull. Chem. Soc. Jpn. 1975, 48, 3573–3575. [Google Scholar] [CrossRef]
- Rao, C.N.R.; Venkataraghavan, R.; Kasturi, T.R. Contribution to the infrared spectra of organosulphur compounds. Can. J. Chem. 1964, 42, 36–42. [Google Scholar] [CrossRef]
- Nicolaï, B.M.; Beullens, K.; Bobelyn, E.; Peirs, A.; Saeys, W.; Theron, K.I.; Lammertyn, J. Nondestructive measurement of fruit and vegetable quality by means of NIR spectroscopy: A review. Postharvest Biol. Technol. 2007, 46, 99–118. [Google Scholar] [CrossRef]
- Ishigaki, M.; Ito, A.; Hara, R.; Miyazaki, S.-i.; Murayama, K.; Yoshikiyo, K.; Yamamoto, T.; Ozaki, Y. Method of Monitoring the Number of Amide Bonds in Peptides Using Near-Infrared Spectroscopy. Anal. Chem. 2021, 93, 2758–2766. [Google Scholar] [CrossRef] [PubMed]
- Tominack, R.L.; Tominack, R. Herbicide Formulations. J. Toxicol. Clin. Toxicol. 2000, 38, 129–135. [Google Scholar] [CrossRef]
- Maleki, M.R.; Mouazen, A.M.; Ramon, H.; De Baerdemaeker, J. Multiplicative Scatter Correction during On-line Measurement with Near Infrared Spectroscopy. Biosyst. Eng. 2007, 96, 427–433. [Google Scholar] [CrossRef]
- Pilorget, C.; Fernando, J.; Ehlmann, B.L.; Schmidt, F.; Hiroi, T. Wavelength dependence of scattering properties in the VIS–NIR and links with grain-scale physical and compositional properties. Icarus 2016, 267, 296–314. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).