Abstract
This interdisciplinary study explores the potential of bioactive compounds from Aronia melanocarpa pomace, a juice industry by-product. The ethanol extract of the pomace was analyzed using HPLC, revealing key polyphenolic acids and anthocyanins. The extract exhibited outstanding antioxidant activity (100% as measured by the ABTS assay and 98.23% as measured by the DPPH assay) and >99% antibacterial efficacy against E. coli and S. aureus. This bioactive extract was utilized in a one-step process to dye and functionalize textiles (wool, silk, cellulose acetate, cotton, and viscose), with cotton and viscose suited for colored disposable bioactive textiles, particularly protective healthcare textiles, due to strong antioxidant (>97% as measured by the ABTS assay and >76% as measured by the DPPH assay) and antibacterial (>75% for E. coli and >80% for S. aureus) properties. The aronia pomace extract was also incorporated into newly synthesized starch/gelatin hydrogels with a compression modulus of 0.041–0.127 MPa and equilibrium swelling ratios of 3.33–4.26 g/g. Functionalized hydrogels demonstrated over 99% ABTS antioxidant activity, while the antibacterial efficacy against E. coli and S. aureus exceeded 70% and 97%, respectively. These properties, combined with the hydrogels’ ability to control the release of extract compounds, make them adequate for wound care applications. The extract’s effectiveness as a green inhibitor for carbon steel, with inhibition efficiency surpassing 94% at a concentration of aronia pomace extract of 100 ppm, was confirmed by electrochemical methods. Moreover, the extract predominantly retards the cathodic reaction. The current research represents the first exploration of alternative and green sustainable technologies for developing novel products based on aronia pomace extract.
1. Introduction
Brand Essence® Market Research valued the aronia market at USD 788.7 million in 2021, with projections to grow to USD 1291.5 million by 2028, with an anticipated compound annual growth rate of 7.3% over the forecast period. Unprocessed Aronia melanocarpa fruit, known as black chokeberry, is utilized in the food industry for the production of juices, wines, jams, and dietary supplements [1], rather than being consumed fresh due to its bitter taste [2]. Leftover material after processing aronia fruit or juicing, called aronia pomace (comprising pulp, peels, and seeds), accounts for approximately 10–35% of the fruit’s weight [3] and contains higher concentrations of nutrients and bioactive compounds than food products made from it. It is important to note that aronia pomace is often composted, repurposed into low-value products like animal feed, or, regrettably, discarded in landfills and oceans [4].
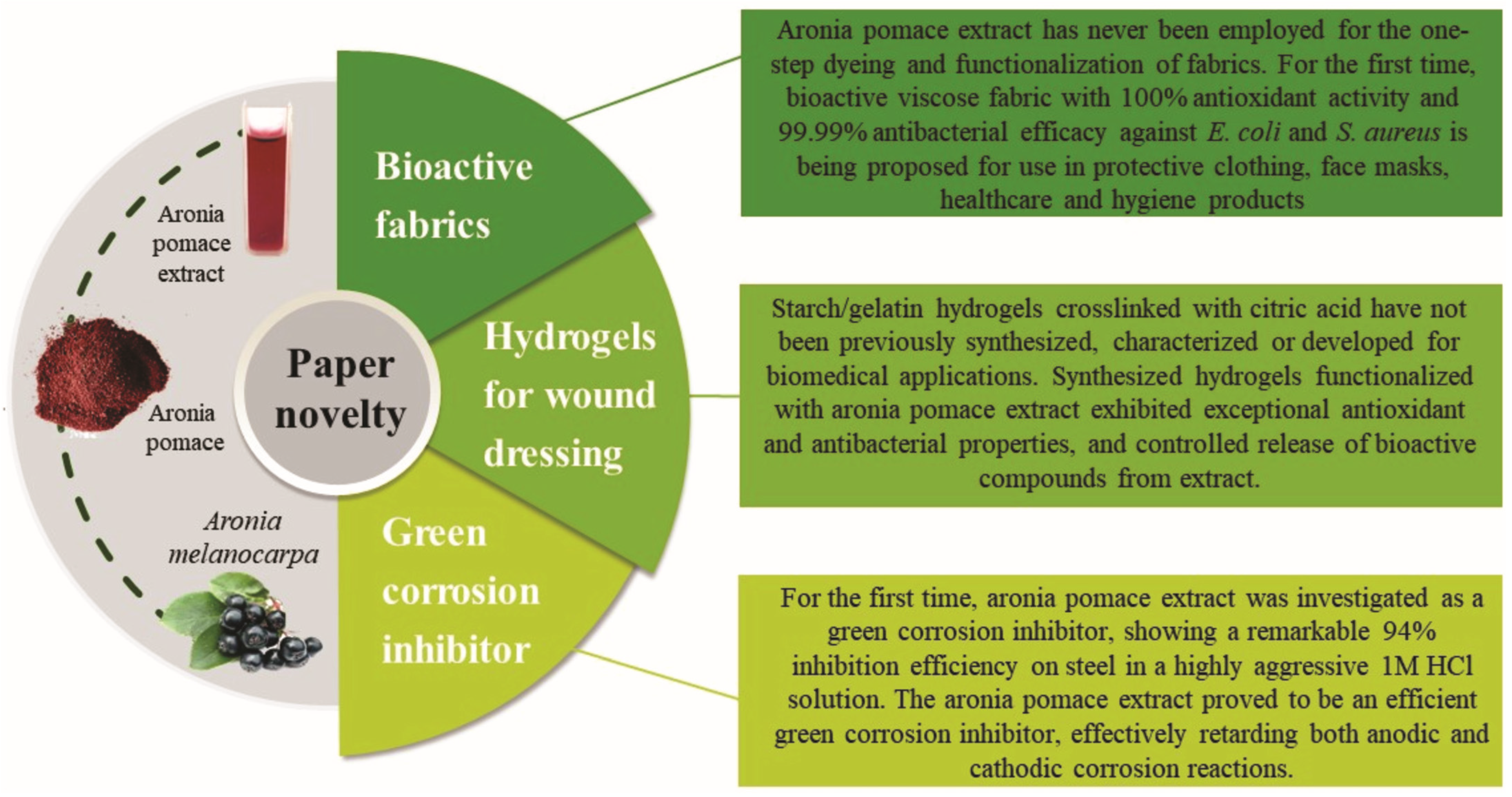
Previous studies [5,6,7,8] have identified aronia pomace as a rich source of polyphenolic compounds, especially flavonoids (like anthocyanins and flavonols), tannins, and phenolic acids. Recently, Zhang et al. [9] highlighted anthocyanins as the main polyphenols responsible for aronia pomace antioxidant activity. Besides antioxidant, the antibacterial properties of phenolic compounds in aronia pomace extract warrant attention. Exactly the well-known abundance of bioactive compounds in aronia pomace lies behind the extensive research over the past decade to explore innovative strategies for its valorization, particularly through the recovery of the bioactive compounds [6,10,11,12]. These efforts are driven by a dual objective: to mitigate the environmental impact and reduce disposal costs [8] while enhancing the product value by incorporating the aronia pomace’s bioactive compounds recovered through various methods. A careful analysis of the mentioned scientific data suggests that most studies focus on the extraction of aronia pomace bioactive compounds, with limited attention given to their applications. To address this research gap, the main objective of this study was to valorize aronia pomace by recovering its bioactive compounds and using them to develop three novel products: (i) colored healthcare textiles with potential applications in protective clothing, face masks, and healthcare and hygiene products, (ii) hydrogels with potential for wound care applications, and (iii) a green corrosion inhibitor for retarding both electrochemical reactions of carbon steel, all of them within the framework of sustainable production. The textile, biomedical, and chemical industries were selected as application areas because they represent complementary fields in which the bioactivity and multifunctional properties of aronia pomace extract can be effectively utilized to replace harmful synthetic compounds and support the transition toward sustainable, circular economy-based technologies. The present study explored the use of aronia pomace extract for fabric dyeing due to its intense coloration (Figure S1, Supplementary Material) and well-documented antioxidant and antibacterial properties [6], positioning it as a promising candidate for one-step dyeing and functionalization of textiles [13]. In the second phase of this research, the aronia pomace extract was incorporated as a functional component in hydrogels, where its antioxidant and antibacterial activities can prevent infection and promote wound healing, addressing the growing demand for biocompatible and sustainable materials in biomedical applications [14,15]. Finally, aronia pomace extract was evaluated as a green corrosion inhibitor in the chemical industry, offering an environmentally benign alternative to conventional toxic inhibitors [16]. The current research represents the first exploration of alternative and sustainable technologies for developing three novel products based on aronia pomace extract, as highlighted in Figure 1.
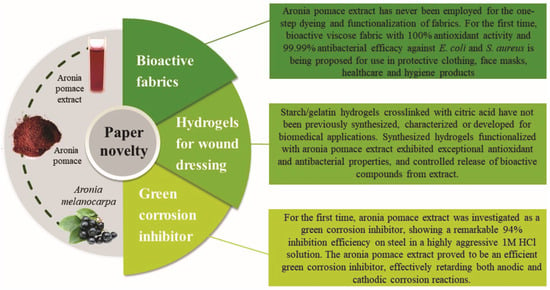
Figure 1.
Novel aspects of this study.
2. Materials and Methods
2.1. Chemicals
HPLC-grade solvents (methanol and water), as well as trifluoroacetic acid (TFA), were purchased from Sigma-Aldrich (Schnelldorf, Germany). HPLC standards, cyanidin-3-glucoside chloride and chlorogenic acid, were obtained from Carl Roth (Karlsruhe, Germany), whereas neochlorogenic acid was supplied by Sigma-Aldrich (Schnelldorf, Germany). Type B bovine skin gelatin (gel strength 70), potato starch, and phosphate-buffered saline (PBS) tablets were acquired from Sigma-Aldrich (Schnelldorf, Germany). Citric acid monohydrate was purchased from Avena Lab (Vršac, Serbia), and hydrochloric acid (HCl) was procured from Mol (Stara Pazova, Serbia).
2.2. Preparation and Characterization of Aronia Pomace Extract
Aronia pomace, sourced from the Vitbery juice industry (Kragujevac, Serbia), was dried in an oven at 40 °C until the percentage of moisture reached 10%. Subsequently, it was milled, and the bioactive compounds were extracted using an ultrasonic liquid processor with 70% ethanol at 60 °C for 30 min (liquid-to-solid ratio of 40 mL/g). The selected experimental conditions for the extraction of bioactive compounds from aronia pomace were chosen based on a combination of literature data and preliminary experiments aimed at maximizing extraction efficiency while preserving bioactive compound stability. The resulting extract was centrifuged, diluted twofold with distilled water, and stored in a refrigerator. The obtained twofold diluted extract was subsequently used for fabric and hydrogel functionalization.
Quantitative analysis of the extract’s anthocyanins was carried out according to the previously described method using a Dionex Ultimate 3000 HPLC system (Thermo Scientific, Waltham, MA, USA) equipped with a reverse-phase ZORBAX Eclipse Plus C18 column (4.6 × 150 mm, 5 µm) [17]. The mobile phase consisted of solvent (A) (H2O with 0.1% TFA) and solvent (B) (MeOH with 0.1% TFA). Separation was carried out using the following gradient elution program: 0–5 min isocratic 5% B, 5–45 min, linear increase from 5% to 50% B, 45–55 min isocratic 50% B, and 55–55.1 min decrease from 50% to 5% B, followed by a 5 min of isocratic elution with 5% B. The flow rate was set to 1 mL/min, and the column temperature was maintained at 45 °C. The sample injection volume was 10 µL. Detection of standards and phenolic compounds present in the twofold diluted aronia pomace extract was performed by UV detection at 520 nm for anthocyanins and 310 nm for neochlorogenic and chlorogenic acid. Concentrations of neochlorogenic and chlorogenic acids in the examined aronia pomace extract were quantified based on a standard curve for commercial standards, with slopes of 4.57 µmol/L and 3.70 µmol/L, respectively. Anthocyanin quantification was performed using standard curve obtained with cyanidin-3-glucoside as standard (slope of 7.85 µmol/L), and the results were expressed as micromoles of cyanidin-3-glucoside equivalents per liter (µmol C3GE/L).
The antioxidant activity of the twofold diluted aronia pomace extract was assessed using ABTS and DPPH assays. Briefly, ABTS•+ radical cation solution was prepared by mixing 4.912 mL of ABTS (7 × 10−3 mol/L in phosphate-buffered saline, PBS) with 0.088 mL of potassium persulfate (0.140 mol/L in distilled water). The mixture was kept in the dark for 16 h to allow the reaction to reach completion. Subsequently, the resulting solution was diluted with methanol to achieve an absorbance of 0.700 ± 0.02 at λ = 734 nm. Two milliliters of this freshly prepared ABTS•+ solution was mixed with 20 µL of twofold diluted aronia pomace extract, shaken, and incubated in the dark for 10 min. The absorbance was then measured at 734 nm. In the DPPH assay, 1 mL of diluted extract was added to 4 mL of a 1 × 10−4 mol/L methanolic solution of DPPH. The mixture was shaken in the dark for 60 min, after which the absorbance was recorded at λ = 517 nm. The antioxidant activity of the twofold diluted aronia pomace extract was calculated according to Equation (1):
where Ac is the absorbance of the control solution (containing all reagents except the extract), while As is the absorbance of the control solution containing the extract. Antioxidant experiments were performed in triplicate.
The antibacterial activity of the twofold diluted aronia pomace extract was evaluated against Escherichia coli ATCC 25922 and Staphylococcus aureus ATCC 25923. The bacterial inoculum was obtained by culturing the strains in tryptic soy broth (TSB) at 37 °C for 18 h and adjusting the suspension in sterile physiological saline to approximately 4–5 × 106 CFU/mL. A volume of 500 µL of aronia pomace extract (or control solution) was added to 9 mL of the bacterial suspension in sterile flasks, followed by incubation at 37 °C for 24 h [18,19]. After incubation, aliquots were serially diluted in sterile physiological saline, spread on tryptic soy agar (TSA), and incubated at 37 °C for 24 h, after which viable colonies (CFU/mL) were counted. Antimicrobial reduction (R, %) was calculated using Equation (2):
where C0 and Ct (CFU/mL) represent bacterial concentrations in the control solution and control solution containing aronia pomace extract, respectively. For conditions in which no colonies were observed on any replicate plates, antibacterial activity was reported as “99.99% (no colonies detected)”, consistent with standard practice in antimicrobial testing. All antimicrobial assays were carried out in triplicate.
2.3. Utilization of Aronia Pomace Extract for One-Step Dyeing and Functionalization of Textiles
A twofold diluted aronia pomace extract was employed for one-step dyeing and functionalization of wool (WO), silk (SILK), cotton (CO), polyamide (PA), and viscose (CV) fabrics. Each 1 g fabric sample was immersed in 40 mL of the twofold diluted extract, shaken for 24 h at 40 °C, rinsed, and air-dried. The reusability of the dyebath consisting of aronia pomace extract was tested for up to three cycles.
Color coordinates (L, a*, and b*) in the CIELab color space were measured using a Color i7 Benchtop Spectrophotometer (X-Rite, Grand Rapids, MI, USA) under illuminant D65 with a 10° standard observer. The color difference between fabrics dyed and functionalized in different cycles was calculated using Equation (3):
where , , and represent the color coordinates after the second dyeing cycle, while , , and are the color coordinates after the first dyeing cycle.
The fabrics’ antioxidant activity was determined using both the DPPH and ABTS assays. The ABTS assay was performed following the method described by Glaser et al. [20]. Briefly, 0.1 g of each fabric was added to a test tube containing 3.9 mL of freshly prepared ABTS•+ radical solution in PBS, and the reaction took place in the dark at 25 °C for 30 min. The radical scavenging activity was determined by measuring the absorbance of the solution at 734 nm and calculated according to Equation (1). The DPPH assay was conducted following the procedure described by Hong [21] wherein 0.5 g of the fabric was added to 30 mL of a freshly prepared DPPH methanolic solution (1.5 × 10−4 mol/L). The mixture was incubated in the dark for 1 h, after which the absorbance was measured at λ = 517 nm. The radical scavenging activity was then calculated using Equation (1). The results of the fabric antioxidant activity represent the mean values of three independent measurements.
For the determination of the fabrics’ antibacterial activity against E. coli and S. aureus, each fabric (0.2 g mass) was immersed in 9 mL of bacterial suspension (prepared according to the procedure previously described for the extract assay, Section 2.1), incubated in a shaking water bath at 37 °C for 24 h. After incubation, the antibacterial activity of the fabrics was determined using the same procedure as described for the extract. The reported results represent mean values of three independent measurements.
2.4. Synthesis of Starch/Gelatin (SG) Hydrogels, Functionalization, and Characterization
SG hydrogels were synthesized based on this protocol: 0.1 g of gelatin, 0.2 g of starch, and 0.02 g of citric acid were added to a reaction glass containing 2 mL of distilled water. The mixture was heated to 50 °C, and after 10 min of stirring at that temperature, it was poured into a Teflon mold and frozen at −20 °C for 24 h. Obtained hydrogels were subsequently lyophilized and then crosslinked at 160 °C for 5, 7, 9, or 12 min. Prepared hydrogels were labeled as SG-X, where X represents the crosslinking time (0, 5, 7, 9, or 12 min). The synthesized hydrogels (except SG-5, which lost its structural integrity during swelling) were immersed in twofold diluted aronia pomace extract for 24 h at room temperature (sample codes SG-7+AE, SG-9+AE, and SG-12+AE).
Fourier transform infrared (FT-IR) spectra of gelatin, starch, and SG hydrogels were recorded using a Nicolet™ iS™ 10 FT-IR Spectrometer (Thermo Fisher Scientific, Waltham, MA, USA) equipped with Smart iTR™ Attenuated Total Reflectance (ATR) accessories. The morphology of the hydrogels was characterized with a MIRA 3 XMU field emission scanning electron microscope (FE-SEM) (Tescan, Brno, Czech Republic).
The compression modulus of the hydrogels was evaluated after 24 h equilibration in PBS at room temperature. Measurements were performed using a Universal Testing Machine (AG-Xplus, Shimadzu, Kyoto, Japan) equipped with a 1000 N load cell (force range 0.01–1000 N). Unconfined compression tests were conducted on cylindrical specimens (8 × 6 mm) up to 100% deformation at a compression rate of 6 mm/min. Contact between the compression plate and the hydrogel was automatically detected by applying a contact force of 0.01 N. The compression modulus was calculated as the slope of the stress–strain curve within the linear region between 0% and 10% strain. Four samples were tested for each hydrogel.
The equilibrium swelling ratio (ESR) of hydrogels was determined after 24 h of swelling in PBS (pH 7.4) at 37 °C, using Equation (4):
where meq (g) is the mass of the swollen hydrogel, and m0 (g) is the mass of the dry hydrogel. Four samples were tested for each hydrogel.
The procedure for evaluating the release of extract from the functionalized hydrogels was performed according to a previously reported and validated method [22]. Phosphate-buffered saline (PBS, pH 7.4) was selected as the release medium because it simulates the physiological ionic strength and pH of human body fluids, providing relevant conditions for assessing the potential biomedical application of the hydrogels in wound care. The temperature of 37 °C was chosen to replicate normal human body temperature. UV-Vis spectroscopy was used to monitor the release kinetics, i.e., the absorbance changes at predefined time intervals (1, 2, 4, 6, 8, 10, 24, and 30 h) at the characteristic wavelength of the extract (λ = 273).
Hydrogels’ antioxidant activity (ABTS method) and antibacterial activity against E. coli and S. aureus were evaluated using the same procedures as described in Section 2.2.
2.5. Preparation of the Working Electrode and Solutions and Electrochemical Testing
All electrochemical tests were carried out on carbon steel plates of the following composition: Mn (0.276%), Cr (0.030%), C (0.080%), Cu (0.025%), Ni (0.012%), and Fe rest. The surfaces of the working electrodes were cleaned and polished using silicon carbide (SiC) paper (grit 150 to 1500). The carbon steel plates were subsequently immersed in distilled water and 99.9% ethyl alcohol, then air-dried immediately. A commercial-grade HCl solution (37%) diluted with deionized water was used for solution preparation. Electrochemical tests were performed in 1 M HCl solution, with varying concentrations of aronia pomace extract (0, 50, 100, or 200 ppm) prepared by dissolving the solid residue (remaining after evaporation of aronia pomace extract). Solutions are labeled as 1 M HCl, 50 ppm AE, 100 ppm AE, and 200 ppm AE, respectively.
Electrochemical measurements were conducted using a GAMRY Reference 600 Potentiostat/Galvanostat/ZRA (Gamry Instruments, Warminster, PA, USA) to evaluate the corrosion inhibition efficiency of aronia pomace extract. All experiments were performed in a three-electrode electrochemical cell, consisting of a carbon steel plate as the working electrode, a saturated calomel electrode (SCE) as the reference electrode, and a platinum electrode as the counter electrode. Prior to each measurement, the working electrodes were immersed for 1 h in both inhibited and uninhibited test solutions to allow stabilization of the open-circuit potential. Electrochemical impedance spectroscopy (EIS) was then performed at open-circuit potential over the frequency range of 0.01 Hz to 100 kHz, with 10 points per decade, using a sinusoidal voltage amplitude of 10 mV. Polarization measurements were subsequently carried out within a potential range from −250 mV to +250 mV vs. the corrosion potential (Ecorr), at a sweep rate of 1 mV/s. All experiments were conducted in triplicate, and the resulting data were processed and analyzed using the Gamry Echem Analyst software suite. The fitting quality accomplished by the suggested equivalent electrical circuit (EEC) was assessed by the “Goodness of Fit” parameter, which was below 1.3 × 10−4 for all samples.
2.6. Statistical Analysis
The results obtained in this study were statistically analyzed using the t-test, whereby parameter t was determined according to Equation (5):
where and are the samples’ mean values of the determining characteristic, and are the standard deviations of determining characteristic, n1 and n2 are the sample sizes.
3. Results and Discussion
3.1. Characterization of Aronia Pomace Ethanol Extract
Chemical analysis of the aronia pomace extract (Table 1 and Figure S2, Supplementary Material) revealed six dominant phenolic compounds, classified into two groups, phenolic acids (chlorogenic and neochlorogenic acid) and anthocyanins (cyanidin-3-galactoside, cyanidin-3-arabinoside, cyanidin-3-glucoside, and cyanidin-3-xyloside), which is in line with the results outlined by Zielińska et al. [23]. The performed t-test analysis (Table S1, Supplementary Material) revealed statistically significant differences among the concentrations of all analyzed compounds, except between chlorogenic acid and cyanidin-3-xyloside. While phenolic acids are colorless, cyanidin derivatives exhibit red to purple colors, depending on the pH of the solution, enabling their application as natural colorants. In addition to its strong antioxidant capacity (100% as measured by the ABTS assay and 98.23 ± 1.34% as measured by the DPPH assay), originating from present phenolics, the extract also demonstrated remarkable antibacterial effectiveness, achieving a 99.99% efficacy against both tested pathogenic strains. It seems this is the right place to mention that aronia pomace extract has been recently suggested as an “emerging” skin prebiotic capable of inhibiting harmful S. aureus, while simultaneously promoting the growth of beneficial skin microbiota representatives [6]. The findings of this section are consistent with previous studies reporting that neochlorogenic acid, chlorogenic acid, and aronia anthocyanins exhibit strong antioxidant properties along with antimicrobial activities against various microbial pathogens, making them promising agents for modulating human microbiota [24,25].

Table 1.
Concentration of phenolic compounds in aronia pomace extract expressed as mean values of triplicate measurements (n = 3) ± standard deviation (λ—wavelength, Rt—retention time).
3.2. Obtaining Colored Bioactive Fabrics
There has been extensive discussion about the negative effects of synthetic dyes on textile workers, consumers, and the environment, particularly through wastewater discharge [26]. In response, the textile industry is looking to substitute synthetic dyes with natural alternatives. Although several reviews highlight the utilization of natural colorants from sustainable sources for fabric dyeing [27,28], the challenge remains in selecting suitable natural colorants that meet modern demands for multifunctional textiles.
This study explored aronia pomace extract for fabric dyeing due to its intense coloration (Figure S1, Supplementary Material) and well-documented antioxidant and antibacterial properties, positioning it as a promising option for one-step dyeing and functionalization of three natural (WO, SILK, and CO), one synthetic (PA), and one regenerated cellulose (CV) fiber. Building on previous findings that highly pigmented extracts can be reused for multiple dyeing cycles [13], this study also investigated the reusability of the dyebath of aronia pomace extract. The objective was to assess whether the same dyebath could be employed for up to three dyeing cycles.
Visual inspection of the dyed fabrics (Figure 2) shows that diluted aronia pomace extract imparts shades ranging from light to dark purple and gray, with variations dependent on the fabric type and dyeing cycle. The fabric coloration is predominantly attributed to the fixation of anthocyanins (Table 1) present in the aronia pomace extract.

Figure 2.
Fabric appearance after different dyeing cycles using aronia pomace extract (WO—wool, SILK—silk, CO—cotton, PA—polyamide, CV—viscose, I cycle—first dyeing cycle, II cycle—second dyeing cycle, and III cycle—third dyeing cycle).
After each dyeing cycle, the fabric color was assessed in the CIELab color space. As shown in Figure 2 and Table 2, regardless of the dyeing cycle, WO fabrics consistently displayed the most intense coloration and yellowish appearance, characterized by the lowest L* and highest b* values. In contrast, CO (I cycle), PA (II cycle), and PA (III cycle) exhibited the least color intensity, reflected by their highest L* values. Additionally, SILK fabrics consistently manifest a bluer appearance, indicated by the lowest b* values, while CV fabrics were characterized by the reddest appearance, with the highest a* values among the fabrics tested. The measured color coordinates were used to calculate the color difference (ΔE) for the same fabric across different dyeing cycles. The lowest ΔE found between the I and II cycles, as well as between the II and III dyeing cycles for CO, suggests that a relatively consistent amount of colored compounds was fixed to that fabric. Furthermore, as shown in Table 2, the ΔE value for CO (II, III) is below 1, indicating that any changes in fabric color are visually imperceptible, which is consistent with the appearance of those fabrics in Figure 2.

Table 2.
Fabric color coordinates (L*—lightness (ranging from 0 for dark to 100 for light), a*—red-green coordinate (positive values indicate redness, negative values greenness), b*—yellow-blue coordinate (positive values indicate yellowness, negative values blueness)), ΔE—color difference for the same fabric across different dyeing cycles using aronia pomace extract. All data are expressed as mean values of triplicate measurements (n = 3), with coefficients of variation below 1.17%.
Depending on the extraction method and molecular structure, some natural colorants not only impart color to textiles but also provide valuable bioactive properties, such as antioxidant and antibacterial activities. These attributes make them highly suitable for producing colored multifunctional textiles, particularly for medical and protective clothing. The antioxidant activity of dyed fabrics was assessed, as oxidative stress, characterized by an overproduction of free radicals and reactive oxygen species (ROS), is a primary contributor to lifestyle diseases in humans [29]. This imbalance surpasses the body’s antioxidant defenses, resulting in damage to cellular biomolecules.
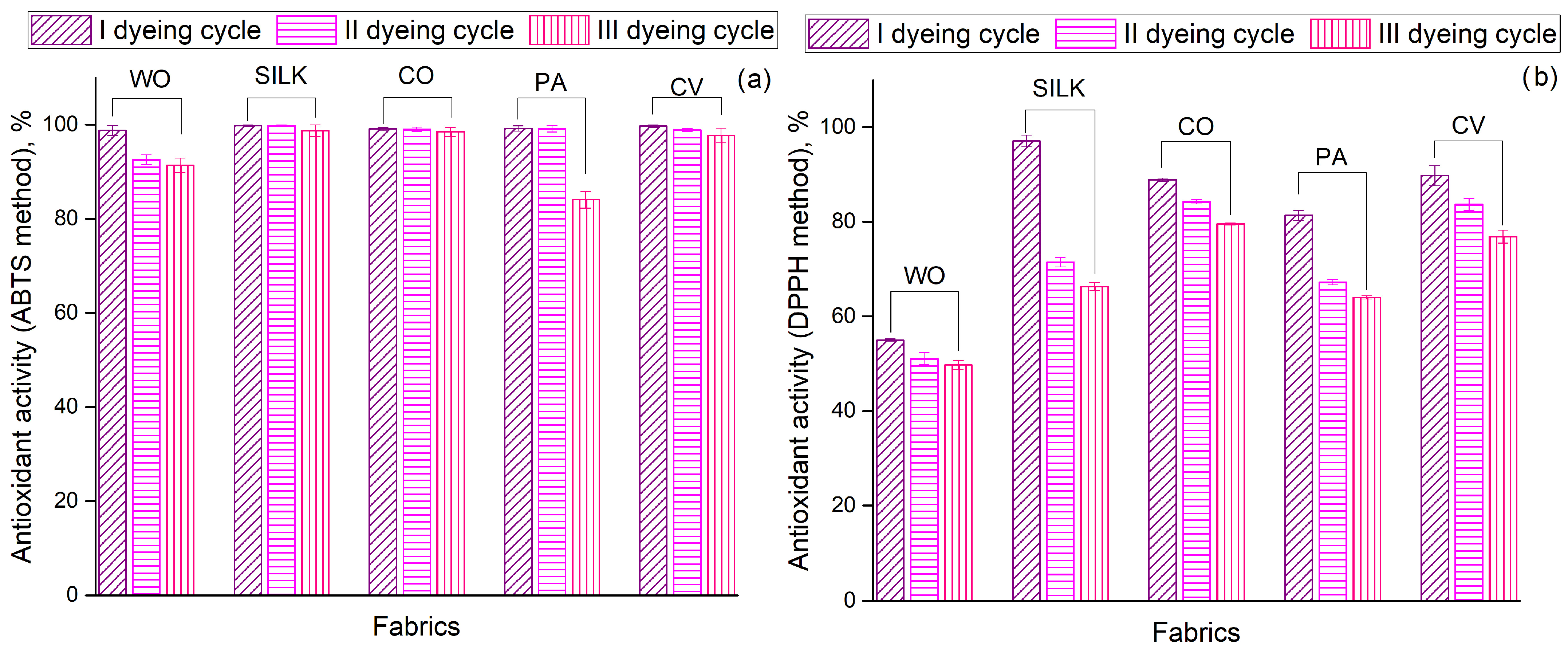
Figure 3 compares the antioxidant activity (determined according to the ABTS and DPPH methods) of fabrics dyed in the I, II, and III cycles. Regardless of the chemical composition or dyeing cycle, all fabrics (except for the PA III cycle) behave similarly, displaying excellent free radical scavenging ability (>91%) and effectively neutralizing nearly all ABTS radicals in the test solutions (Figure 3a). Surprisingly, the antioxidant activity of SILK, CO, and CV remained nearly unchanged after the II and III dyeing cycles. As previously discussed, Zhang et al. [9] identified anthocyanins as the primary polyphenols responsible for this antioxidant activity. Hong [21] revealed that the antioxidant activity (measured via DPPH assay) of wool and cotton fabrics mordanted with aronia leaf aqueous extract and functionalized with aronia fruit aqueous extract over three cycles ranged from approximately 5–70% and 2–75%, respectively. Comparing these findings with those presented in Figure 3b, it is evident that the ethanol extract of aronia pomace imparts superior antioxidant activity to the CO fabric (79.55–88.88% according to DPPH method), even when utilizing the same dyebath for three dyeing cycles. In the case of WO, the DPPH antioxidant activity was 55.00, 51.07, and 49.76% for I, II, and III dyeing cycles, respectively. Although these values are lower than those reported by Hong [21], the difference between the I and III dyeing cycles is less than 10%, which is not the case in the referenced study. The observed differences in the antioxidant activity of the same fabric when evaluated using different assays (ABTS and DPPH) can be described by the distinct reaction mechanisms involved, as well as the use of different model radicals [30].
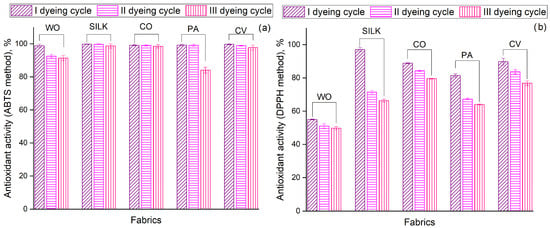
Figure 3.
Antioxidant activity of fabrics determined according to: (a) ABTS method and (b) DPPH method. All data are expressed as mean values of triplicate measurements (n = 3), error bars represent standard deviations.
Two bacterial strains, Escherichia coli and Staphylococcus aureus, were used for testing the antimicrobial activity of fabrics. As shown in Table 3, CV fabrics demonstrated outstanding antibacterial efficacy, achieving over 97% inhibition of Staphylococcus aureus. The same fabric dyed in the first cycle also exhibited excellent activity against Escherichia coli. This activity decreased to 75.45% after the third cycle. These results are significant, as the enhanced antibacterial performance of CV fabrics may contribute to infection prevention, potentially reducing the need for antibiotics and lowering healthcare costs. For CO fabric, exceptional antibacterial activity against Staphylococcus aureus was observed after the first dyeing cycle. However, this activity decreased after the second cycle and was completely lost after the third cycle. WO, SILK, and PA fabrics showed no antibacterial activity against either bacterium (Table 3).

Table 3.
Antibacterial activity of the examined fabrics expressed as mean values of triplicate measurements (n = 3) ± standard deviation. The standard deviation of 0 was recorded for 99.99% reductions (no viable colonies detected).
The dyeing protocol for WO, SILK, CO, PA, and CV fabrics with diluted ethanol extract of aronia pomace is one of the key innovations in this part of the study. This is the first investigation in which this extract has been utilized to impart antioxidant properties to the mentioned fabrics, as well as antibacterial properties to CO and CV fabrics. The results suggest that functionalized CO and CV fabrics hold potential as disposable bioactive textiles for protective medical applications. Further research will focus on the broader application of these fabrics as reusable medical textiles, with an emphasis on evaluating their color fastness and the durability of antioxidant and antibacterial properties after washing. This section lays the groundwork for identifying sustainable alternatives to harmful synthetic dyes and agents employed in the textile industry, with a goal of minimizing water pollution from the industry while promoting healthier and safer practices for consumers and workers.
3.3. Bioactive Hydrogels
In the second part of this study, the potential of diluted aronia pomace extract to impart bioactive properties to natural polymer-based hydrogels was investigated. This was accomplished in two steps: (1) the synthesis and characterization of novel hydrogels, and (2) their functionalization with aronia pomace extract, followed by an evaluation of the hydrogels’ antioxidant and antibacterial properties and the ability to release the extract’s bioactive compounds.
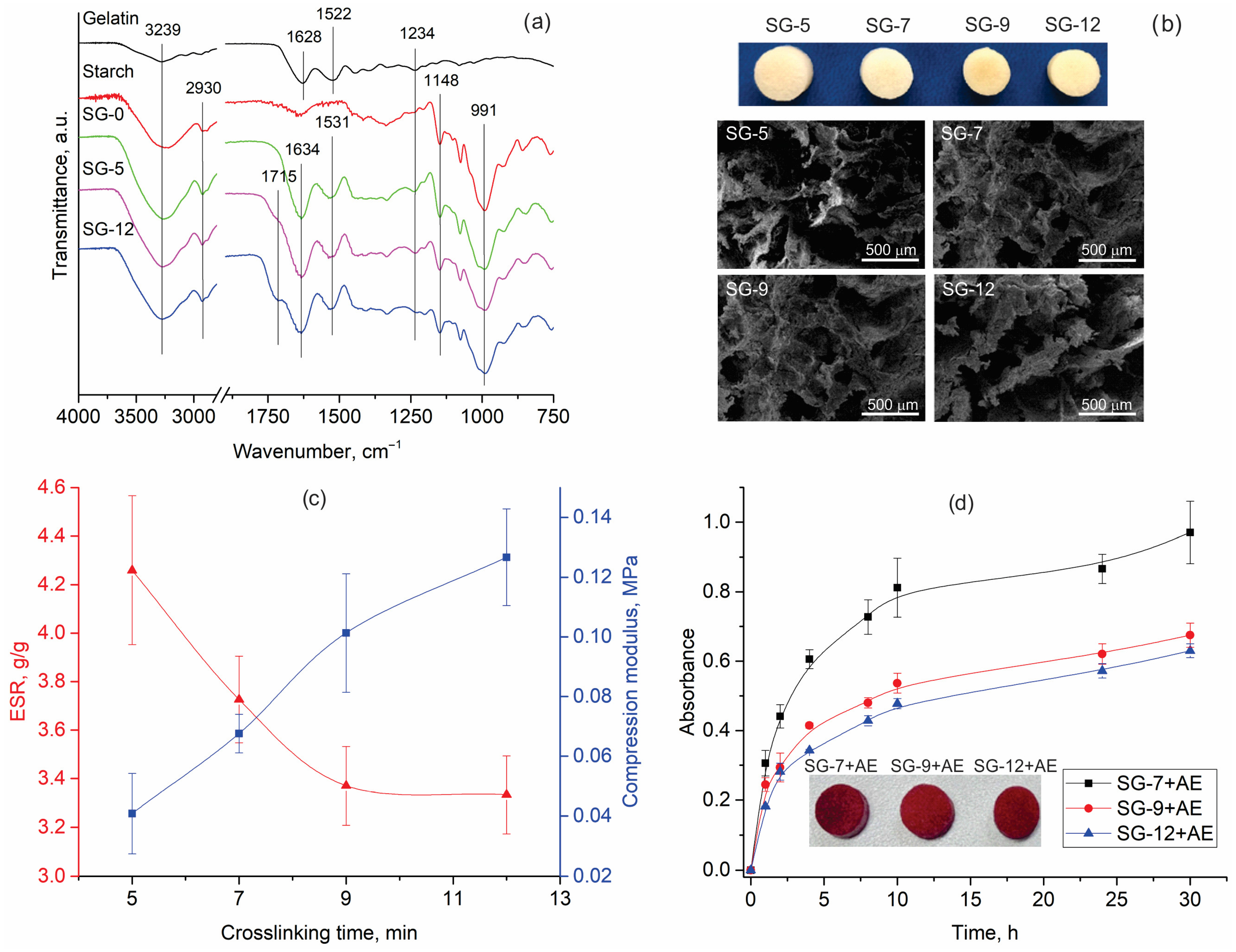
Novel starch/gelatin (SG) hydrogels were synthesized using a combination of solvent casting and freeze-drying techniques, followed by crosslinking with citric acid. Starch is an abundant natural polysaccharide, which finds extensive use in pharmaceuticals, food packaging, and agriculture, thanks to its low cost, biocompatibility, and biodegradability [31]. Gelatin, derived from collagen, is widely utilized in biomedicine for hydrogel preparation due to its biocompatibility, biodegradability, nonimmunogenicity, abundance, and cost-effectiveness [32]. It contains Arg-Gly-Asp (RGD)-like sequences that enhance cell adhesion, proliferation, migration, and differentiation. At elevated temperatures, this cost-effective, eco-friendly, and naturally derived crosslinker promotes the esterification of starch’s hydroxyl groups, resulting in the formation of crosslinks [33]. Figure 4a compares the ATR-FTIR spectra of the non-crosslinked hydrogel (SG-0) with those of the crosslinked ones for the shortest (SG-5) and longest (SG-12) times. The appearance of a shoulder at 1715 cm−1 (assigned to the ester groups), which is more intensified in the case of SG-12, pointed out the successful synthesis and progressive esterification between the citric acid carboxylic groups and starch hydroxyl groups [34]. The SG hydrogels’ FTIR spectra also contain bands originating from starch, such as those at 991 and 1148 cm−1 (C-O stretching vibrations), and 2930 cm−1 (C-H stretching) [35,36]. Furthermore, gelatin characteristic bands are centered at 1628 cm−1 (νC = O and νCN vibrations of polypeptide backbone), 1522 cm−1 (δNH and νCN vibrations of Amide II), and 1234 cm−1 (νCN and δNH vibrations of Amide III band) [37]. The minor shift in the band from 1628 cm to 1634 cm−1 in the spectra of SG hydrogels is due to a redistribution of charge and a reduction in the p–π conjugation effect of the amide group, induced by the formation of hydrogen bonds [38]. This observation is further supported by shifts in the band originating from δNH in the gelatin backbone (from 1522 to 1531 cm−1) and changes in the shape of the starch O-H stretching vibration band (centered at 3239 cm−1) in the spectra of SG-0, SG-5, and SG-12 hydrogels (Figure 4a). Discussed changes in the band positions and shape indicate interactions between the gelatin’s amide groups and the starch’s hydroxyl groups, confirming significant physical interactions [36], even in the non-crosslinked hydrogel.
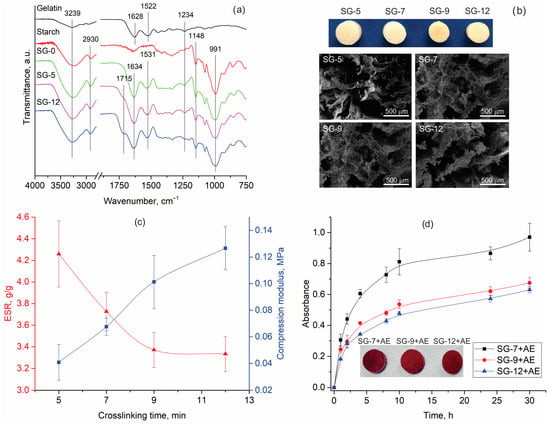
Figure 4.
(a) ATR-FTIR spectra of gelatin, starch, and synthesized hydrogels, (b) visual appearance and microstructure of the lyophilized hydrogels, (c) equilibrium swelling ratio (ESR) and compression modulus of hydrogels, and (d) visual appearance and kinetic of release extract’s bioactive compounds from functionalized hydrogels (SG-X—starch/gelatin hydrogels, where X represents the crosslinking time (5, 7, 9, or 12 min), SG-X+AE—starch/gelatin hydrogels functionalized with aronia pomace extract). The data are expressed as mean values of four parallel measurements (n = 4) ± standard deviation.
The differences in the visual appearance and microstructure (investigated by FE-SEM) (Figure 4b) of the lyophilized hydrogels were less pronounced than the variations observed in their surface chemistry (Figure 4a). Despite differing crosslinking time (5, 7, 9, or 12 min), the hydrogels consistently displayed a porous structure with irregular pores ranging from 200–500 µm.
The hydrogels, once characterized, were evaluated for their mechanical and swelling properties. Among the various mechanical properties, compression modulus was tested as an essential parameter for hydrogels intended for biomedical applications since it influences their performance, patient comfort, and overall therapeutic efficacy. Specifically, a higher compression modulus reflects enhanced mechanical strength, which helps in maintaining the three-dimensional shape and structural integrity of the hydrogels during application and use. The data presented in Figure 4c demonstrate that the compression moduli of SG-5, SG-7, SG-9, and SG-12 are 0.041, 0.068, 0.101, and 0.127 MPa, respectively. Performed t-test analysis proves statistically significant differences between the compression moduli of all SG hydrogels (Table S2, Supplementary Material). The over threefold increase in compression modulus between SG-5 and SG-12 can be explained by the greater number of crosslinks formed in SG-12 as a consequence of the longer crosslinking time, which was supported by the strong linear correlation (r = 0.990) between these two parameters.
Given the intended application of the synthesized SG hydrogels, a logical next step in their evaluation was to investigate the equilibrium swelling ratio (ESR), which indicates the hydrogel’s capacity to absorb and retain fluid under equilibrium conditions [39]. For wound dressings, a high ESR is desirable, as it signifies the hydrogel’s ability to absorb a substantial amount of wound exudate, thereby maintaining a moist environment that promotes the healing process [40]. A decreasing trend in hydrogels’ ESR was observed with increasing crosslinking time (Figure 4c), as evidenced by the negative linear correlation (r = −0.902) between these two parameters. Furthermore, the differences in ESR of SG-5, SG-7, SG-9, and SG-12, measured as 4.26, 3.73, 3.37, and 3.33 g/g, respectively, are statistically significant (Table S2, Supplementary Material).
It should not be overlooked that, in addition to absorbing and retaining fluid, a hydrogel’s swelling behavior also influences its capacity to control the release kinetics of bioactive compounds, ensuring a sustained and effective therapeutic effect. Various hydrogel applications require different release rates of bioactive compounds into the surrounding medium; for instance, in wound healing, a rapid release over a short period is often desirable. Therefore, the synthesized hydrogels (except SG-5, which lost its structural integrity during swelling) were immersed in diluted aronia pomace extract for 24 h at room temperature (sample codes SG-7+AE, SG-9+AE, and SG-12+AE). After drying, the release of bioactive compounds in PBS was monitored over a period of 30 h. Regardless of the crosslinking time, all hydrogels demonstrated a continuous release of bioactive compounds throughout the study period, as evidenced by the increasing trend in the solution absorbance maximum (Figure 4d). The in vitro release kinetics revealed that SG-7+AE exhibited the highest absorbance at the targeted maximum, indicating that it had the greatest ability to release bioactive compounds among the studied hydrogels. This phenomenon is likely attributed to its higher ESR (Figure 4c) and the observation that shorter crosslinking times correlated with faster release rates. In contrast, SG-9+AE and SG-12+AE, which were crosslinked for longer times and have lower ESR values, showed a reduced ability to release bioactive compounds compared to SG-7+AE. No changes in the visual appearance or structural integrity of the examined samples were observed after the release period, a factor that is important for their practical applications.
Following the release of bioactive compounds from the functionalized hydrogels, their antioxidant and antibacterial properties were tested. Antioxidant activity is very important in wound care, as dressings are frequently exposed to body fluids and tissues where oxidative stress, induced by excessive ROS, can impede the healing process [41]. However, a more prevalent factor that disrupts and prolongs wound healing is the occurrence of infections, primarily caused by bacterial microorganisms. These infections can, in severe cases, lead to sepsis or even necessitate the amputation of affected body parts [42]. Table 4 presents a comparison of the antioxidant and antibacterial properties of the studied hydrogels after functionalization with diluted aronia pomace extract. All functionalized hydrogels exhibited over 99% effectiveness in neutralizing free oxygen radicals, attributed to the presence of different polyphenolic compounds, especially anthocyanins (Table 1). This likely explains the observed >97% bacterial reduction of SG-7+AE, SG-9+AE, and SG-12+AE against Staphylococcus aureus, as well as their slightly lower effectiveness against Escherichia coli, with bacterial reduction ranging from 70.91 to 78.18% (Table 4). As recently documented by Ivanovska et al. [43], these differences in antibacterial activity against Escherichia coli and Staphylococcus aureus can be ascribed to the distinct structures of the cell wall, compositions of the membrane, and metabolic routes, which result in differences in their vulnerability to antibacterial mechanisms.

Table 4.
Antibacterial and antioxidant properties of functionalized hydrogels expressed as mean values of triplicate measurements (n = 3) ± standard deviation. The standard deviation of 0 was recorded for 99.99% bacterial reductions (no viable colonies detected).
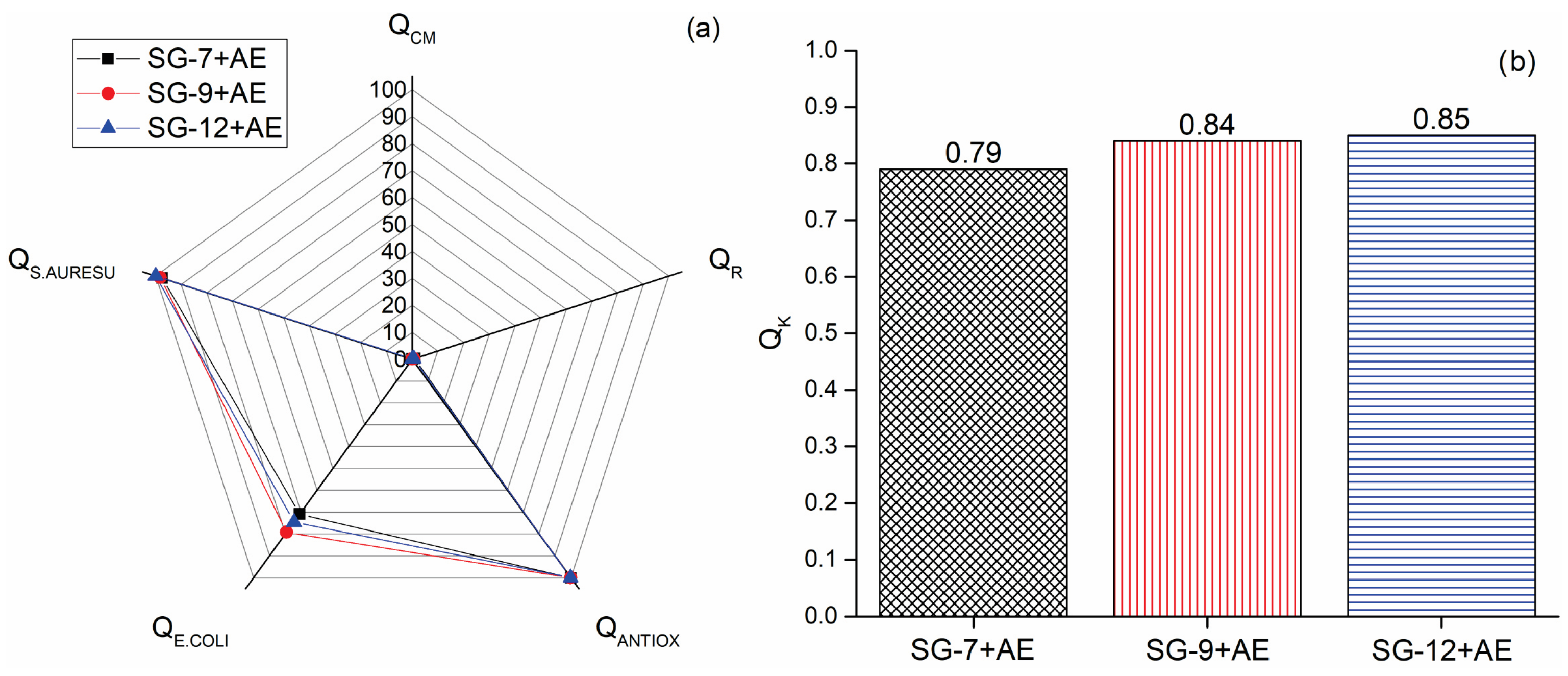
The final stage in evaluating functionalized hydrogels for wound dressing applications involved testing their overall quality (Supplementary Material). The dimensionless indicators for compression modulus (QCM), bioactive compounds release (QR), antioxidant activity (QANTIOX), and antimicrobial effect against Escherichia coli (QE.COLI) and Staphylococcus aureus (QS.AUREUS) were used for the assessment of hydrogels’ suitability for wound dressings. These indicators were then used to calculate the complex criterion of hydrogel quality (QK). The histogram presented in Figure 5b discloses that SG-7+AE, SG-9+AE, and SG-12+AE have excellent quality, with QK values of 0.79, 0.84, and 0.85, respectively.
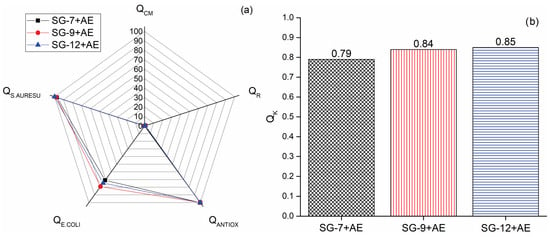
Figure 5.
(a) Dimensionless indicators for hydrogels’ quality (QCM—compression modulus, QR—bioactive compounds release, QANTIOX—antioxidant activity, QE.COLI—antimicrobial effect against E. coli, and QS.AUREUS—S. aureus, and (b) complex criterion (QK) of hydrogels’ quality.
This part of the study demonstrated that aronia pomace extract successfully functionalized newly synthesized SG hydrogels, imparting significant bioactive properties and rendering them highly suitable for biomedical applications, particularly in wound care. Future investigations will focus on evaluating the potential of these hydrogels as scaffolds for tissue engineering applications, where the controlled release of bioactive compounds could facilitate tissue regeneration and repair.
3.4. Green Corrosion Inhibitor Based on Aronia Pomace Extract
Aronia pomace extract, previously utilized for the development of bioactive fabrics and hydrogels, was evaporated to obtain a solid residue. Different masses of this residue were dissolved in 1 M HCl to prepare solutions with varying aronia pomace extract concentrations. The rationale for evaluating the aronia pomace extract bioactive compounds as a green inhibitor of carbon steel corrosion stems from its polyphenol-rich composition (Table 1), making this by-product a promising candidate for that purpose. After the preparation of the extracts, electrochemical impedance and polarisation measurements were conducted.
3.4.1. Electrochemical Impedance Spectroscopy (EIS) Measurements
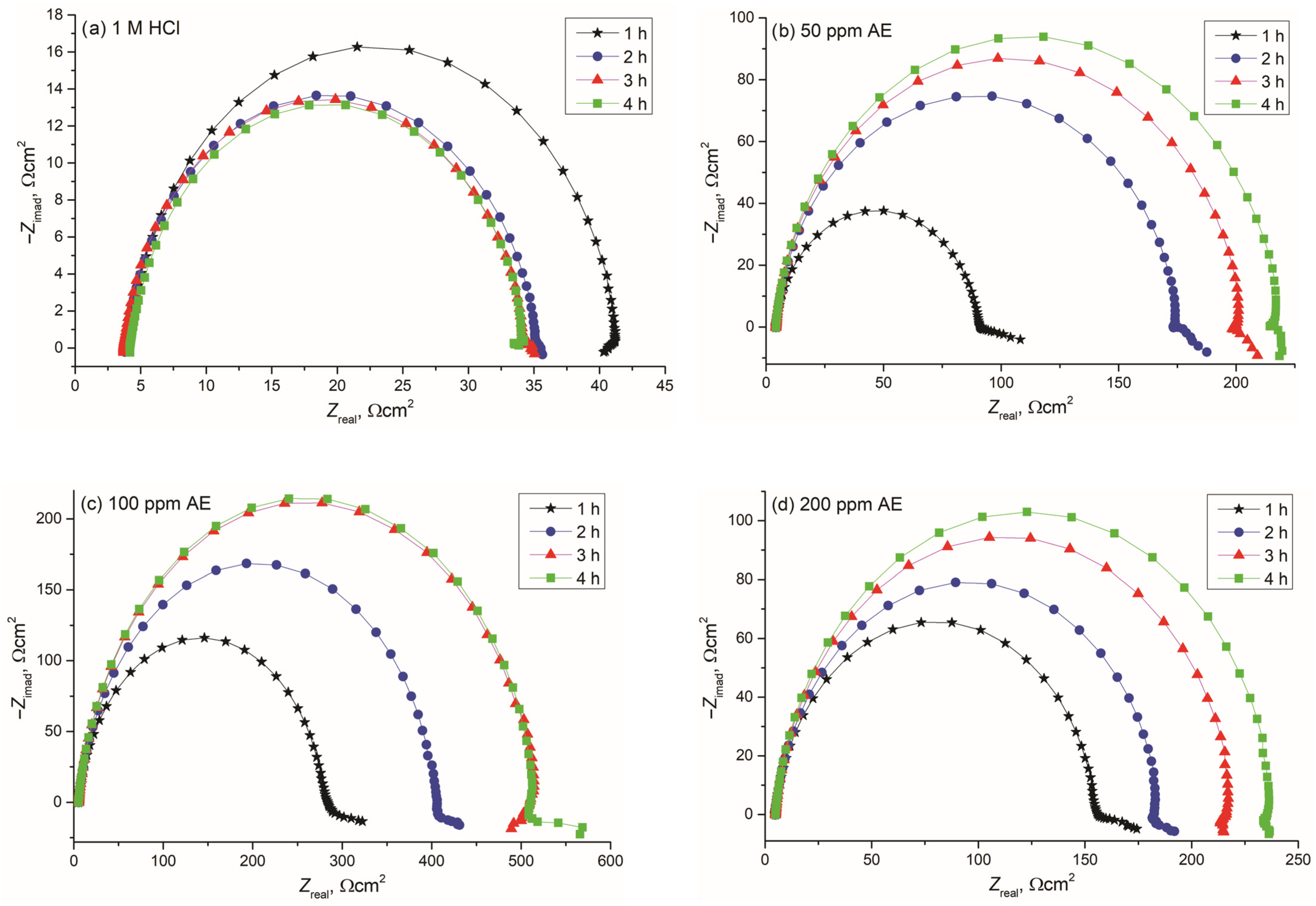
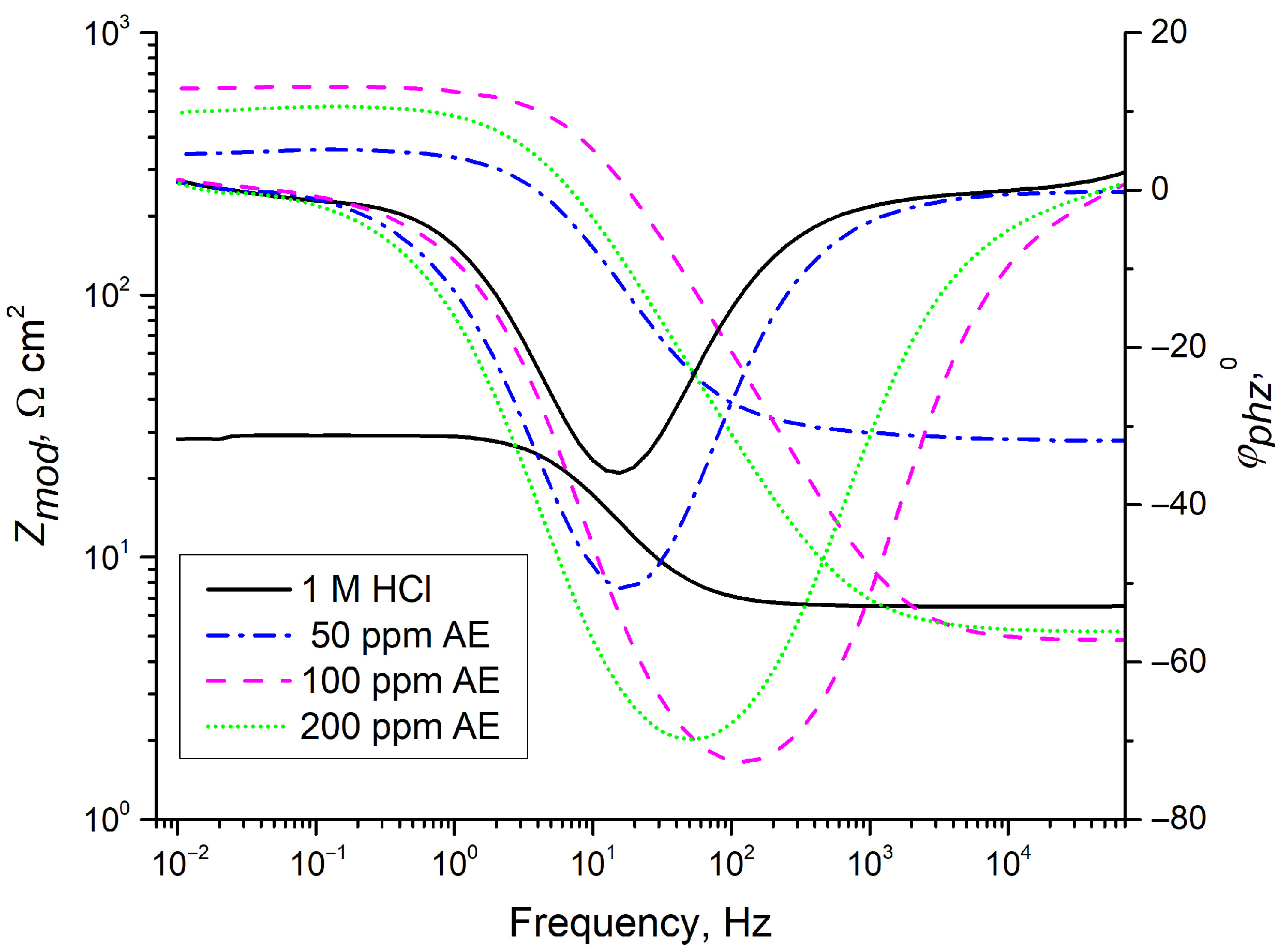
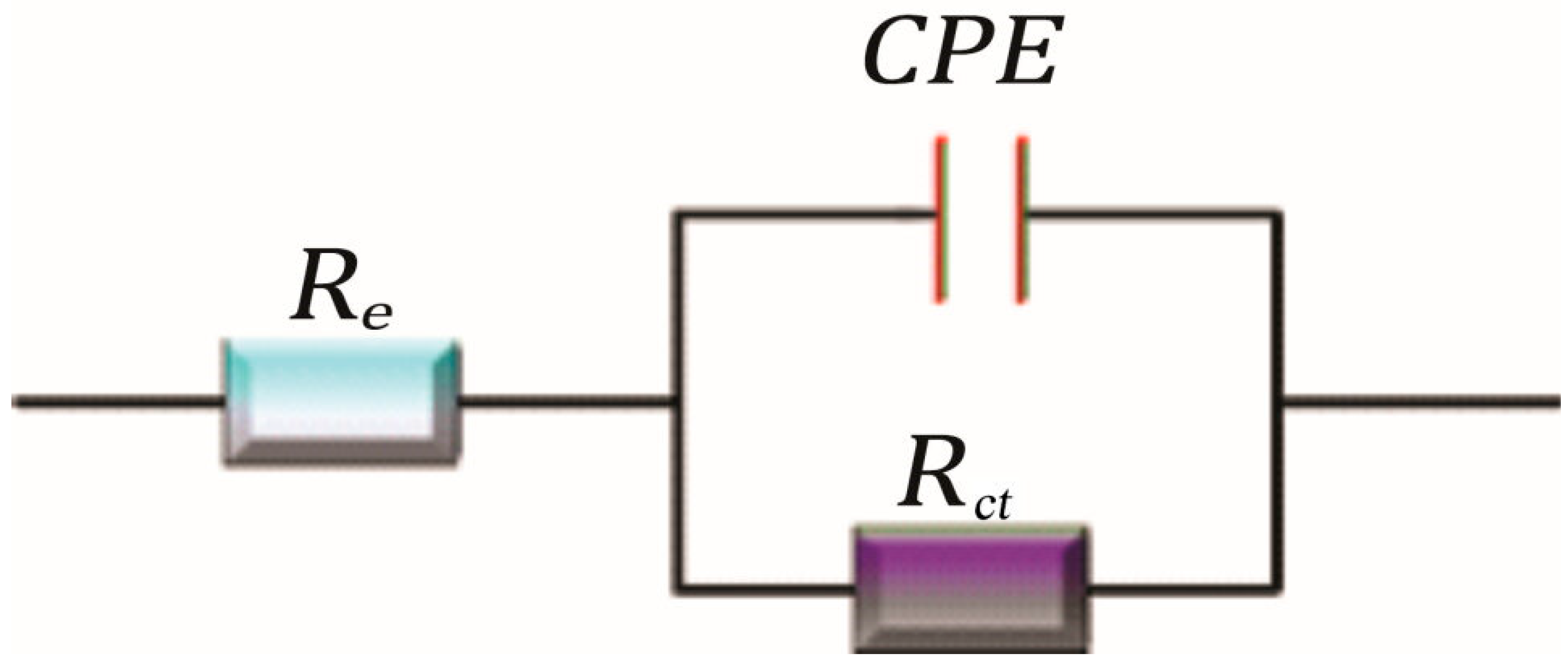
EIS was employed to analyze the inhibitor’s effect over different exposure times (1, 2, 3, 4, or 24 h). Nyquist and Bode diagrams (Figure 6 and Figure 7) were obtained for different concentrations of aronia pomace extract. The EIS spectra exhibit a single time constant, suggesting that the addition of aronia pomace extract does not alter the corrosion mechanism, and the corrosion process remains controlled by charge transfer kinetics. The impedance data were modeled using the equivalent electric circuit (EEC) in Figure 8. The elements of EEC are described in the paper [44].
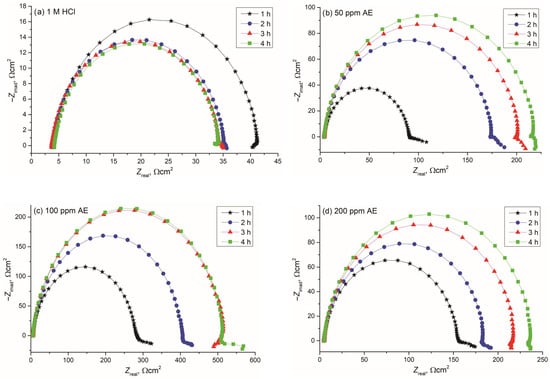
Figure 6.
Nyquist diagrams (dependence of imaginary, −Zimag, on the real impedance component, Zreal) for carbon steel during 1–4 h of immersion in 1 M HCl solution containing: (a) 0 ppm, (b) 50 ppm, (c) 100 ppm, and (d) 200 ppm of AE (aronia pomace extract). The data are expressed as mean values of four parallel measurements (n = 3).
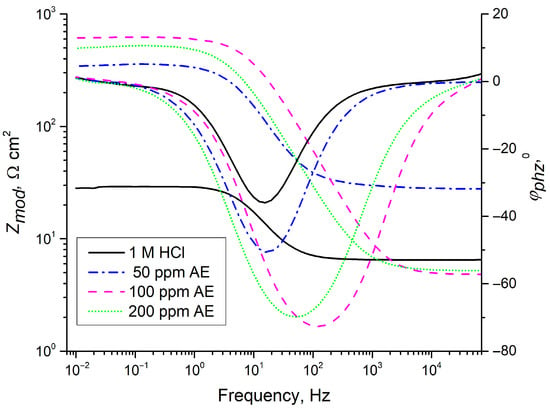
Figure 7.
Bode diagrams (dependence of impedance modulus, Zmod, and phase angle, φ, on the frequency) for carbon steel after 24 h of immersion in 1 M HCl with different concentrations of AE (aronia pomace extract). The data are expressed as mean values of four parallel measurements (n = 3).
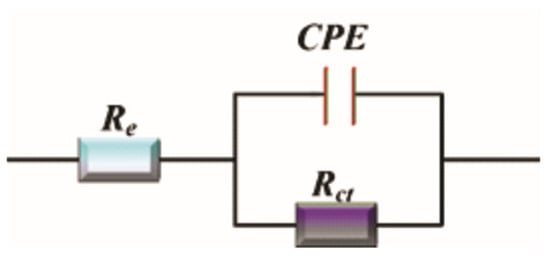
Figure 8.
One-time constant EEC used for modeling the impedance data.
Analysis of the results presented in Figure 6 shows that the diameter of Nyquist plots is substantially larger for carbon steel samples treated with aronia pomace extract compared to the ones in inhibitor-free HCl. This observation clearly signifies a reduced corrosion rate, which can be explained by the adsorption of the aronia extract’s bioactive compounds on the reaction sites of the carbon steel surface. The differences in the diameters of the Nyquist plots are also observed between carbon steel samples treated with varying concentrations of aronia pomace extract. Namely, increasing the inhibitor concentration from 50 to 100 ppm (Figure 6b,c) leads to a corresponding enlargement in the diameter of the impedance semicircles, which is attributed to the enhanced adsorption of organic bioactive compounds onto the metal surface, resulting in improved surface coverage. However, at an extract’s concentration of 200 ppm (Figure 6d), a decrease in the diameter is observed, a commonly reported behavior for green inhibitors of higher concentrations [45,46]. From an economic perspective, optimizing inhibitor concentration is crucial, as an effective inhibitor should demonstrate significant performance at minimal concentrations. Furthermore, the effect of immersion time on the diameter of Nyquist semicircles should not be neglected. In the case of the carbon steel samples treated with an inhibitor concentration of 50 or 200 ppm, it continues to increase with prolonged sample immersion of up to 4 h, indicating the formation of a more compact and protective inhibitory film on the metal surface.
A significant enhancement in inhibitor efficiency with an extended sample immersion time to 24 h is visible from the Bode plots in Figure 7. The magnitude of impedance Zmod at 10 mHz for inhibited samples is over ten times higher than when immersed in 1 M HCl solution. This enhancement further corroborates the effectiveness of aronia pomace extract in improving metal corrosion resistance. Moreover, the observed increase in the phase angle (φphz) and its broadening for the inhibited samples, relative to the uninhibited one (1 M HCl), provide additional evidence for the formation of a protective organic film on the steel substrate.
The inhibition efficiency () of aronia pomace extract of various concentrations was calculated using Equation (6) [47]:
where and are the charge transfer resistances in non-inhibited aggressive solution and in solution with aronia extract, respectively, as determined by fitting the experimental data to the EEC (Figure 8).
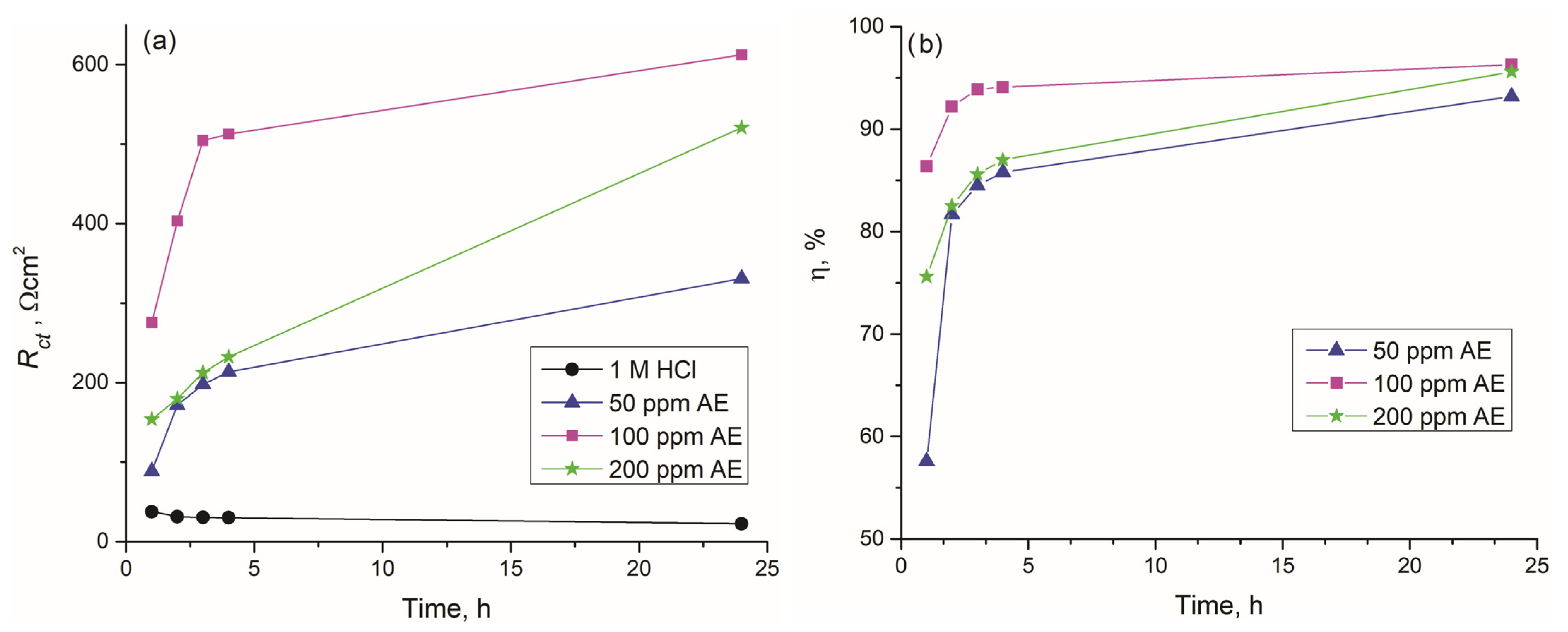
The variation in Rct of all samples over exposure time to the corrosive agent is presented in Figure 9a. The uninhibited sample maintained consistently low Rct values throughout the entire examination period. In contrast, the Rct of samples immersed in solutions containing 50, 100, or 200 ppm aronia pomace extract as an inhibitor increased progressively over time. Regardless of immersion time, the highest Rct values were detected for the solution containing 100 ppm of aronia pomace extract, reaching 275.7, 403.4, 504.4, 512.4, and 612.4 Ωcm2 after 1, 2, 3, 4, and 24 h, respectively. In addition to Rct, the inhibition efficiency (η) of aronia pomace extract is another time-dependent parameter (Figure 9b). Remarkably, after the second hour, all concentrations of aronia pomace extract demonstrated η exceeding 80%, with performance improving over time. The maximum η of 96.3% was achieved with 100 ppm aronia pomace extract after 24 h of immersion.
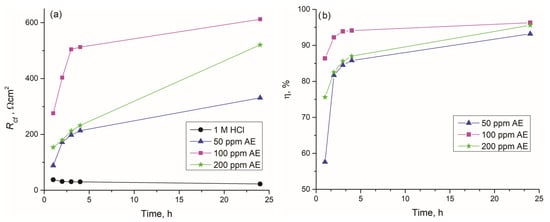
Figure 9.
The effect of immersion time on: (a) charge transfer resistances (Rct) in the inhibited solutions, and (b) inhibitor efficiency () during immersion of carbon steel in 1 M HCl solutions with different concentrations of aronia pomace extract. The data are expressed as mean values of three parallel measurements (n = 3).
3.4.2. Polarization Measurements
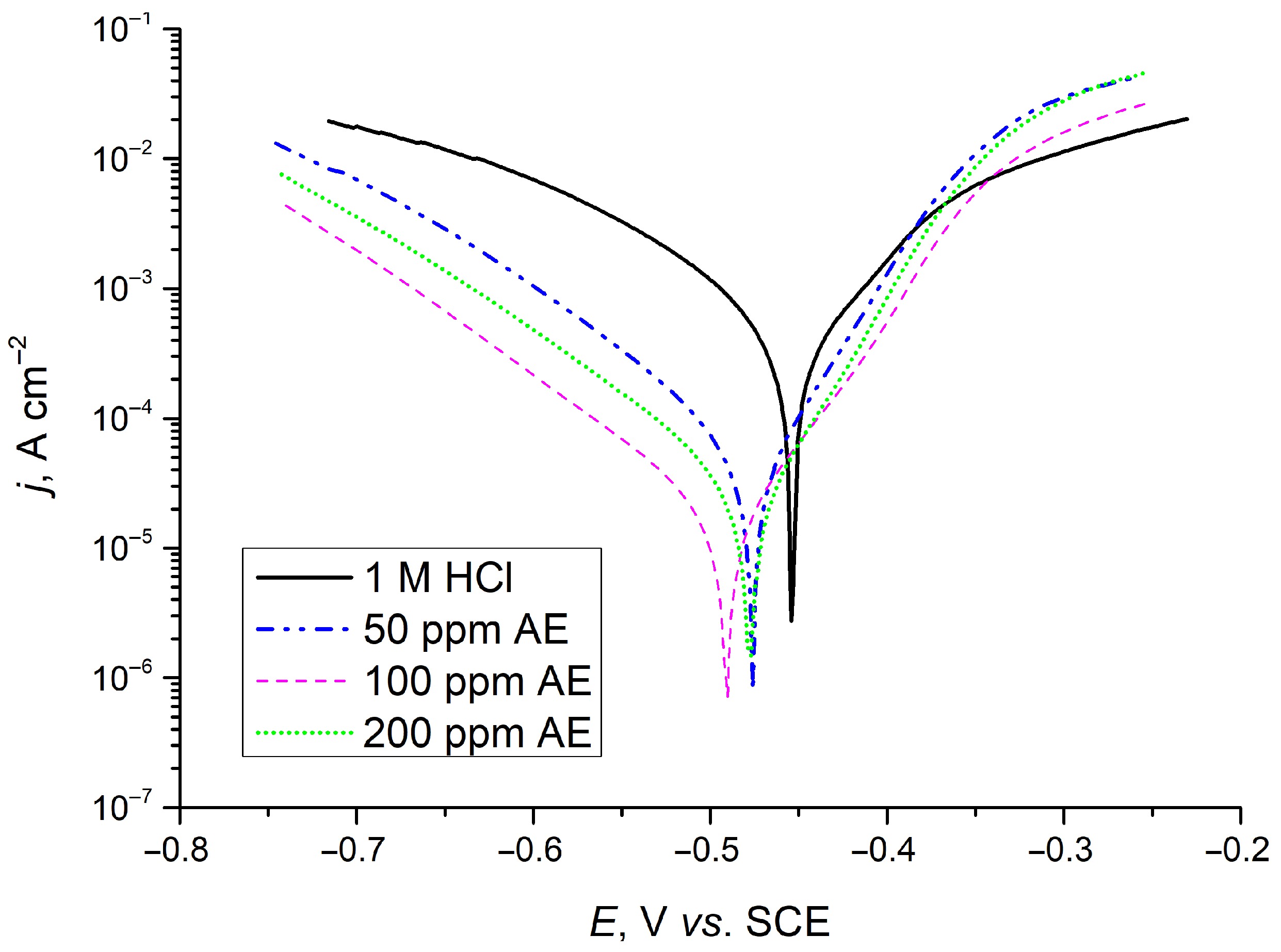
Figure 10 presents the Tafel polarization curves for carbon steel samples immersed for 24 h in solution with 0 ppm, 50 ppm, 100 ppm, and 200 ppm aronia extract. The polarization curves reveal that the introduction of aronia pomace extract into the corrosive environment (1 M HCl) leads to a notable reduction in both cathodic and anodic current densities with respect to the sample in 1 M HCl. This shift in current density is the most prominent in the case of 100 ppm aronia pomace extract, indicating that the examined green inhibitor effectively reduces the corrosion rate of carbon steel after 24 h of exposure. Additionally, the observed behavior suggests the mixed behavior of the aronia corrosion inhibitor, influencing both the anodic and cathodic reaction processes.
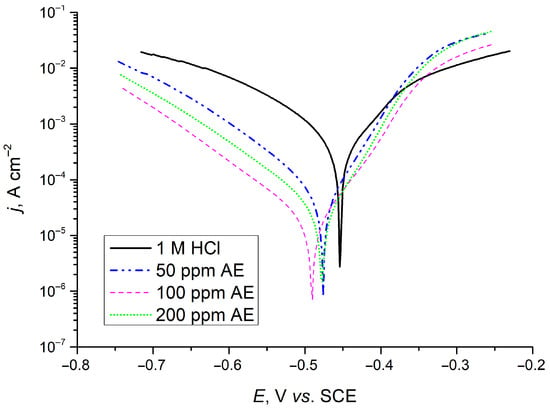
Figure 10.
Polarization plots for carbon steel after 24 h of immersion in inhibitor-free solution (1 M HCl) and 1 M HCl solution containing 50, 100 or 200 ppm of AE (aronia pomace extract). The data are expressed as mean values of three parallel measurements (n = 3).
Table 5 presents key electrochemical parameters obtained from the Tafel curves’ extrapolation, and the inhibition efficiency (η) of aronia pomace extract of different concentrations after 24 h of immersion. The η was calculated using Equation (7) [48]:
where jcorr(o) and jcorr(i) are the corrosion current densities in the absence and presence of the inhibitor, respectively.
η (%) = (1 − (jcorr(i)/jcorr(o)) · 100

Table 5.
Electrochemical parameters (corrosion potential and corrosion current density) derived from the Tafel polarization plots for carbon steel after 24 h immersion in different solutions.
Table 5 indicates that the differences in Ecorr between the uninhibited and inhibited metal samples are less than ± 85 mV after 24 h of immersion, supporting the conclusion of a mixed inhibition mechanism [49]. However, the greater suppression of the cathodic corrosion reaction compared to the anodic reaction, along with the shift in corrosion potential towards more negative (cathodic) values relative to the uninhibited steel sample, suggests that aronia pomace extract primarily acts as a mixed-type inhibitor with a dominant influence on the cathodic reaction. The presence of adsorbed aronia active components on the steel surface significantly reduces jcorr by 12.6 to 30.7 times for metal samples exposed to various inhibitor concentrations (Table 5). The lowest jcorr was achieved with 100 ppm aronia pomace. The similar shapes of the anodic and cathodic Tafel branches for inhibited and uninhibited samples mean that organic compounds from the aronia pomace extract adsorb onto the steel surface, effectively reducing the corrosion rate without altering the fundamental anodic and cathodic reaction mechanisms [50]. Comparing the results in Figure 9b and Table 5, it is clear that the η values from EIS measurements align with those from polarization measurements.
Compared to commercial synthetic inhibitors such as benzimidazole, 2-mercaptobenzoxazole, and 2,5-bis(2-pyridyl)-1,3,4-oxadiazole, which exhibit lower inhibition efficiencies at higher concentrations [51], the aronia pomace extract demonstrates an exceptionally favorable balance between efficiency and minimal concentration. Owing to its biodegradable and non-toxic nature, as well as its origin from renewable agro-industrial by-product, it represents a sustainable and environmentally benign alternative that simultaneously reduces environmental impact and production costs. These findings highlight the strong potential of aronia pomace extract as an effective green corrosion inhibitor and support further investigation into its inhibition mechanism, process optimization, and industrial scalability.
In summary, aronia pomace extract, rich in bioactive polyphenolic compounds, serves as an efficient green corrosion inhibitor for carbon steel in 1 M HCl. Its inhibition potential depends both on its concentration and exposure time, exhibiting the optimal aronia pomace extract concentration of 100 ppm, with a maximum efficiency of about 96% after 24 h. This performance surpasses that of many other plant-based green inhibitors reported in the literature [52], which typically require higher concentrations to achieve similar protection.
3.5. Advances Beyond Existing Methods and Connection of the Findings with Industrial Feasibility or Scalability
The main scientific and practical advances of the proposed approach, highlighting how each part of the study extends beyond what is typically reported in the literature and connecting the findings with their industrial feasibility or scalability, are summarized below:
- From extraction to novel products
Most prior work focuses either on improving extraction yields or on developing a single value-added product (e.g., food ingredients, purified anthocyanins, etc.). The present study moves beyond single-product outcomes by extracting bioactive compounds from aronia pomace and using them for developing three novel products: colored healthcare textiles, biomedical hydrogels, and green corrosion inhibitor. This approach demonstrates how a single low-cost by-product stream can feed multiple higher-value supply chains, improving resource efficiency and commercial feasibility.
- Choice and validation of a scalable and mild extraction method
We used ultrasound-assisted extraction under conditions that balance the extraction of bioactive compounds from aronia pomace, solvent safety, and scalability, and then performed the identification of colored bioactive compounds and the extract’s bioactivity. This coupling of an industry-compatible extraction protocol (using existing equipment) with extract characterization supports reproducible application and techno-economic reasoning that many analytical studies omit.
- One-step dyeing and functionalization of textiles with a focus on dyebath reusability
The manuscript introduces a one-step protocol that simultaneously dyes and functionalizes fabrics of different chemical compositions using aronia pomace extract under moderate temperature and without adjusting dyebath pH. Unlike many reports that require mordanting, multi-stage finishing, or harsh chemicals, our protocol is simple, operates at modest temperature/time, and preserves the extract’s bioactivity on the fabric. Importantly, we tested dyebath reusability for up to three cycles and quantified the color difference between the dyed fabrics, practical metrics that directly address industrial sustainability and reduction in dyes and chemicals in the textile industry wastewaters.
- Development and characterization of starch/gelatin hydrogels functionalized with aronia pomace extract with tunable properties and controlled release
The starch/gelatin hydrogels synthesis route uses abundant biopolymers and a non-toxic crosslinker suitable for biomedical applications, thus supporting easy scale-up and regulatory acceptance. The hydrogels show tunable compression modulus and equilibrium swelling, maintaining outstanding antioxidant activity and high antibacterial efficacy, and importantly, they sustainably control the release of the aronia pomace extract’s bioactive compounds over 24 h. Combining low-cost natural polymers, a green crosslinker, and functionalization with aronia pomace extract to achieve preclinical relevant hydrogels represents a meaningful advance over studies that only screen extracts or report only characterized scaffolds. The work also introduces an evaluation of the hydrogels’ quality for wound dressing suitability.
- Electrochemical demonstration of a green corrosion inhibitor
We demonstrate that aronia pomace extract acts as an effective green inhibitor for carbon steel in 1 M HCl, with optimal performance at 100 ppm. Compared to conventional synthetic inhibitors, aronia pomace extract exhibits superior efficiency at lower concentrations, combining technical effectiveness with economic benefits, making it a scalable and sustainable alternative for industrial applications.
3.6. Study Limitations
Variations in the chemical composition of Aronia melanocarpa pomace due to cultivation, processing, or storage conditions may influence extract reproducibility. Further optimization and standardization of raw material preprocessing are required to ensure consistent industrial-scale performance. The one-step dyeing and functionalization of fabrics effectively imparts coloration and bioactivity. However, the color fastness and long-term stability of antioxidant and antibacterial properties after multiple washing cycles should be tested. Additional in vivo testing is necessary to fully assess the functionalized hydrogels’ performance under physiological conditions. Future work should include evaluations of aronia pomace extract in different industrial media and under dynamic flow conditions to validate inhibitor stability and long-term protective performance in real systems.
4. Conclusions
This research highlights the potential of aronia pomace ethanol extract as a source of antioxidants and antibacterial agents, addressing both environmental concerns related to its disposal and the need for innovative, sustainable products. Using ultrasound-assisted extraction, we recovered the bioactive compounds from aronia pomace and made a pioneering exploration of alternative, sustainable technologies for product development. Specifically, the aronia pomace extract was used in:
- Sustainable textile dyeing and functionalization, wherein cotton and viscose fabrics were found suitable for colored disposable bioactive textiles, including protective clothing, face masks, and healthcare and hygiene products, due to their strong antioxidant activity (>97% ABTS, >76% DPPH) and antibacterial efficacy (>75% against E. coli, >80% against S. aureus).
- Development of novel starch/gelatin hydrogels for wound dressing, with compression modulus of 0.068–0.127 MPa and equilibrium swelling ratios of 3.33–3.73 g/g. Hydrogels functionalized with aronia pomace extract exhibited over 99% ABTS antioxidant activity and antibacterial efficacy exceeding 70% against E. coli and 97% against S. aureus, along with controlled release of bioactive compounds, demonstrating suitability for wound care applications.
- Green corrosion inhibition, where the extract demonstrated inhibition efficiency above 96% for carbon steel at 100 ppm, predominantly retarding the cathodic reaction, confirming its effectiveness as a sustainable corrosion inhibitor.
If the juice industry becomes more aware of by-product valorization and further research is conducted on the feasibility of valorization techniques, manufacturers could benefit by selling their byproducts instead of incurring disposal costs, leading to cost savings that could allow them to reduce their prices.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/suschem6040046/s1, Figure S1: Appearance of twofold diluted aronia pomace extract; Figure S2: Chromatogram of twofold diluted aronia pomace extract recorded at 310 nm (black line) and 520 nm (blue line); Table S1: Statistical results of the determination of concentration of bioactive compounds in the aronia pomace extract (df = n1 + n2 − 2 = 4, n1 = n2 = 3), Table S2: Statistical results of the determination of compression modulus and equilibrium swelling ratio (df = n1 + n2 − 2 = 4, n1 = n2 = 3).
Author Contributions
V.U.: Methodology, Formal analysis, Investigation, Visualization, Writing—original draft preparation. A.S.: Methodology, Formal analysis, Investigation, Visualization, Writing—original draft preparation. M.Ć.: Formal analysis, Investigation, Review and editing. K.M.: Formal analysis, Investigation. J.L.: Formal analysis, Investigation, Visualization, Review and editing. J.B.: Review and editing. A.I.: Conceptualization, Methodology, Formal analysis, Investigation, Visualization, Writing—original draft preparation, Supervision. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the Ministry of Science, Technological Development and Innovation of the Republic of Serbia, grant numbers 451-03-136/2025-03/200287, 451-03-136/2025-03/200135, and 451-03-136/2025-03/200026.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data will be made available on request.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Sidor, A.; Gramza-Michałowska, A. Black chokeberry Aronia melanocarpa L.—A qualitative composition, phenolic profile and antioxidant potential. Molecules 2019, 24, 3710. [Google Scholar] [CrossRef]
- Lee, K.H.; Kim, S.; Lee, J.; Son, H.; Lee, J.U.; Park, C.; Yoo, H.Y. Improved recovery of antioxidants from aronia juice processing residue via optimization of extraction variables based on multi-prediction models. Sustain. Chem. Pharm. 2024, 39, 10156. [Google Scholar] [CrossRef]
- Melo, P.S.; Massarioli, A.P.; Denny, C.; dos Santos, L.F.; Franchin, M.; Pereira, G.E.; Vieira, T.M.; Rosalen, P.L.; de Alencar, S.M. Winery by-products: Extraction optimization, phenolic composition and cytotoxic evaluation to act as a new source of scavenging of reactive oxygen species. Food Chem. 2015, 181, 160–169. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.Z.; Ju, Y.; Bao, N.; Luo, Y.L.; Huang, L.L.; Cao, N.X.; Liu, M.Z.; Bo, J.N.; Zhang, S.; Yan, Y. Effects of polyphenol-rich Aronia melanocarpa pomace feeding on growth performance, biochemical profile, and meat quality in pigs at weaned and finishing stages. Livest. Sci. 2021, 252, 104674. [Google Scholar] [CrossRef]
- Petreska Stanoeva, J.; Damjanovski, V.; Cichna-Markl, M.; Stefova, M. Anthocyanin fingerprinting as an authentication testing tool for blueberry, aronia, and pomegranate juices. Eur. Food Res. Technol. 2024, 250, 751–762. [Google Scholar] [CrossRef]
- Petrov Ivanković, A.; Ćorović, M.; Milivojević, A.; Simović, M.; Banjanac, K.; Veljković, M.; Bezbradica, D. Berries pomace valorization: From waste to potent antioxidants and emerging skin prebiotics. Int. J. Fruit Sci. 2024, 2, 85–101. [Google Scholar] [CrossRef]
- Gao, N.; Sun, X.; Li, D.; Gong, E.; Tian, J.; Si, X.; Jiao, X.; Xing, J.; Wang, Y.; Meng, X.; et al. Optimization of anthocyanidins conversion using chokeberry pomace rich in polymeric proanthocyanidins and cellular antioxidant activity analysis. LWT 2020, 133, 109889. [Google Scholar] [CrossRef]
- Witczak, T.; Stępień, A.; Gumul, D.; Witczak, M.; Fiutak, G.; Zięba, T. The influence of the extrusion process on the nutritional composition, physical properties and storage stability of black chokeberry pomaces. Food Chem. 2021, 334, 127548. [Google Scholar] [CrossRef]
- Zhang, M.-Q.; Zhang, J.; Zhang, Y.-T.; Sun, J.-Y.; Prieto, M.A.; Simal-Gandara, J.; Putnik, P.; Li, N.-Y.; Liu, C. The link between the phenolic composition and the antioxidant activity in different small berries: A metabolomic approach. LWT 2023, 182, 114853. [Google Scholar] [CrossRef]
- Kitryte, V.; Kraujaliene, V.; Sulniute, V.; Pukalskas, A.; Venskutonis, P.R. Chokeberry pomace valorization into food ingredients by enzyme-assisted extraction: Process optimization and product characterization. Food Bioprod. Process. 2017, 105, 36–50. [Google Scholar] [CrossRef]
- Simić, V.M.; Rajković, K.M.; Stojičević, S.S.; Veličković, D.T.; Nikolić, N.C.; Lasić, M.L.; Karabegović, I.T. Optimization of microwave-assisted extraction of total polyphenolic compounds from chokeberries by response surface methodology and artificial neural network. Sep. Purif. Technol. 2016, 160, 89–97. [Google Scholar] [CrossRef]
- Andrade, T.A.; Hamerski, F.; Fetzer, D.E.L.; Roda-Serrat, M.C.; Corazza, M.L.; Norddahl, B.; Errico, M. Ultrasound-assisted pressurized liquid extraction of anthocyanins from Aronia melanocarpa pomace. Sep. Purif. Technol. 2021, 276, 119290. [Google Scholar] [CrossRef]
- Ivanovska, A.; Savić Gajić, I.; Mravik, Ž.; Reljić, M.; Ilić-Tomić, T.; Savić, I.; Luxbacher, T.; Lađarević, J. Transforming discarded walnut green husk into a resource of valuable compounds for colored bioactive textiles with a focus on circular economy concept. Dyes Pigments 2024, 231, 112406. [Google Scholar] [CrossRef]
- Jurendić, T.; Ščetar, M. Aronia melanocarpa products and by-products for health and nutrition: A review. Antioxidants 2021, 10, 1052. [Google Scholar] [CrossRef]
- Aprodu, I.; Chitescu, C.L.; Grigore-Gurgu, L.; Dumitrașcu, L. Investigation of the antioxidant and antimicrobial properties of ultrasound-assisted extracted phenolics from Aronia melanocarpa pomace. Appl. Sci. 2025, 15, 7070. [Google Scholar] [CrossRef]
- Winkler, D.A.; Breedon, M.; White, P.; Hughes, A.E.; Sapper, E.D.; Cole, I. Using high throughput experimental data and in silico models to discover alternatives to toxic chromate corrosion inhibitors. Corros. Sci. 2016, 106, 229–235. [Google Scholar] [CrossRef]
- Petrov Ivanković, A.; Milivojević, A.; Ćorović, M.; Simović, M.; Banjanac, K.; Jansen, P.; Vukoičić, A.; van den Bogaard, E.; Bezbradica, D. In Vitro evaluation of enzymatically derived blackcurrant extract as prebiotic cosmetic ingredient: Extraction conditions optimization and effect on cutaneous microbiota representatives. Chem. Biol. Technol. Agric. 2023, 10, 125. [Google Scholar] [CrossRef]
- ASTM E2149-20; Standard Test Method for Determining the Antimicrobial Activity of Antimicrobial Agents Under Dynamic Contact Conditions. ASTM International: West Conshohocken, PA, USA, 2020.
- AATCC 100-2019; Antibacterial Finishes on Textile Materials. AATCC: Research Triangle Park, NC, USA, 2019.
- Glaser, T.K.; Plohl, O.; Vesel, A.; Ajdnik, U.; Ulrih, N.P.; Hrnčič, M.K.; Bren, U.; Fras Zemljič, L. Functionalization of polyethylene (PE) and polypropylene (PP) material using chitosan nanoparticles with incorporated resveratrol as potential active packaging. Materials 2019, 12, 2118. [Google Scholar] [CrossRef]
- Hong, K.H. Effect of biomordanting with Aronia melanocarpa leaf extract on coloring and functionalizing of wool and cotton fabrics dyed with A. melanocarpa fruit extract. Polym. Bull. 2024, 81, 9235–9251. [Google Scholar] [CrossRef]
- Ugrinović, V.; Marković, M.; Božić, B.; Panić, V.; Veljović, Đ. Poly(methacrylic acid) hydrogels crosslinked by poly(ethylene glycol) diacrylate as pH-responsive systems for drug delivery applications. Hem. Ind. 2023, 77, 235–249. [Google Scholar] [CrossRef]
- Zielińska, A.; Siudem, P.; Paradowska, K.; Gralec, M.; Kaźmierski, S.; Wawer, I. Aronia melanocarpa fruits as a rich dietary source of chlorogenic acids and anthocyanins: 1H-NMR, HPLC-DAD, and chemometric studies. Molecules 2020, 25, 3234. [Google Scholar] [CrossRef]
- Wang, L.; Pan, X.; Jiang, L.; Chu, Y.; Gao, S.; Jiang, X.; Zhang, Y.; Chen, Y.; Luo, S.; Peng, C. The biological activity mechanism of chlorogenic acid and its applications in food industry: A review. Front Nutr. 2022, 9, 943911. [Google Scholar] [CrossRef] [PubMed]
- Deng, H.; Zhu, J.; Tong, Y.; Kong, Y.; Tan, C.; Wang, M.; Wan, M.; Meng, X. Antibacterial characteristics and mechanisms of action of Aronia melanocarpa anthocyanins against Escherichia coli. LWT 2021, 150, 112018. [Google Scholar] [CrossRef]
- Pavun, L.; Spasojević, D.; Ivanovska, A.; Lađarević, J.; Milenković, M.; Uskoković-Marković, S. Characterization of tea water extracts and their utilization for dyeing and functionalization of fabrics of different chemical compositions. Maced. J. Chem. Chem. Eng. 2023, 42, 263–273. [Google Scholar] [CrossRef]
- Venil, C.K.; Velmurugan, P.; Dufossé, L.; Renuka Devi, P.; Veera Ravi, A. Fungal pigments: Potential coloring compounds for wide ranging applications in textile dyeing. J. Fungi. 2020, 6, 68. [Google Scholar] [CrossRef]
- Srivastava, P.; Ramesh, M.; Kaushik, P.; Kumari, A.; Aggarwal, S. Pyocyanin pigment from Pseudomonas species: Source of a dye and antimicrobial textile finish—A review. Proc. Indian Natl. Sci. Acad. 2022, 88, 542–550. [Google Scholar] [CrossRef]
- Hamieau, M.; Loulergue, P.; Szydłowska-Czerniak, A. A green solvent extraction of antioxidants from herbs and agro-food wastes: Optimization and capacity determination. Appl. Sci. 2024, 14, 2936. [Google Scholar] [CrossRef]
- Platzer, M.; Kiese, S.; Tybussek, T.; Herfellner, T.; Schneider, F.; Schweiggert-Weisz, U.; Eisner, P. Radical scavenging mechanisms of phenolic compounds: A quantitative structure-property relationship (QSPR) study. Front. Nutr. 2022, 9, 882458. [Google Scholar] [CrossRef]
- Qamruzzaman, M.; Ahmed, F.; Mondal, M.I.H. An overview on starch-based sustainable hydrogels: Potential applications and aspects. J. Polym. Environ. 2022, 30, 19–50. [Google Scholar] [CrossRef]
- Sisso, A.M.; Boit, M.O.; De Forest, C.A. Self-healing injectable gelatin hydrogels for localized therapeutic cell delivery. J. Biomed. Mater. Res. A 2020, 108, 1112–1121. [Google Scholar] [CrossRef]
- Lipatova, I.M.; Yusova, A.A. Effect of mechanical activation on starch crosslinking with citric acid. Int. J. Biol. Macromol. 2021, 185, 688–695. [Google Scholar] [CrossRef]
- Reddy, N.; Yang, Y. Citric acid cross-linking of starch films. Food Chem. 2010, 118, 702–711. [Google Scholar] [CrossRef]
- Mallick, S.P.; Sagiri, S.S.; Singh, V.K.; Pal, K.; Pradhan, D.K.; Bhattacharya, M.K. Effect of processed starches on the properties of gelatin-based physical hydrogels: Characterization, in vitro drug release and antimicrobial studies. Polym. Plast. Technol. Eng. 2014, 53, 700–715. [Google Scholar] [CrossRef]
- Chhabra, R.; Peshattiwar, V.; Pant, T.; Deshpande, A.; Modi, D.; Sathaye, S.; Tibrewala, A.; Dyawanapelly, S.; Jain, R.; Dandekar, P. In Vivo studies of 3D starch-gelatin scaffolds for full-thickness wound healing. ACS Appl. Bio Mater. 2020, 18, 2920–2929. [Google Scholar] [CrossRef] [PubMed]
- Derkach, S.R.; Voron’ko, N.G.; Sokolan, N.I.; Kolotova, D.S.; Kuchina, Y.A. Interactions between gelatin and sodium alginate: UV and FTIR studies. J. Disper. Sci. Technol. 2019, 41, 690–698. [Google Scholar] [CrossRef]
- Zhang, H.J.; Sun, T.L.; Zhang, A.K.; Ikura, Y.; Nakajima, T.; Nonoyama, T.; Kurokawa, T.; Ito, O.; Ishitobi, H.; Gong, J.P. Tough physical double-network hydrogels based on amphiphilic triblock copolymers. Adv. Mater. 2016, 28, 4884–4890. [Google Scholar] [CrossRef] [PubMed]
- Thang, N.H.; Chien, T.B.; Cuong, D.X. Polymer-based hydrogels applied in drug delivery: An overview. Gels 2023, 9, 523. [Google Scholar] [CrossRef]
- Liu, J.; Chinga-Carrasco, G.; Cheng, F. Hemicellulose-reinforced nanocellulose hydrogels for wound healing application. Cellulose 2016, 23, 3129–3143. [Google Scholar] [CrossRef]
- Wang, G.; Yang, F.; Zhou, W.; Xiao, N.; Luo, M.; Tang, Z. The initiation of oxidative stress and therapeutic strategies in wound healing. Biomed. Pharmacother. 2023, 157, 114004. [Google Scholar] [CrossRef]
- Petkovska, J.; Geskovski, N.; Marković, D.; Dimova, V.; Mirakovski, D.; Radetić, M.; Jordanov, I. Chitosan-pectin multilayer coating with anthocyanin grape dye as pH indicating wound dressing: Synthesis and characterization. Carbohydr. Polym. Technol. Appl. 2024, 7, 100438. [Google Scholar] [CrossRef]
- Ivanovska, A.; Milenković, J.; Lađarević, J.; Mihajlovski, K.; Dojčinović, B.; Ugrinović, V.; Škaro Bogojević, S.; Kostić, M. Harnessing the power of green and rooibos tea aqueous extracts for obtaining colored bioactive cotton and cotton/flax fabrics intended for disposable and reusable medical textiles. Cellulose 2024, 31, 9523–9542. [Google Scholar] [CrossRef]
- Wang, Q.; Zhou, X.; Sun, X.; Zhang, Q.; Wang, R.; Zhao, J.; Aslam, R.; Sun, Y.; Yan, Y.; Li, X. Seaweed extract as green corrosion inhibitor for carbon steel in hydrochloric acid solution. Colloid. Surf. A 2020, 700, 134751. [Google Scholar] [CrossRef]
- Guo, L.; Tan, B.; Li, W.; Li, Q.; Zheng, X.; Obot, I.B. Banana leaves water extracts as inhibitor for X70 steel corrosion in HCl medium. J. Mol. Liq. 2021, 327, 114828. [Google Scholar] [CrossRef]
- Shahini, M.H.; Ramezanzadeh, M.; Ramezanzadeh, B.; Bahlakeh, G. The role of ethanolic extract of Stachys byzantina’s leaves for effective decreasing the mild-steel (MS) degradation in the acidic solution; coupled theoretical/experimental assessments. J. Mol. Liq. 2021, 329, 115571. [Google Scholar] [CrossRef]
- El Azzouzi, M.; Azzaoui, K.; Warad, I.; Hammouti, B.; Shityakov, S.; Sabbahi, R.; Saoiabi, S.; Youssoufi, M.H.; Akartasse, N.; Jodeh, S.; et al. Moroccan, Mauritania, and senegalese gum Arabic variants as green corrosion inhibitors for mild steel in HCl: Weight loss, electrochemical, AFM and XPS studies. J. Mol. Liq. 2022, 347, 118354. [Google Scholar] [CrossRef]
- Zhang, Q.H.; Yang, X.B.; Zhang, Y.; Xu, N. Artemisia verlotiorum extract as green corrosion inhibitor for enhanced corrosion resistance of mild steel in acidic solution. J. Mol. Liq. 2025, 419, 126811. [Google Scholar] [CrossRef]
- Fernandes, C.M.; Ferreira Fagundes, T.d.S.; dos Santos, N.E.; Rocha, T.S.d.M.; Garrett, R.; Borges, R.M.; Muricy, G.; Valverde, A.L.; Ponzio, E.A. Ircinia strobilina crude extract as corrosion inhibitor for mild steel in acid medium. Electrochim. Acta 2019, 312, 137–148. [Google Scholar] [CrossRef]
- Cherrad, S.; Alrashdi, A.A.; Lee, H.S.; El Aoufir, Y.; Lgaz, H.; Satrani, B.; Ghanmi, M.; Aouane, E.M.; Chaouch, A. Cupressus arizonica fruit essential oil: A novel green inhibitor for acid corrosion of carbon steel. Arab. J. Chem. 2022, 15, 103849. [Google Scholar] [CrossRef]
- Alibakhshi, E.; Ramezanzadeh, M.; Haddadi, S.A.; Bahlakeh, G.; Ramezanzadeh, B.; Mahdavian, M. Persian liquorice extract as a highly efficient sustainable corrosion inhibitor for mild steel in sodium chloride solution. J. Clean. Prod. 2019, 210, 660–672. [Google Scholar] [CrossRef]
- Li, Y.; Chen, Y.; Wang, C.; Li, Y.; Wu, Y. Exploring the potential of plant extracts as corrosion inhibitors: A comprehensive review. Prog. Org. Coat. 2025, 198, 108915. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).