Photoacoustic Imaging of Human Skin for Accurate Diagnosis and Treatment Guidance
Abstract
1. Introduction
2. Key Features of PAI
| OR-PAM | AR-PAM | PACT | |
|---|---|---|---|
| Depth | <1.5 mm [46,47,48] | ≤5 mm [49,50] | ≤40 mm [20] |
| Penetrated skin layers | Epidermis and partially dermis | Epidermis, dermis, and subcutaneous tissue | Epidermis, dermis, and subcutaneous tissue |
| Resolution | Lateral: 0.3–5 μm Axial: 15–30 μm [64,65] | Lateral: 20–80 μm Axial: 20–60 μm [66] | 30–400 μm [20,55] |
| Resolved features | Capillaries | Arterioles and venules | Arterioles, venules, and larger vessels |
| Frame rate | A few hertz [48,64,67] | Several tens of hertz [49,50] | Several tens or even hundreds of hertz [20,55] |
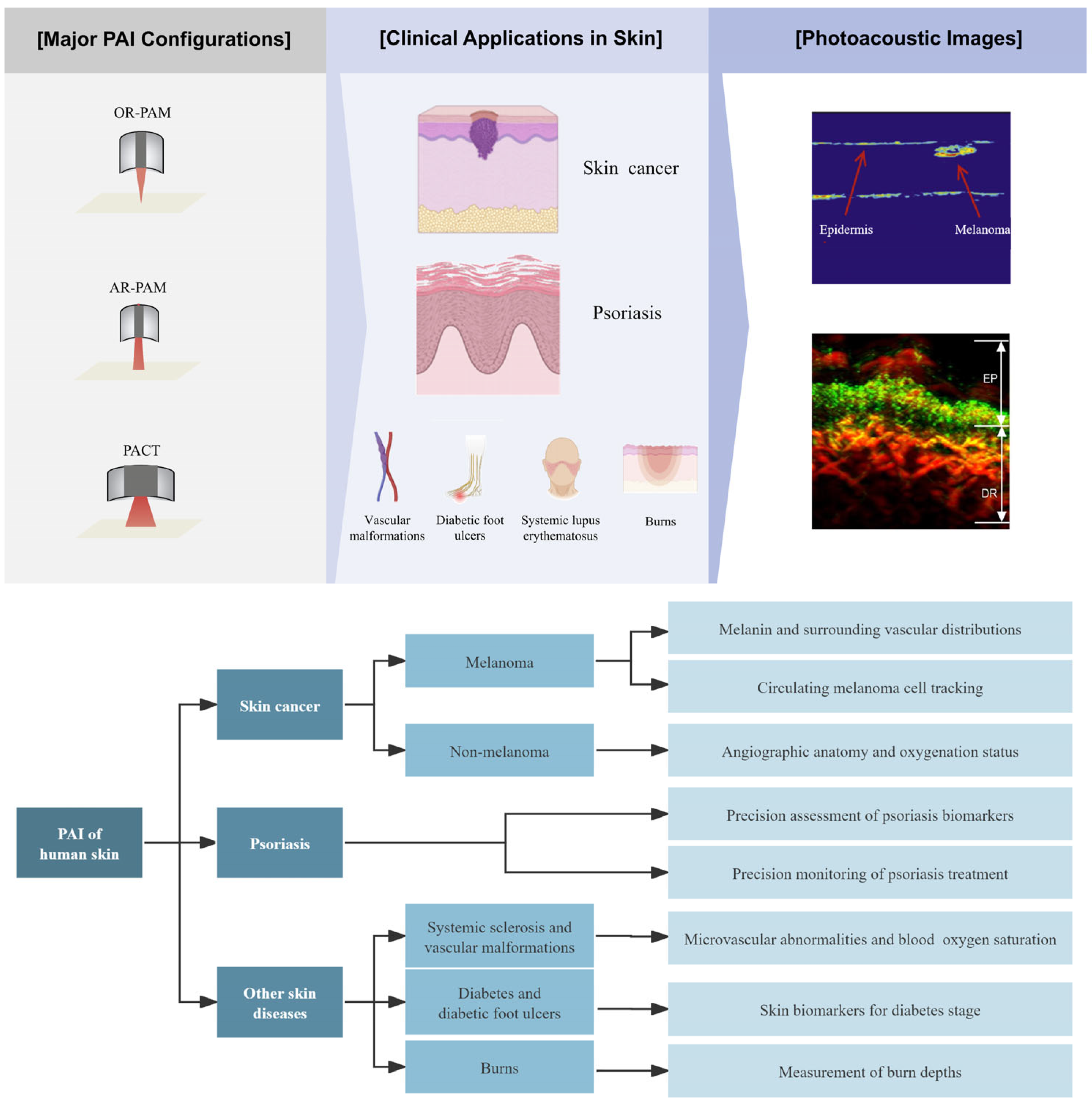
3. Clinical Applications in Skin Imaging
3.1. Skin Cancer Imaging
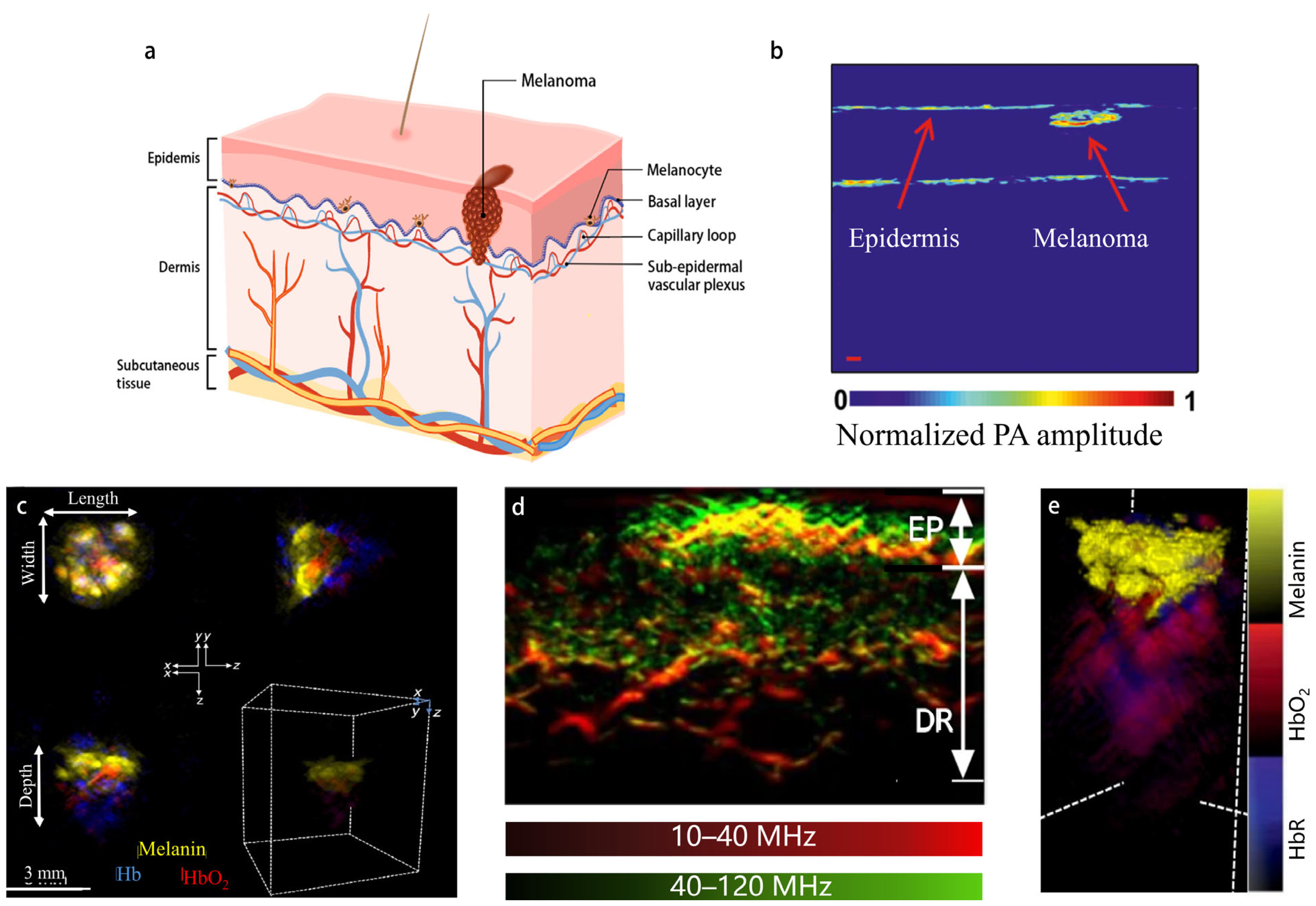
3.1.1. PAI of Melanoma
3.1.2. Detection and Treatment of Circulating Melanoma Cells
3.1.3. Non-Invasive Tumor Margin Imaging of Non-Melanoma
3.2. Psoriasis

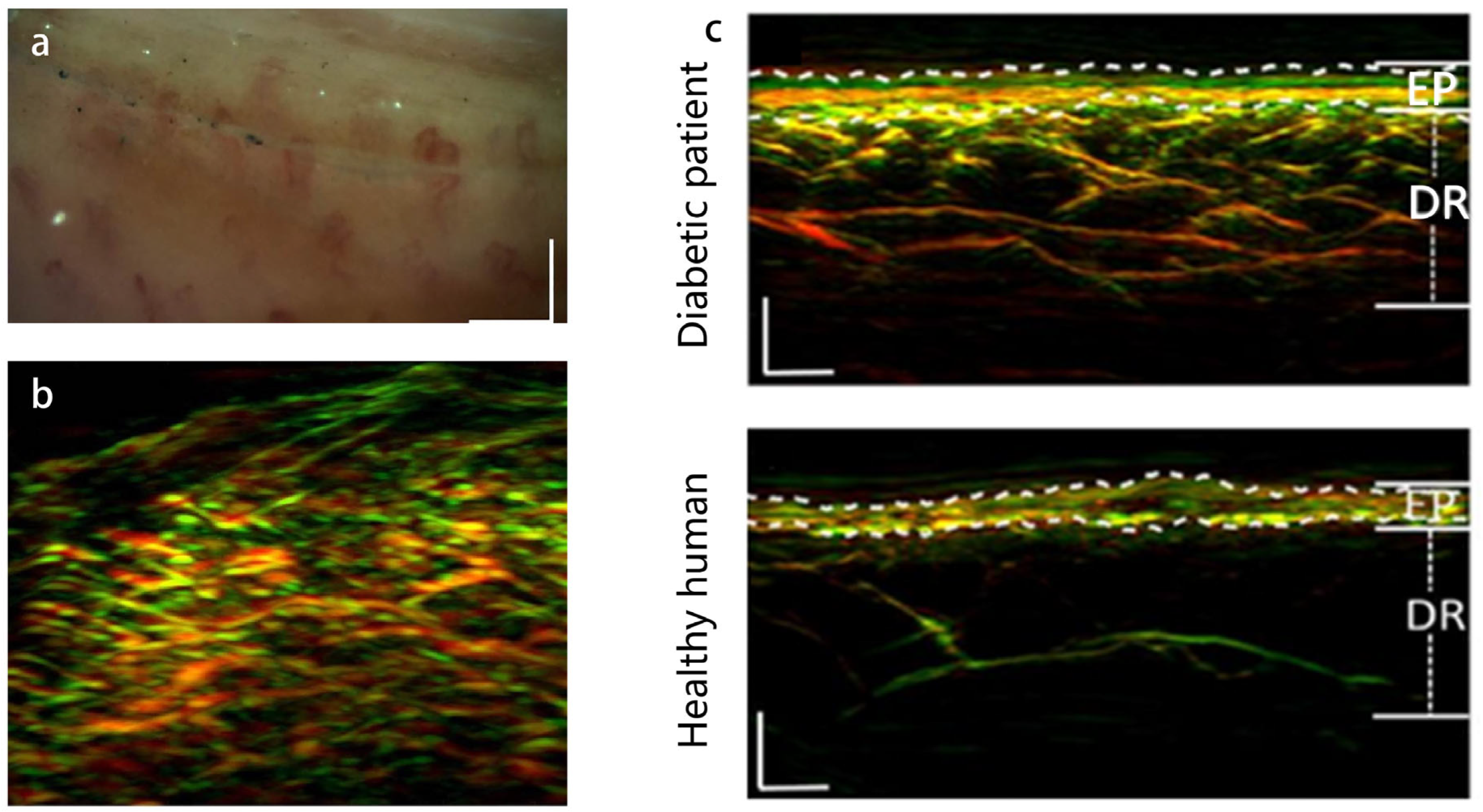
3.3. PAI of Some Other Skin Diseases
4. Conclusions and Perspective
Author Contributions
Funding
Conflicts of Interest
References
- Schneider, S.L.; Kohli, I.; Hamzavi, I.H.; Council, M.L.; Rossi, A.M.; Ozog, D.M. Emerging imaging technologies in dermatology. J. Am. Acad. Dermatol. 2019, 80, 1114–1120. [Google Scholar] [CrossRef]
- Marchetti, M.A.; Cowen, E.A.; Kurtansky, N.R.; Weber, J.; Dauscher, M.; DeFazio, J.; Deng, L.; Dusza, S.W.; Haliasos, H.; Halpern, A.C.; et al. Prospective validation of dermoscopy-based open-source artificial intelligence for melanoma diagnosis (PROVE-AI study). NPJ Digit. Med. 2023, 6, 127. [Google Scholar] [CrossRef]
- Alenezi, F.; Armghan, A.; Polat, K. A multi-stage melanoma recognition framework with deep residual neural network and hyperparameter optimization-based decision support in dermoscopy images. Expert Syst. Appl. 2023, 215, 119352. [Google Scholar] [CrossRef]
- Infante, V.H.; Maia Campos, P. Application of a Reflectance Confocal Microscopy Imaging Analysis Score for the Evaluation of Non-Melanogenic Changes in Male Photoaged Skin. Photochem. Photobiol. 2023, 99, 993–1002. [Google Scholar] [CrossRef]
- Perino, F.; Suarez, R.; Perez-Anker, J.; Carrera, C.; Rezze, G.G.; Primiero, C.A.; Alos, L.L.; Díaz, A.; Barreiro, A.; Puig, S.; et al. Concordance of in vivo reflectance confocal microscopy and horizontal-sectioning histology in skin tumours. Acad. Dermatol. Venereol. 2024, 38, 124–135. [Google Scholar] [CrossRef]
- Cinotti, E.; Brunetti, T.; Cartocci, A.; Tognetti, L.; Suppa, M.; Malvehy, J.; Perez-Anker, J.; Puig, S.; Perrot, J.L.; Rubegni, P. Diagnostic Accuracy of Line-Field Confocal Optical Coherence Tomography for the Diagnosis of Skin Carcinomas. Diagnostics 2023, 13, 361. [Google Scholar] [CrossRef]
- Kim, H.; Kang, D.; Seong, D.; Saleah, S.A.; Luna, J.A.; Kim, Y.; Kim, H.; Han, S.; Jeon, M.; Kim, J. Skin pore imaging using spectral-domain optical coherence tomography: A case report. Biomed. Eng. Lett. 2023, 13, 729–737. [Google Scholar] [CrossRef]
- Wortsman, X. Top applications of dermatologic ultrasonography that can modify management. Ultrasonography 2023, 42, 183–202. [Google Scholar] [CrossRef]
- Alex, A.; Chaney, E.J.; Žurauskas, M.; Criley, J.M.; Spillman, D.R., Jr.; Hutchison, P.B.; Li, J.; Marjanovic, M.; Frey, S.; Arp, Z.; et al. In vivo characterization of minipig skin as a model for dermatological research using multiphoton microscopy. Exp. Dermatol. 2020, 29, 953–960. [Google Scholar] [CrossRef]
- Marconi, A.; Quadri, M.; Farnetani, F.; Ciardo, S.; Palazzo, E.; Lotti, R.; Cesinaro, A.M.; Fabbiani, L.; Vaschieri, C.; Puviani, M.; et al. In Vivo Melanoma Cell Morphology Reflects Molecular Signature and Tumor Aggressiveness. J. Investig. Dermatol. 2022, 142, 2205–2216.e6. [Google Scholar] [CrossRef]
- Schmid-Wendtner, M.-H.; Dill-Müller, D. Ultrasound Technology in Dermatology. Semin. Cutan. Med. Surg. 2008, 27, 44–51. [Google Scholar] [CrossRef]
- Haedicke, K.; Agemy, L.; Omar, M.; Berezhnoi, A.; Roberts, S.; Longo-Machado, C.; Skubal, M.; Nagar, K.; Hsu, H.-T.; Kim, K.; et al. High-resolution optoacoustic imaging of tissue responses to vascular-targeted therapies. Nat. Biomed. Eng. 2020, 4, 286–297. [Google Scholar] [CrossRef]
- Li, Y.; Wong, T.T.W.; Shi, J.; Hsu, H.-C.; Wang, L.V. Multifocal photoacoustic microscopy using a single-element ultrasonic transducer through an ergodic relay. Light. Sci. Appl. 2020, 9, 135. [Google Scholar] [CrossRef]
- Wang, L.V.; Yao, J. A practical guide to photoacoustic tomography in the life sciences. Nat. Methods 2016, 13, 627–638. [Google Scholar] [CrossRef]
- Iskander-Rizk, S.; Visscher, M.; Moerman, A.M.; Korteland, S.-A.; Van Der Heiden, K.; Van Der Steen, A.F.W.; Van Soest, G. Micro Spectroscopic Photoacoustic (μsPA) imaging of advanced carotid atherosclerosis. Photoacoustics 2021, 22, 100261. [Google Scholar] [CrossRef]
- Iskander-Rizk, S.; Kruizinga, P.; Beurskens, R.; Springeling, G.; Mastik, F.; De Groot, N.M.S.; Knops, P.; Van Der Steen, A.F.W.; Van Soest, G. Real-time photoacoustic assessment of radiofrequency ablation lesion formation in the left atrium. Photoacoustics 2019, 16, 100150. [Google Scholar] [CrossRef]
- Li, W.; Sun, X.; Wang, Y.; Niu, G.; Chen, X.; Qian, Z.; Nie, L. In vivo quantitative photoacoustic microscopy of gold nanostar kinetics in mouse organs. Biomed. Opt. Express 2014, 5, 2679. [Google Scholar] [CrossRef]
- Lv, J.; Xu, Y.; Xu, L.; Nie, L. Quantitative Functional Evaluation of Liver Fibrosis in Mice with Dynamic Contrast-enhanced Photoacoustic Imaging. Radiology 2021, 300, 89–97. [Google Scholar] [CrossRef]
- Huang, G.; Lv, J.; He, Y.; Yang, J.; Zeng, L.; Nie, L. In vivo quantitative photoacoustic evaluation of the liver and kidney pathology in tyrosinemia. Photoacoustics 2022, 28, 100410. [Google Scholar] [CrossRef]
- Lin, L.; Hu, P.; Tong, X.; Na, S.; Cao, R.; Yuan, X.; Garrett, D.C.; Shi, J.; Maslov, K.; Wang, L.V. High-speed three-dimensional photoacoustic computed tomography for preclinical research and clinical translation. Nat. Commun. 2021, 12, 882. [Google Scholar] [CrossRef]
- Attia, A.B.E.; Balasundaram, G.; Moothanchery, M.; Dinish, U.S.; Bi, R.; Ntziachristos, V.; Olivo, M. A review of clinical photoacoustic imaging: Current and future trends. Photoacoustics 2019, 16, 100144. [Google Scholar] [CrossRef]
- Deán-Ben, X.L.; Fehm, T.F.; Ford, S.J.; Gottschalk, S.; Razansky, D. Spiral volumetric optoacoustic tomography visualizes multi-scale dynamics in mice. Light. Sci. Appl. 2017, 6, e16247. [Google Scholar] [CrossRef]
- Wang, L.V. Multiscale photoacoustic microscopy and computed tomography. Nat. Photonics 2009, 3, 503–509. [Google Scholar] [CrossRef]
- Wang, L.V.; Hu, S. Photoacoustic Tomography: In Vivo Imaging from Organelles to Organs. Science 2012, 335, 1458–1462. [Google Scholar] [CrossRef]
- Lin, L.; Wang, L.V. The emerging role of photoacoustic imaging in clinical oncology. Nat. Rev. Clin. Oncol. 2022, 19, 365–384. [Google Scholar] [CrossRef]
- Wang, Z.; Yang, F.; Zhang, W.; Xiong, K.; Yang, S. Towards in vivo photoacoustic human imaging: Shining a new light on clinical diagnostics. Fundam. Res. 2023; in press. [Google Scholar] [CrossRef]
- Rajendran, P.; Sharma, A.; Pramanik, M. Photoacoustic imaging aided with deep learning: A review. Biomed. Eng. Lett. 2022, 12, 155–173. [Google Scholar] [CrossRef]
- Liu, W.-W.; Li, P.-C. Photoacoustic imaging of cells in a three-dimensional microenvironment. J. Biomed. Sci. 2020, 27, 3. [Google Scholar] [CrossRef]
- Chen, Q.; Qin, W.; Qi, W.; Xi, L. Progress of clinical translation of handheld and semi-handheld photoacoustic imaging. Photoacoustics 2021, 22, 100264. [Google Scholar] [CrossRef]
- Steinberg, I.; Huland, D.M.; Vermesh, O.; Frostig, H.E.; Tummers, W.S.; Gambhir, S.S. Photoacoustic clinical imaging. Photoacoustics 2019, 14, 77–98. [Google Scholar] [CrossRef]
- Seong, M.; Chen, S.-L. Recent advances toward clinical applications of photoacoustic microscopy: A review. Sci. China Life Sci. 2020, 63, 1798–1812. [Google Scholar] [CrossRef]
- Hindelang, B.; Aguirre, J.; Schwarz, M.; Berezhnoi, A.; Eyerich, K.; Ntziachristos, V.; Biedermann, T.; Darsow, U. Non-invasive imaging in dermatology and the unique potential of raster-scan optoacoustic mesoscopy. J. Eur. Acad. Dermatol. Venereol. 2019, 33, 1051–1061. [Google Scholar] [CrossRef]
- Messas, T.; Messas, A.; Kroumpouzos, G. Optoacoustic imaging and potential applications of raster-scan optoacoustic mesoscopy in dermatology. Clin. Dermatol. 2022, 40, 85–92. [Google Scholar] [CrossRef]
- Von Knorring, T.; Mogensen, M. Photoacoustic tomography for assessment and quantification of cutaneous and metastatic malignant melanoma—A systematic review. Photodiagnosis Photodyn. Ther. 2021, 33, 102095. [Google Scholar] [CrossRef]
- Li, D.; Humayun, L.; Vienneau, E.; Vu, T.; Yao, J. Seeing through the Skin: Photoacoustic Tomography of Skin Vasculature and Beyond. JID Innov. 2021, 1, 100039. [Google Scholar] [CrossRef]
- Yao, J.; Wang, L.V. Photoacoustic microscopy: Photoacoustic microscopy. Laser Photonics Rev. 2013, 7, 758–778. [Google Scholar] [CrossRef]
- Xia, J.; Li, G.; Wang, L.; Nasiriavanaki, M.; Maslov, K.; Engelbach, J.A.; Garbow, J.R.; Wang, L.V. Wide-field two-dimensional multifocal optical-resolution photoacoustic-computed microscopy. Opt. Lett. 2013, 38, 5236. [Google Scholar] [CrossRef]
- Yao, J.; Song, L.; Wang, L.V. Photoacoustic Microscopy: Superdepth, superresolution, and superb contrast. IEEE Pulse 2015, 6, 34–37. [Google Scholar] [CrossRef]
- Strohm, E.M.; Berndl, E.S.L.; Kolios, M.C. High frequency label-free photoacoustic microscopy of single cells. Photoacoustics 2013, 1, 49–53. [Google Scholar] [CrossRef] [PubMed]
- Xu, G.; Meng, Z.-X.; Lin, J.D.; Yuan, J.; Carson, P.L.; Joshi, B.; Wang, X. The Functional Pitch of an Organ: Quantification of Tissue Texture with Photoacoustic Spectrum Analysis. Radiology 2014, 271, 248–254. [Google Scholar] [CrossRef]
- Aguirre, J.; Schwarz, M.; Soliman, D.; Buehler, A.; Omar, M.; Ntziachristos, V. Broadband mesoscopic optoacoustic tomography reveals skin layers. Opt. Lett. 2014, 39, 6297. [Google Scholar] [CrossRef] [PubMed]
- Jathoul, A.P.; Laufer, J.; Ogunlade, O.; Treeby, B.; Cox, B.; Zhang, E.; Johnson, P.; Pizzey, A.R.; Philip, B.; Marafioti, T.; et al. Deep in vivo photoacoustic imaging of mammalian tissues using a tyrosinase-based genetic reporter. Nat. Photonics 2015, 9, 239–246. [Google Scholar] [CrossRef]
- Buehler, A.; Deán-Ben, X.L.; Claussen, J.; Ntziachristos, V.; Razansky, D. Three-dimensional optoacoustic tomography at video rate. Opt. Express 2012, 20, 22712. [Google Scholar] [CrossRef] [PubMed]
- Merčep, E.; Deán-Ben, X.L.; Razansky, D. Imaging of blood flow and oxygen state with a multi-segment optoacoustic ultrasound array. Photoacoustics 2018, 10, 48–53. [Google Scholar] [CrossRef] [PubMed]
- Ivankovic, I.; Merčep, E.; Schmedt, C.-G.; Deán-Ben, X.L.; Razansky, D. Real-time Volumetric Assessment of the Human Carotid Artery: Handheld Multispectral Optoacoustic Tomography. Radiology 2019, 291, 45–50. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.-L.; Guo, L.J.; Wang, X. All-optical photoacoustic microscopy. Photoacoustics 2015, 3, 143–150. [Google Scholar] [CrossRef]
- Hu, S.; Maslov, K.; Wang, L.V. Second-generation optical-resolution photoacoustic microscopy with improved sensitivity and speed. Opt. Lett. 2011, 36, 1134. [Google Scholar] [CrossRef]
- Lin, L.; Zhang, P.; Xu, S.; Shi, J.; Li, L.; Yao, J.; Wang, L.; Zou, J.; Wang, L.V. Handheld optical-resolution photoacoustic microscopy. J. Biomed. Opt. 2016, 22, 041002. [Google Scholar] [CrossRef]
- Favazza, C.P.; Wang, L.V.; Jassim, O.W.; Cornelius, L.A. In vivo photoacoustic microscopy of human cutaneous microvasculature and a nevus. J. Biomed. Opt. 2011, 16, 016015. [Google Scholar] [CrossRef]
- Zhang, H.F.; Maslov, K.; Stoica, G.; Wang, L.V. Functional photoacoustic microscopy for high-resolution and noninvasive in vivo imaging. Nat. Biotechnol. 2006, 24, 848–851. [Google Scholar] [CrossRef] [PubMed]
- Qin, W.; Chen, Q.; Xi, L. A handheld microscope integrating photoacoustic microscopy and optical coherence tomography. Biomed. Opt. Express 2018, 9, 2205. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Shcherbakova, D.M.; Kurupassery, N.; Li, Y.; Zhou, Q.; Verkhusha, V.V.; Yao, J. Quad-mode functional and molecular photoacoustic microscopy. Sci. Rep. 2018, 8, 11123. [Google Scholar] [CrossRef]
- Zabihian, B.; Weingast, J.; Liu, M.; Zhang, E.; Beard, P.; Pehamberger, H.; Drexler, W.; Hermann, B. In vivo dual-modality photoacoustic and optical coherence tomography imaging of human dermatological pathologies. Biomed. Opt. Express 2015, 6, 3163. [Google Scholar] [CrossRef]
- Ford, S.J.; Bigliardi, P.L.; Sardella, T.C.P.; Urich, A.; Burton, N.C.; Kacprowicz, M.; Bigliardi, M.; Olivo, M.; Razansky, D. Structural and Functional Analysis of Intact Hair Follicles and Pilosebaceous Units by Volumetric Multispectral Optoacoustic Tomography. J. Investig. Dermatol. 2016, 136, 753–761. [Google Scholar] [CrossRef] [PubMed]
- Lin, L.; Hu, P.; Shi, J.; Appleton, C.M.; Maslov, K.; Li, L.; Zhang, R.; Wang, L.V. Single-breath-hold photoacoustic computed tomography of the breast. Nat. Commun. 2018, 9, 2352. [Google Scholar] [CrossRef] [PubMed]
- Diot, G.; Metz, S.; Noske, A.; Liapis, E.; Schroeder, B.; Ovsepian, S.V.; Meier, R.; Rummeny, E.; Ntziachristos, V. Multispectral Optoacoustic Tomography (MSOT) of Human Breast Cancer. Clin. Cancer Res. 2017, 23, 6912–6922. [Google Scholar] [CrossRef] [PubMed]
- Kothapalli, S.-R.; Sonn, G.A.; Choe, J.W.; Nikoozadeh, A.; Bhuyan, A.; Park, K.K.; Cristman, P.; Fan, R.; Moini, A.; Lee, B.C.; et al. Simultaneous transrectal ultrasound and photoacoustic human prostate imaging. Sci. Transl. Med. 2019, 11, eaav2169. [Google Scholar] [CrossRef]
- Gargiulo, S.; Albanese, S.; Mancini, M. State-of-the-Art Preclinical Photoacoustic Imaging in Oncology: Recent Advances in Cancer Theranostics. Contrast Media Mol. Imaging 2019, 2019, 5080267. [Google Scholar] [CrossRef] [PubMed]
- Deán-Ben, X.L.; Razansky, D. Optoacoustic imaging of the skin. Exp. Dermatol. 2021, 30, 1598–1609. [Google Scholar] [CrossRef] [PubMed]
- Upputuri, P.K.; Pramanik, M. Recent advances toward preclinical and clinical translation of photoacoustic tomography: A review. J. Biomed. Opt. 2016, 22, 041006. [Google Scholar] [CrossRef]
- Regensburger, A.P.; Wagner, A.L.; Claussen, J.; Waldner, M.J.; Knieling, F. Shedding light on pediatric diseases: Multispectral optoacoustic tomography at the doorway to clinical applications. Mol. Cell Pediatr. 2020, 7, 3. [Google Scholar] [CrossRef]
- Ravina, K.; Lin, L.; Liu, C.Y.; Thomas, D.; Hasson, D.; Wang, L.V.; Russin, J.J. Prospects of Photo- and Thermoacoustic Imaging in Neurosurgery. Neurosurgery 2020, 87, 11–24. [Google Scholar] [CrossRef]
- Wang, Y.; Thompson, J.M.; Ashbaugh, A.G.; Khodakivskyi, P.; Budin, G.; Sinisi, R.; Heinmiller, A.; Van Oosten, M.; Van Dijl, J.M.; Van Dam, G.M.; et al. Preclinical Evaluation of Photoacoustic Imaging as a Novel Noninvasive Approach to Detect an Orthopaedic Implant Infection. J. Am. Acad. Orthop. Surg. 2017, 25, S7–S12. [Google Scholar] [CrossRef]
- Yao, J.; Wang, L.; Yang, J.-M.; Maslov, K.I.; Wong, T.T.W.; Li, L.; Huang, C.-H.; Zou, J.; Wang, L.V. High-speed label-free functional photoacoustic microscopy of mouse brain in action. Nat. Methods 2015, 12, 407–410. [Google Scholar] [CrossRef] [PubMed]
- Lin, L.; Yao, J.; Zhang, R.; Chen, C.; Huang, C.; Li, Y.; Wang, L.; Chapman, W.; Zou, J.; Wang, L.V. High-speed photoacoustic microscopy of mouse cortical microhemodynamics. J. Biophoton. 2017, 10, 792–798. [Google Scholar] [CrossRef] [PubMed]
- Stein, E.W.; Maslov, K.; Wang, L.V. Noninvasive, in vivo imaging of blood-oxygenation dynamics within the mouse brain using photoacoustic microscopy. J. Biomed. Opt. 2009, 14, 020502. [Google Scholar] [CrossRef] [PubMed]
- Lan, B.; Liu, W.; Wang, Y.; Shi, J.; Li, Y.; Xu, S.; Sheng, H.; Zhou, Q.; Zou, J.; Hoffmann, U.; et al. High-speed widefield photoacoustic microscopy of small-animal hemodynamics. Biomed. Opt. Express 2018, 9, 4689. [Google Scholar] [CrossRef]
- Rao, A.P.; Bokde, N.; Sinha, S. Photoacoustic Imaging for Management of Breast Cancer: A Literature Review and Future Perspectives. Appl. Sci. 2020, 10, 767. [Google Scholar] [CrossRef]
- Nyayapathi, N.; Xia, J. Photoacoustic imaging of breast cancer: A mini review of system design and image features. J. Biomed. Opt. 2019, 24, 121911. [Google Scholar] [CrossRef]
- Wong, T.T.W.; Zhang, R.; Hai, P.; Zhang, C.; Pleitez, M.A.; Aft, R.L.; Novack, D.V.; Wang, L.V. Fast label-free multilayered histology-like imaging of human breast cancer by photoacoustic microscopy. Sci. Adv. 2017, 3, e1602168. [Google Scholar] [CrossRef]
- Attia, A.B.E.; Chuah, S.Y.; Razansky, D.; Ho, C.J.H.; Malempati, P.; Dinish, U.S.; Bi, R.; Fu, C.Y.; Ford, S.J.; Lee, J.S.-S.; et al. Noninvasive real-time characterization of non-melanoma skin cancers with handheld optoacoustic probes. Photoacoustics 2017, 7, 20–26. [Google Scholar] [CrossRef] [PubMed]
- Stoffels, I.; Morscher, S.; Helfrich, I.; Hillen, U.; Leyh, J.; Burton, N.C.; Sardella, T.C.P.; Claussen, J.; Poeppel, T.D.; Bachmann, H.S.; et al. Metastatic status of sentinel lymph nodes in melanoma determined noninvasively with multispectral optoacoustic imaging. Sci. Transl. Med. 2015, 7, 317ra199. [Google Scholar] [CrossRef]
- Zhang, J.; Duan, F.; Liu, Y.; Nie, L. High-Resolution Photoacoustic Tomography for Early-Stage Cancer Detection and Its Clinical Translation. Radiol. Imaging Cancer 2020, 2, e190030. [Google Scholar] [CrossRef] [PubMed]
- Valluru, K.S.; Willmann, J.K. Clinical photoacoustic imaging of cancer. Ultrasonography 2016, 35, 267–280. [Google Scholar] [CrossRef] [PubMed]
- Taruttis, A.; Van Dam, G.M.; Ntziachristos, V. Mesoscopic and Macroscopic Optoacoustic Imaging of Cancer. Cancer Res. 2015, 75, 1548–1559. [Google Scholar] [CrossRef]
- Mehrmohammadi, M.; Joon Yoon, S.; Yeager, D.; Emelianov, S.Y. Photoacoustic Imaging for Cancer Detection and Staging. Curr. Mol. Imaging 2013, 2, 89–105. [Google Scholar] [CrossRef]
- Park, K.; Kim, J.Y.; Lee, C.; Jeon, S.; Lim, G.; Kim, C. Handheld Photoacoustic Microscopy Probe. Sci. Rep. 2017, 7, 13359. [Google Scholar] [CrossRef]
- Na, S.; Russin, J.J.; Lin, L.; Yuan, X.; Hu, P.; Jann, K.B.; Yan, L.; Maslov, K.; Shi, J.; Wang, D.J.; et al. Massively parallel functional photoacoustic computed tomography of the human brain. Nat. Biomed. Eng. 2021, 6, 584–592. [Google Scholar] [CrossRef]
- Kalaora, S.; Nagler, A.; Wargo, J.A.; Samuels, Y. Mechanisms of immune activation and regulation: Lessons from melanoma. Nat. Rev. Cancer 2022, 22, 195–207. [Google Scholar] [CrossRef]
- Schadendorf, D.; van Akkooi, A.C.J.; Berking, C.; Griewank, K.G.; Gutzmer, R.; Hauschild, A.; Stang, A.; Roesch, A.; Ugurel, S. Melanoma. Lancet 2018, 392, 971–984. [Google Scholar] [CrossRef]
- US Preventive Services Task Force; Bibbins-Domingo, K.; Grossman, D.C.; Curry, S.J.; Davidson, K.W.; Ebell, M.; Epling, J.W.; García, F.A.R.; Gillman, M.W.; Kemper, A.R.; et al. Screening for Skin Cancer: US Preventive Services Task Force Recommendation Statement. JAMA 2016, 316, 429. [Google Scholar] [CrossRef] [PubMed]
- Switzer, B.; Puzanov, I.; Skitzki, J.J.; Hamad, L.; Ernstoff, M.S. Managing Metastatic Melanoma in 2022: A Clinical Review. JCO Oncol. Pract. 2022, 18, 335–351. [Google Scholar] [CrossRef]
- Zhou, Y.; Tripathi, S.V.; Rosman, I.; Ma, J.; Hai, P.; Linette, G.P.; Council, M.L.; Fields, R.C.; Wang, L.V.; Cornelius, L.A. Noninvasive Determination of Melanoma Depth using a Handheld Photoacoustic Probe. J. Investig. Dermatol. 2017, 137, 1370–1372. [Google Scholar] [CrossRef]
- Chuah, S.Y.; Attia, A.B.E.; Long, V.; Ho, C.J.H.; Malempati, P.; Fu, C.Y.; Ford, S.J.; Lee, J.S.S.; Tan, W.P.; Razansky, D.; et al. Structural and functional 3D mapping of skin tumours with non-invasive multispectral optoacoustic tomography. Ski. Res. Technol. 2017, 23, 221–226. [Google Scholar] [CrossRef]
- He, H.; Schönmann, C.; Schwarz, M.; Hindelang, B.; Berezhnoi, A.; Steimle-Grauer, S.A.; Darsow, U.; Aguirre, J.; Ntziachristos, V. Fast raster-scan optoacoustic mesoscopy enables assessment of human melanoma microvasculature in vivo. Nat. Commun. 2022, 13, 2803. [Google Scholar] [CrossRef]
- Schadendorf, D.; Fisher, D.E.; Garbe, C.; Gershenwald, J.E.; Grob, J.-J.; Halpern, A.; Herlyn, M.; Marchetti, M.A.; McArthur, G.; Ribas, A.; et al. Melanoma. Nat. Rev. Dis. Primers 2015, 1, 15003. [Google Scholar] [CrossRef]
- Kelly, J.W.; Henderson, M.A.; Thursfield, V.J.; Slavin, J.; Ainslie, J.; Giles, G.G. The management of primary cutaneous melanoma in Victoria in 1996 and 2000. Med. J. Aust. 2007, 187, 511–514. [Google Scholar] [CrossRef]
- Hieken, T.J.; Hernández-Irizarry, R.; Boll, J.M.; Jones Coleman, J.E. Accuracy of Diagnostic Biopsy for Cutaneous Melanoma: Implications for Surgical Oncologists. Int. J. Surg. Oncol. 2013, 2013, 196493. [Google Scholar] [CrossRef]
- Ng, J.C.; Swain, S.; Dowling, J.P.; Wolfe, R.; Simpson, P.; Kelly, J.W. The Impact of Partial Biopsy on Histopathologic Diagnosis of Cutaneous Melanoma: Experience of an Australian Tertiary Referral Service. Arch. Dermatol. 2010, 146, 234–239. [Google Scholar] [CrossRef]
- Breathnach, A.; Concannon, E.; Dorairaj, J.J.; Shaharan, S.; McGrath, J.; Jose, J.; Kelly, J.L.; Leahy, M.J. Preoperative measurement of cutaneous melanoma and nevi thickness with photoacoustic imaging. J. Med. Imaging 2018, 5, 015004. [Google Scholar] [CrossRef]
- Zhou, W.; Chen, Z.; Yang, S.; Xing, D. Optical biopsy approach to basal cell carcinoma and melanoma based on all-optically integrated photoacoustic and optical coherence tomography. Opt. Lett. 2017, 42, 2145. [Google Scholar] [CrossRef]
- Zhou, W.; Chen, Z.; Zhou, Q.; Xing, D. Optical Biopsy of Melanoma and Basal Cell Carcinoma Progression by Noncontact Photoacoustic and Optical Coherence Tomography: In Vivo Multi-Parametric Characterizing Tumor Microenvironment. IEEE Trans. Med. Imaging 2020, 39, 1967–1974. [Google Scholar] [CrossRef]
- Omar, M.; Schwarz, M.; Soliman, D.; Symvoulidis, P.; Ntziachristos, V. Pushing the Optical Imaging Limits of Cancer with Multi-Frequency-Band Raster-Scan Optoacoustic Mesoscopy (RSOM). Neoplasia 2015, 17, 208–214. [Google Scholar] [CrossRef]
- Folkman, J. Role of angiogenesis in tumor growth and metastasis. Semin. Oncol. 2002, 29, 15–18. [Google Scholar] [CrossRef]
- Weidner, N.; Semple, J.P.; Welch, W.R.; Folkman, J. Tumor Angiogenesis and Metastasis—Correlation in Invasive Breast Carcinoma. N. Engl. J. Med. 1991, 324, 1–8. [Google Scholar] [CrossRef]
- Hanahan, D.; Weinberg, R.A. Hallmarks of Cancer: The Next Generation. Cell 2011, 144, 646–674. [Google Scholar] [CrossRef]
- Massagué, J.; Obenauf, A.C. Metastatic colonization by circulating tumour cells. Nature 2016, 529, 298–306. [Google Scholar] [CrossRef]
- Fidler, I.J. The pathogenesis of cancer metastasis: The “seed and soil” hypothesis revisited. Nat. Rev. Cancer 2003, 3, 453–458. [Google Scholar] [CrossRef]
- Cristofanilli, M.; Budd, G.T.; Ellis, M.J.; Stopeck, A.; Matera, J.; Miller, M.C.; Reuben, J.M.; Doyle, G.V.; Allard, W.J.; Terstappen, L.W.M.M.; et al. Circulating Tumor Cells, Disease Progression, and Survival in Metastatic Breast Cancer. N. Engl. J. Med. 2004, 351, 781–791. [Google Scholar] [CrossRef]
- Edgar, R.H.; Tarhini, A.; Sander, C.; Sanders, M.E.; Cook, J.L.; Viator, J.A. Predicting Metastasis in Melanoma by Enumerating Circulating Tumor Cells Using Photoacoustic Flow Cytometry. Lasers Surg. Med. 2021, 53, 578–586. [Google Scholar] [CrossRef] [PubMed]
- Shoji, Y.; Bustos, M.A.; Gross, R.; Hoon, D.S.B. Recent Developments of Circulating Tumor Cell Analysis for Monitoring Cutaneous Melanoma Patients. Cancers 2022, 14, 859. [Google Scholar] [CrossRef] [PubMed]
- Weight, R.M.; Dale, P.S.; Viator, J.A. Detection of circulating melanoma cells in human blood using photoacoustic flowmetry. In Proceedings of the 2009 Annual International Conference of the IEEE Engineering in Medicine and Biology Society, Minneapolis, MN, USA, 3–6 September 2009; pp. 106–109. [Google Scholar]
- Galanzha, E.I.; Menyaev, Y.A.; Yadem, A.C.; Sarimollaoglu, M.; Juratli, M.A.; Nedosekin, D.A.; Foster, S.R.; Jamshidi-Parsian, A.; Siegel, E.R.; Makhoul, I.; et al. In vivo liquid biopsy using Cytophone platform for photoacoustic detection of circulating tumor cells in patients with melanoma. Sci. Transl. Med. 2019, 11, eaat5857. [Google Scholar] [CrossRef] [PubMed]
- Hai, P.; Qu, Y.; Li, Y.; Zhu, L.; Shmuylovich, L.; Cornelius, L.A.; Wang, L.V. Label-free high-throughput photoacoustic tomography of suspected circulating melanoma tumor cells in patients in vivo. J. Biomed. Opt. 2020, 25, 036002. [Google Scholar] [CrossRef] [PubMed]
- He, Y.; Wang, L.; Shi, J.; Yao, J.; Li, L.; Zhang, R.; Huang, C.-H.; Zou, J.; Wang, L.V. In vivo label-free photoacoustic flow cytography and on-the-spot laser killing of single circulating melanoma cells. Sci. Rep. 2016, 6, 39616. [Google Scholar] [CrossRef] [PubMed]
- Watts, C.; Price, S.J.; Santarius, T. Current Concepts in the Surgical Management of Glioma Patients. Clin. Oncol. 2014, 26, 385–394. [Google Scholar] [CrossRef]
- Etzkorn, J.R.; Alam, M. What Is Mohs Surgery? JAMA Dermatol. 2020, 156, 716. [Google Scholar] [CrossRef]
- Folkman, J. Angiogenesis in cancer, vascular, rheumatoid and other disease. Nat. Med. 1995, 1, 27–30. [Google Scholar] [CrossRef]
- Carmeliet, P.; Jain, R.K. Angiogenesis in cancer and other diseases. Nature 2000, 407, 249–257. [Google Scholar] [CrossRef]
- Plumb, A.A.; Huynh, N.T.; Guggenheim, J.; Zhang, E.; Beard, P. Rapid volumetric photoacoustic tomographic imaging with a Fabry-Perot ultrasound sensor depicts peripheral arteries and microvascular vasomotor responses to thermal stimuli. Eur. Radiol. 2018, 28, 1037–1045. [Google Scholar] [CrossRef]
- Chen, Z.; Rank, E.; Meiburger, K.M.; Sinz, C.; Hodul, A.; Zhang, E.; Hoover, E.; Minneman, M.; Ensher, J.; Beard, P.C.; et al. Non-invasive multimodal optical coherence and photoacoustic tomography for human skin imaging. Sci. Rep. 2017, 7, 17975. [Google Scholar] [CrossRef]
- Ron, A.; Deán-Ben, X.L.; Gottschalk, S.; Razansky, D. Volumetric Optoacoustic Imaging Unveils High-Resolution Patterns of Acute and Cyclic Hypoxia in a Murine Model of Breast Cancer. Cancer Res. 2019, 79, 4767–4775. [Google Scholar] [CrossRef]
- Greb, J.E.; Goldminz, A.M.; Elder, J.T.; Lebwohl, M.G.; Gladman, D.D.; Wu, J.J.; Mehta, N.N.; Finlay, A.Y.; Gottlieb, A.B. Psoriasis. Nat. Rev. Dis. Primers 2016, 2, 16082. [Google Scholar] [CrossRef]
- Branisteanu, D.E.; Cojocaru, C.; Diaconu, R.; Porumb, E.A.; Alexa, A.I.; Nicolescu, A.C.; Brihan, I.; Bogdanici, C.M.; Branisteanu, G.; Dimitriu, A.; et al. Update on the etiopathogenesis of psoriasis (Review). Exp. Ther. Med. 2022, 23, 201. [Google Scholar] [CrossRef] [PubMed]
- Griffiths, C.E.; Barker, J.N. Pathogenesis and clinical features of psoriasis. Lancet 2007, 370, 263–271. [Google Scholar] [CrossRef]
- Ryan, C.; Korman, N.J.; Gelfand, J.M.; Lim, H.W.; Elmets, C.A.; Feldman, S.R.; Gottlieb, A.B.; Koo, J.Y.M.; Lebwohl, M.; Leonardi, C.L.; et al. Research gaps in psoriasis: Opportunities for future studies. J. Am. Acad. Dermatol. 2014, 70, 146–167. [Google Scholar] [CrossRef] [PubMed]
- Aguirre, J.; Schwarz, M.; Garzorz, N.; Omar, M.; Buehler, A.; Eyerich, K.; Ntziachristos, V. Precision assessment of label-free psoriasis biomarkers with ultra-broadband optoacoustic mesoscopy. Nat. Biomed. Eng. 2017, 1, 0068. [Google Scholar] [CrossRef]
- Hindelang, B.; Nau, T.; Englert, L.; Berezhnoi, A.; Lauffer, F.; Darsow, U.; Biedermann, T.; Eyerich, K.; Aguirre, J.; Ntziachristos, V. Enabling precision monitoring of psoriasis treatment by optoacoustic mesoscopy. Sci. Transl. Med. 2022, 14, eabm8059. [Google Scholar] [CrossRef]
- Ossadnik, K.; Philipp, S.; Bost, W.; Fournelle, M.; Richter, H.; Lademann, J. Application of Photoacoustic Methods and Confocal Microscopy for Monitoring of Therapeutic Response in Plaque Psoriasis. Ski. Pharmacol. Physiol. 2018, 31, 308–315. [Google Scholar] [CrossRef] [PubMed]
- Aguirre, J.; Hindelang, B.; Berezhnoi, A.; Darsow, U.; Lauffer, F.; Eyerich, K.; Biedermann, T.; Ntziachristos, V. Assessing nailfold microvascular structure with ultra-wideband raster-scan optoacoustic mesoscopy. Photoacoustics 2018, 10, 31–37. [Google Scholar] [CrossRef]
- Hofstee, H.M.A.; Serné, E.H.; Roberts, C.; Hesselstrand, R.; Scheja, A.; Moore, T.L.; Wildt, M.; Manning, J.B.; Vonk Noordegraaf, A.; Voskuyl, A.E.; et al. A multicentre study on the reliability of qualitative and quantitative nail-fold videocapillaroscopy assessment. Rheumatology 2012, 51, 749–755. [Google Scholar] [CrossRef]
- Masthoff, M.; Helfen, A.; Claussen, J.; Karlas, A.; Markwardt, N.A.; Ntziachristos, V.; Eisenblätter, M.; Wildgruber, M. Use of Multispectral Optoacoustic Tomography to Diagnose Vascular Malformations. JAMA Dermatol. 2018, 154, 1457. [Google Scholar] [CrossRef] [PubMed]
- Barrett, E.J.; Liu, Z.; Khamaisi, M.; King, G.L.; Klein, R.; Klein, B.E.K.; Hughes, T.M.; Craft, S.; Freedman, B.I.; Bowden, D.W.; et al. Diabetic Microvascular Disease: An Endocrine Society Scientific Statement. J. Clin. Endocrinol. Metab. 2017, 102, 4343–4410. [Google Scholar] [CrossRef] [PubMed]
- Cheung, C.Y.; Ikram, M.K.; Klein, R.; Wong, T.Y. The clinical implications of recent studies on the structure and function of the retinal microvasculature in diabetes. Diabetologia 2015, 58, 871–885. [Google Scholar] [CrossRef] [PubMed]
- He, H.; Fasoula, N.-A.; Karlas, A.; Omar, M.; Aguirre, J.; Lutz, J.; Kallmayer, M.; Füchtenbusch, M.; Eckstein, H.-H.; Ziegler, A.; et al. Opening a window to skin biomarkers for diabetes stage with optoacoustic mesoscopy. Light Sci. Appl. 2023, 12, 231. [Google Scholar] [CrossRef] [PubMed]
- Karlas, A.; Katsouli, N.; Fasoula, N.-A.; Bariotakis, M.; Chlis, N.-K.; Omar, M.; He, H.; Iakovakis, D.; Schäffer, C.; Kallmayer, M.; et al. Dermal features derived from optoacoustic tomograms via machine learning correlate microangiopathy phenotypes with diabetes stage. Nat. Biomed. Eng. 2023, 7, 1667–1682. [Google Scholar] [CrossRef] [PubMed]
- Greenman, R.L.; Panasyuk, S.; Wang, X.; Lyons, T.E.; Dinh, T.; Longoria, L.; Giurini, J.M.; Freeman, J.; Khaodhiar, L.; Veves, A. Early changes in the skin microcirculation and muscle metabolism of the diabetic foot. Lancet 2005, 366, 1711–1717. [Google Scholar] [CrossRef] [PubMed]
- Caballero, A.E.; Arora, S.; Saouaf, R.; Lim, S.C.; Smakowski, P.; Park, J.Y.; King, G.L.; LoGerfo, F.W.; Horton, E.S.; Veves, A. Microvascular and macrovascular reactivity is reduced in subjects at risk for type 2 diabetes. Diabetes 1999, 48, 1856–1862. [Google Scholar] [CrossRef]
- Mennes, O.A.; van Netten, J.J.; Slart, R.H.J.A.; Steenbergen, W. Novel Optical Techniques for Imaging Microcirculation in the Diabetic Foot. Curr. Pharm. Des. 2018, 24, 1304–1316. [Google Scholar] [CrossRef]
- McDermott, K.; Fang, M.; Boulton, A.J.M.; Selvin, E.; Hicks, C.W. Etiology, Epidemiology, and Disparities in the Burden of Diabetic Foot Ulcers. Diabetes Care 2023, 46, 209–221. [Google Scholar] [CrossRef]
- Huang, F.; Lu, X.; Yang, Y.; Yang, Y.; Li, Y.; Kuai, L.; Li, B.; Dong, H.; Shi, J. Microenvironment-Based Diabetic Foot Ulcer Nanomedicine. Adv. Sci. 2023, 10, 2203308. [Google Scholar] [CrossRef]
- Wang, Z.; Tong, Z.; Chen, H.; Nie, G.; Hu, J.; Liu, W.; Wang, E.; Yuan, B.; Wang, Z.; Hu, J. Photoacoustic/ultrasonic dual-mode imaging for monitoring angiogenesis and synovial erosion in rheumatoid arthritis. Photoacoustics 2023, 29, 100458. [Google Scholar] [CrossRef]
- Peng, X.; Xu, Z.; Dentinger, A.; Kewalramani, S.; Jo, J.; Xu, G.; Chamberland, D.; Abdulaziz, N.; Gandikota, G.; Mills, D.; et al. Longitudinal volumetric assessment of inflammatory arthritis via photoacoustic imaging and Doppler ultrasound imaging. Photoacoustics 2023, 31, 100514. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.F.; Maslov, K.; Stoica, G.; Wang, L.V. Imaging acute thermal burns by photoacoustic microscopy. J. Biomed. Opt. 2006, 11, 054033. [Google Scholar] [CrossRef] [PubMed]
- Yamazaki, M.; Sato, S.; Ashida, H.; Saito, D.; Okada, Y.; Obara, M. Measurement of burn depths in rats using multiwavelength photoacoustic depth profiling. J. Biomed. Opt. 2005, 10, 064011. [Google Scholar] [CrossRef] [PubMed]
- Hochreiner, A.; Bauer-Marschallinger, J.; Burgholzer, P.; Jakoby, B.; Berer, T. Non-contact photoacoustic imaging using a fiber based interferometer with optical amplification. Biomed. Opt. Express 2013, 4, 2322–2331. [Google Scholar] [CrossRef] [PubMed]
- Deán-Ben, X.L.; Pang, G.A.; Montero de Espinosa, F.; Razansky, D. Non-contact optoacoustic imaging with focused air-coupled transducers. Appl. Phys. Lett. 2015, 107, 051105. [Google Scholar] [CrossRef]
- Song, W.; Xu, Q.; Zhang, Y.; Zhan, Y.; Zheng, W.; Song, L. Fully integrated reflection-mode photoacoustic, two-photon and second harmonic generation microscopy in vivo. Sci. Rep. 2016, 6, 32240. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Qi, W.; Xi, L. Deep-learning-based motion-correction algorithm in optical resolution photoacoustic microscopy. Vis. Comput. Ind. Biomed. Art 2019, 2, 12. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.; Ke, Z.; Yang, F.; Li, K.; Chen, N.; Song, L.; Zheng, C.; Liang, D.; Liu, C. Deep Learning Enables Superior Photoacoustic Imaging at Ultralow Laser Dosages. Adv. Sci. 2021, 8, 2003097. [Google Scholar] [CrossRef]
- Li, J.; Wang, C.; Chen, T.; Lu, T.; Li, S.; Sun, B.; Gao, F.; Ntziachristos, V. Deep learning-based quantitative optoacoustic tomography of deep tissues in the absence of labeled experimental data. Optica 2022, 9, 32. [Google Scholar] [CrossRef]
- Li, X.; Zhang, S.; Wu, J.; Huang, S.; Feng, Q.; Qi, L.; Chen, W. Multispectral Interlaced Sparse Sampling Photoacoustic Tomography. IEEE Trans. Med. Imaging 2020, 39, 3463–3474. [Google Scholar] [CrossRef]
- Wang, G.; Ye, J.C.; De Man, B. Deep learning for tomographic image reconstruction. Nat. Mach. Intell. 2020, 2, 737–748. [Google Scholar] [CrossRef]
- Chen, Y.; Xu, C.; Zhang, Z.; Zhu, A.; Xu, X.; Pan, J.; Liu, Y.; Wu, D.; Huang, S.; Cheng, Q. Prostate cancer identification via photoacoustic spectroscopy and machine learning. Photoacoustics 2021, 23, 100280. [Google Scholar] [CrossRef]
- Forbrich, A.; Heinmiller, A.; Zemp, R.J. Photoacoustic imaging of lymphatic pumping. J. Biomed. Opt. 2017, 22, 106003. [Google Scholar] [CrossRef] [PubMed]
- Toi, M.; Asao, Y.; Matsumoto, Y.; Sekiguchi, H.; Yoshikawa, A.; Takada, M.; Kataoka, M.; Endo, T.; Kawaguchi-Sakita, N.; Kawashima, M.; et al. Visualization of tumor-related blood vessels in human breast by photoacoustic imaging system with a hemispherical detector array. Sci. Rep. 2017, 7, 41970. [Google Scholar] [CrossRef] [PubMed]
- Hosseinaee, Z.; Tummon Simmons, J.A.; Reza, P.H. Dual-Modal Photoacoustic Imaging and Optical Coherence Tomography [Review]. Front. Phys. 2021, 8, 616618. [Google Scholar] [CrossRef]
- Park, J.; Park, B.; Kim, T.Y.; Jung, S.; Choi, W.J.; Ahn, J.; Yoon, D.H.; Kim, J.; Jeon, S.; Lee, D.; et al. Quadruple ultrasound, photoacoustic, optical coherence, and fluorescence fusion imaging with a transparent ultrasound transducer. Proc. Natl. Acad. Sci. USA 2021, 118, e1920879118. [Google Scholar] [CrossRef] [PubMed]
- Rao, B.; Soto, F.; Kerschensteiner, D.; Wang, L.V. Integrated photoacoustic, confocal, and two-photon microscope. J. Biomed. Opt. 2014, 19, 036002. [Google Scholar] [CrossRef] [PubMed]

| Imaging Modality | Sensitivity | Resolution | Imaging Depth |
|---|---|---|---|
| Photoacoustic Imaging | High sensitivity to endogenous chromophores such as hemoglobin and melanin, enabling detection of subtle changes in tissue oxygenation and vascularization. | High spatial resolution, typically ranging from tens to hundreds of micrometers, enabling detailed imaging of skin structures including blood vessels, pigmented lesions, and subcutaneous. | Penetrates several millimeters beneath the skin surface, providing information about structures located deeper in the tissue. |
| Dermoscopy | High sensitivity to surface features and pigmented lesions, aiding in the detection of melanoma and other skin cancers. | Provides magnified views of skin lesions with detailed surface characteristics, such as pigment patterns, vascular structures, and specific dermal structures. | Limited to superficial layers of the skin, providing surface-level information about skin lesions. |
| Confocal Microscopy | Cellular-level sensitivity, visualizing individual skin cells, nuclei, and cellular organelles. | Sub-cellular resolution, providing detailed morphological information about cellular architecture and identifying cellular abnormalities associated with skin diseases. | Limited to superficial layers of the skin, typically up to 100–200 μm deep, depending on the imaging system and objective used. |
| Optical Coherence Tomography | High sensitivity to changes in tissue optical scattering properties, providing detailed cross-sectional images of skin layers. | Micrometer-scale resolution, providing detailed imaging of skin layers and fine structural features such as epidermal-dermal junctions, hair follicles, and sweat glands. | Penetrates up to 1–2 mm into the skin, depending on the wavelength of light used and tissue scattering properties. |
| Ultrasound | Excellent sensitivity to tissue density variations, detecting structural abnormalities such as tumors, cysts, and edema. | Spatial resolution on the order of millimeters, allowing visualization of macroscopic features such as tumor size, shape, and depth within the skin. | Penetrates several centimeters into the tissue, depending on the frequency of the ultrasound probe. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ying, Y.; Zhang, H.; Lin, L. Photoacoustic Imaging of Human Skin for Accurate Diagnosis and Treatment Guidance. Optics 2024, 5, 133-150. https://doi.org/10.3390/opt5010010
Ying Y, Zhang H, Lin L. Photoacoustic Imaging of Human Skin for Accurate Diagnosis and Treatment Guidance. Optics. 2024; 5(1):133-150. https://doi.org/10.3390/opt5010010
Chicago/Turabian StyleYing, Yue, Hong Zhang, and Li Lin. 2024. "Photoacoustic Imaging of Human Skin for Accurate Diagnosis and Treatment Guidance" Optics 5, no. 1: 133-150. https://doi.org/10.3390/opt5010010
APA StyleYing, Y., Zhang, H., & Lin, L. (2024). Photoacoustic Imaging of Human Skin for Accurate Diagnosis and Treatment Guidance. Optics, 5(1), 133-150. https://doi.org/10.3390/opt5010010







