Abstract
This paper provides insight into the various studies that have been carried out to date on liquid crystalline materials based on copper(I) complexes. Although the study of copper(I) complexes with respect to their liquid crystalline property is quite limited, metallomesogens prepared with different structural components and ligands from groups such as azamacrocycles, alkythiolates, ethers, isocyanides, phenanthroline, Schiff bases, pyrazolates, phosphines, biquinolines, and benzoylthioureas are reported and summarized in this review. A special section is dedicated to the discussion of emission properties of copper(I) metallomesogens.
1. Introduction
Liquid crystals are generally referred to as substances that blend the structure and properties of solid and liquid states; they share with liquids the ability to flow but also exhibit some structural arrangement similarities with solids. This intriguing combination of the properties of both liquid and solid states gives liquid crystals the ability to induce certain properties that enable useful applications in the displays of devices such as wristwatches, calculators, portable computers, and flat-screen televisions, as well as in sensors, smart windows, optical switches, etc. [1].
With so many compounds synthesized, liquid crystals containing metals, also known as metallomesogens, have become a major subject of study [2]. The incorporation of metal into organic matrices enhances and induces unique magnetic, spectroscopic, and redox properties of the resulting materials [3,4,5,6,7,8,9]. At least one liquid crystalline complex has been reported in the literature for most metals. However, issues often encountered with these complexes include those relating to the high transition temperatures (usually >100 °C) and the low thermal stability associated with metallomesogens at elevated temperatures, which are major drawbacks that hinder the study of the physical properties of these materials. The performance parameters in electro-optical applications should be enhanced by luminescent liquid crystals, which offer anisotropic long-range order and consequently polarized emission [10,11]. Therefore, the preceding challenges make it difficult to observe light emission (luminescence) at elevated temperatures due to strong tendencies of the excited states to undergo deactivation through non-radiative transitions. Therefore, luminescence studies on metallomesogens in many cases were performed with samples in the solid state or dissolved in organic solvents [12].
Copper(I) complexes have not been studied to a great extent as luminescent materials in the liquid crystalline state, but they do possess luminescence properties with great potentials [13,14,15,16,17,18,19,20,21,22]. Copper, which is somewhat abundant and affordable, is a suitable alternative to noble metal complexes [23,24]. The ratio of triplet to singlet excitons is 3:1; consequently, for luminescent materials to be used in OLEDs, they should essentially be able to harvest all the excitons. Because copper(I) complexes exhibit various metal-to-ligand charge-transfer (MLCT) behaviors, they can induce spin orbital coupling of the triplet and singlet states, leading to small energy separations between the energy levels [25]. This allows for reverse intersystem crossing (RISC), i.e., singlet harvesting, resulting in thermally activated delayed fluorescence (TADF) [26]. Therefore, liquid crystals based on copper(I) complexes have considerable promise for producing effective luminescent materials for a wide range of optical or electro-optical applications due to the large diversity of possible structures, including mononuclear or polynuclear complexes, and the great potential of emission behavior. In addition, the range of coordination geometries (such as linear, plan-trigonal, or tetrahedral) combined with the ligands’ structural design provide a significant benefit for controlling the LC properties, including their enhanced thermal stability and mesophase type related to both calamitic and discotic materials.
A number of reviews have provided an overview of the luminescent properties of copper(I) complexes and their use in various applications [27,28,29,30,31]; however, a systematic review specific to copper (I) metallomesogens has not been reported. Liquid crystalline materials obtained from metal complexes of copper(I) exist in a variety of forms and structural geometries. These complexes have been designed using a wide variety of ligands from various groups, including azamacrocycles, alkythiolates, ethers, isocyanides, phenanthrolines, Schiff bases, pyrazolates, phosphines, biquinolines, and benzoylthioureas. In this paper, we review studies relevant to our interest in research on liquid crystalline materials in the form of copper(I) complexes from 1994 to the present. Furthermore, the known luminescent properties of these copper(I) complexes in their mesophase are highlighted.
2. Copper(I) Metallomesogens with Sulfur-Containing Ligands
A series of cationic macrocyclic copper(I) complexes based on non-mesogenic bis[4-(n-alkyloxy) benzamide derivatives of 1,10-diaza-4,7,13,16-tetrathiacyclooctadecane was reported in 1994 by Ghedini et al. [32]. The studied complexes had transition temperatures from the solid to liquid crystalline states ranging from 93 to 123 °C. Complex 1e (Figure 1) demonstrated the clearest mesomorphism among the series, with sufficient thermal stability, so the research group focused their X-ray analysis on it, and the study revealed an X-ray diffraction pattern consistent with a disordered layered structure associated with a smectic phase of A or C type.
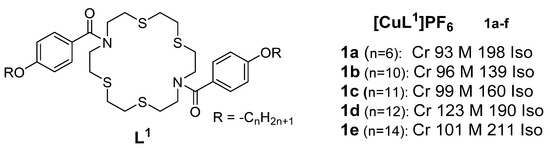
Figure 1.
Copper(I) complexes from azamacrocycle derivatives. Transition temperatures are in °C [32].
Alkyl thiolates (R-SH) are strong ligands that can bind to metals via the donor S atom to obtain metal thiolates. In 1999, Espinet et al. [33] reported the preparation of a series of copper(I) thiolates, [CuSCnH2n+1], where n = 4, 6, 8, 10, 12, 14, 16, and 18. X-ray diffraction analysis of the polycrystalline sample revealed a layered structure in the solid state, contrary to previously reported work by Dance et al. (1991) [34]. However, the authors suggested that the discrepancies might have been due to the methodology used, indicating that the preparative method of the complex influences its stability towards oxidation. The textures of the complexes show a columnar mesophase based on stacking of cyclic [Cu4(µ2-SCnH2n+1)4] aggregates, with transition temperatures ranging from 56 to 210 °C.
A new class of copper(I) metallomesogens based on copper(I) halide complexes with thiourea-based ligands with long-chain alkoxy groups and a perfluorooctyl group were reported in 2018 by Ilis and Cîrcu [35] (Figure 2). They found no liquid crystalline behavior for the ligand but observed a hexagonal columnar phase for both complexes 2a and 2b at high temperatures above 100 °C via a combined study of POM, DSC, and XRD, while the thermal stability studied by TGA indicated a higher stability (180 °C) for the corresponding copper(I) complexes compared to that of the BTU (160 °C) ligand.
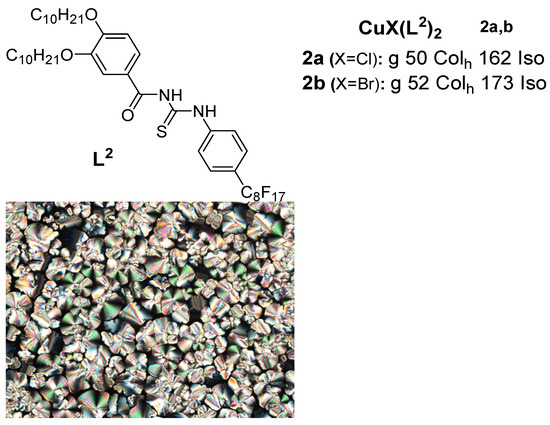
Figure 2.
Copper(I) complexes with benzoylthiourea ligands. Transition temperatures are in °C. Inset: POM image of the Colh phase of 2a at 85 °C [35].
3. Copper(I) Metallomesogens with N-Donor Ligands
Many copper(I) complexes with N-donor ligands integrating pyrazole, 2,2’-bypiridine, 1,10-phenantroline moieties, or Schiff bases have been described to date. Copper(I) metallomesogens with three-coordinate geometry were first reported by Lin et al. in 2001 [36]. The complexes were derived from bis{2-[3’-(3’’,4’’-dialkoxyphenyl)-5’-methyl-1’-pyrazolyl]ethyl} ethers and from bis{2-[3’-(3’’,4’,5’-trialkoxyphenyl)-5’-methyl-1’-pyrazolyl]ethyl} ethers. These novel complexes were obtained by complexing the ethers with [Cu(MeCN)4]BF4 (Figure 3). The reported copper(I) complexes with four or six alkoxy chains exhibited liquid crystalline behavior and are characteristic of columnar discotics. DSC analysis results indicated a higher enthalpy for the melting transitions at lower temperatures and a relatively lower enthalpy for the isotropization transitions at higher temperatures. The attachment of an additional alkoxy chain on the terminal benzene ring resulted in lower transition temperatures, while preserving the mesophase type (Colhd).
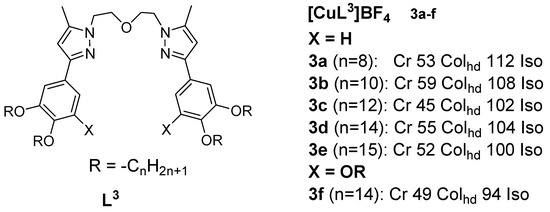
Figure 3.
Copper(I) complexes with ether-type ligands. All temperatures are in °C [36].
Schiff bases are a diverse group of compounds formed by the nucleophilic substitution reaction of an aldehyde or ketone with an amine. These compounds are characterized by the presence of a double bond linking carbon and nitrogen atoms, the functionalities of which are generated in many ways to combine a variety of alkyl or aryl substituents useful for the design of liquid crystalline materials, either organic or metallomesogens [37,38,39,40,41,42]. The lone pair of electrons on nitrogen provides a basis for making complexes with metals, including copper(I). Diverse liquid crystalline copper(I) complexes have been prepared with these ligands; the resulting complexes are indicated in Figure 4. Dinuclear copper(I) complexes (4a and 4b) with Schiff bases based on the α,α’-imino-substituted 2,2’-bipyridine unit were developed by El-ghayoury et al. [43]. The two ligands adopt a rather unusual coordination mode in which the central unit bridges the two copper(I) centers. The tetrahedral surrounding is completed by the coordination of the two imino groups of each Schiff base ligand with the iminopyridine fragment chelated in a cis fashion to provide a symmetric structure. Complex 4a is non-mesomorphic, owing to the unsuitable balance between the aliphatic chains and the aromatic core. The optical textures observed upon slow cooling of the isotropic melt of complex 4b clearly indicate the existence of a viscous columnar phase at temperatures as low as 25 °C.
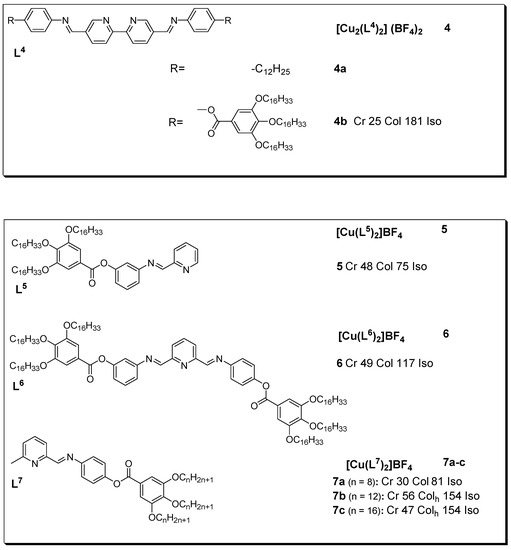
Figure 4.
Copper(I) metallomesogens with Schiff bases. All transition temperatures are in °C (transition temperature for 4a not reported) [43,44,45].
Although non-mesogenic, upon complexation with copper(I), free ligands L5 and L6 showed mesogenic character, as described by the DSC and POM experiments. The results indicated that complex 6 showed high stability after several heating cycles relative to complex 5, which was thought to be due to the lack of substituents at position 6. In addition, the optical textures observed for complex 6 during slow cooling from the isotropic melt are typical of a columnar phase (with pseudo-focal-conic textures [45]. Similarly, Douce and coworkers prepared new complexes (7a–c) by modifying the organic ligands to enable scrutiny of the nature of the packing in the liquid crystalline phase. In their work, the authors reported the preparation of new ligands with various chain lengths (n = 8, 12, and 16) bearing an additional methyl fragment in the α-position of the pyridine ring in order to protect the copper complex (5) against oxidation and decomplexation. The prepared complexes displayed hexagonal columnar mesophases with a transition temperature range of 30 to 56 °C [44].
A study by Ziessel et al. (2004) [46] presented mesomorphic materials based on copper(I) complexes with phenanthroline-based ligands (Figure 5). The authors aimed to engineer a structural framework with additional supramolecular binding factors (hydrogen bonding) so as to stabilize the mesophase both as a free ligand and within the complex. The thermotropic properties of the ligands and complexes of the phenanthroline derivatives were investigated via a combination of POM, DSC, and XRD methods. The ligand used for complexes 8b and 8c showed distinct cubic and disordered lamellar mesophases at different temperature regions in the reported XRD measurements. Neither the ligand with the shortest chain (n = 8) nor the corresponding copper(I) complex (8a) displayed any mesomorphic behavior in the investigated temperature region, but as expected, the related copper(I) complexes with longer chains (8b (n = 12) and 8c (n = 16)) showed mesomorphic behaviors, displaying mesophases characteristic of an oblique columnar phase. In effect, the complexation of the metal center with the organic ligand resulted in a distinct change in the mesomorphic properties. It was observed that the mesophase stability was enhanced upon coordination with copper(I), as reflected by the large increase in the clearing temperature by nearly 50 °C, whereas the melting temperature remained almost the same for the ligands, as well as for the corresponding complexes.
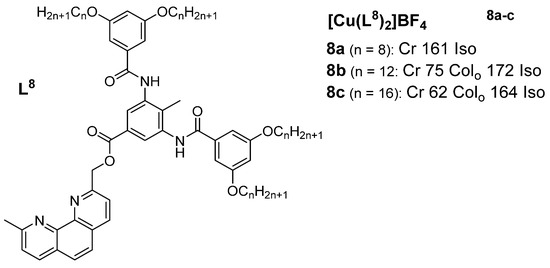
Figure 5.
Copper(I) complexes with phenanthroline-based ligands. Temperatures are in (°C) [46].
4. Copper(I) Metallomesogens with Isocyanide Ligands
Isocyanides are a class of organic compounds of the R-NC type, where R is a combination of groups obtained by the removal of a hydrogen atom from an organic compound and the carbon therein is triply bonded to nitrogen, the site of which is also capable of coordinating with metals. The isocyanides are isomers of the nitriles and are largely used in coordination chemistry [47]. These kinds of ligands have been used to design a large variety of transition metal complexes [48] and copper(I); metallomesogens are also well known for this. In general, the reaction of isocyanides with CuX (X is halide) resulted in a mononuclear complex; on the contrary, reaction of two equivalents of the isocyanide derivatives with CuX yielded binuclear copper(I) complexes, as depicted in Figure 6.
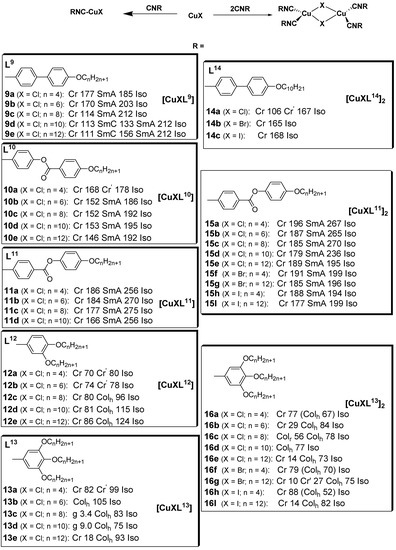
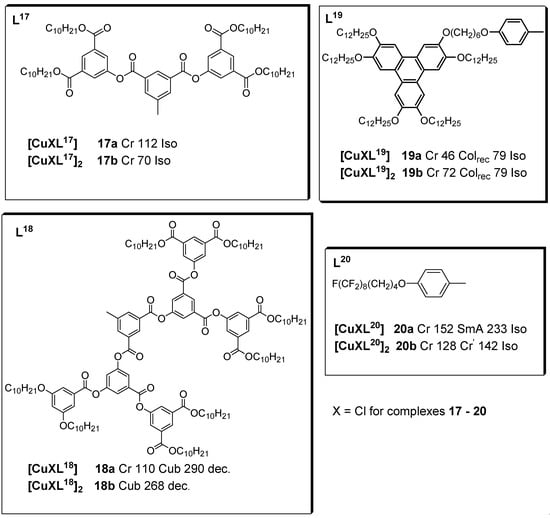
Figure 6.
Mononuclear and dinuclear copper(I) isocyanide complexes. Transition temperatures are in °C [49,50,51,52,53].
The first set of liquid crystals based on copper(I) isocyanide complexes was reported in 2001 by Benouazzane et al. [52]. Some of the isocyanide ligands (L9–11) were reported to display nematic and/or smectic A phases. The copper complexes (9a–e) showed SmA and SmC mesophases, while complexes 10b–e and 11a–d were found to display only SmA phases [52]. Further study indicated that the range of the SmC phase increases and that of the SmA phase decreases as the length of the chain increases. All the copper isocyanides reported in Figure 6 are mesogenic, except complexes 10a, 12a, 12b, 13a, 14a–c, 20, 17a,b, and 20b. The isocyanide ligands L12 and L13, with a single aromatic ring, did not appear to be mesogenic, but upon complexation with copper(I), all the complexes (except 13a with the shortest chain (n = 4)) showed liquid crystalline behavior.
After the successful preparation of stable linear copper(I) liquid crystals (complexes 9–13) with isocyanide ligands reported in [52], in 2002, the same research group subsequently reported another series of binuclear copper(I) complexes (14–16) with mesogenic properties. The free isocyanide ligands were reported as promesogenic, with nematic and/or smectic A phases. Copper complexes 14a–c lack mesogenic properties, whereas, copper complexes 15a–i were found to be mesogenic. On the other hand, although the uncomplexed isocyanide ligands (L13) are not liquid crystals, all their dinuclear copper isocyanide complexes (16a–i) had liquid crystalline properties, displaying columnar mesophases [53]. These liquid crystalline binuclear copper(I) complexes with isocyanide ligands reported by Benouazzane et al. were the first examples of liquid crystals with a core formed by two tetrahedral structures sharing an edge. Dendrimers based on isocyanides were first reported by Coco et al. (2008) [51]. The authors found that whereas all the free, highly branched isocyanide ligands (L17 and L18) and the metal complexes (17a and 17b) were not liquid crystals, complexes 18a and 18b showed a cubic mesophase.
Chico et al. [50] reported two isocyano-triphenylene copper(I) complexes (19a and 19b), both of which displayed good thermal stability in the range of study. The free isocyanide ligand appeared not to be mesomorphic, as observed by POM. The identification of the columnar mesophase for 19a and 19b was achieved by small-angle X-ray scattering on powder samples, which was measured as a function of temperature, consistent with the DSC and POM experiments. For these complexes, the columnar mesophases were stable in the temperature range of 46 to 79 °C.
The effect of incorporating a fluorinated chain in an isocyanide ligand with respect to its mesomorphic behavior when compared to its hydrocarbon derivative was studied by Dembinski and coworkers [49]. Previously, several studies [54,55,56] had investigated the fluorophobic effect of single aromatic ring-containing organic molecules containing a perfluoroalkyl chain; as such, a study was carried out on fluorinated analogs of hydrocarbon complexes in which mesomorphic behaviors had not been previously observed. The semi-perfluorinated isocyanide ligand (L20), in contrast to its alkyl analog, exhibited liquid crystalline properties, showing a smectic A mesophase upon both heating and cooling. This was attributed to the fluorophobic effect, allowing for the mesogenicity of the isocyanide compound with a single benzene unit. The corresponding mononuclear copper complex retained the mesophase but with a high crystalline–mesophase transition temperature (152 °C), while the dinuclear analog did not show liquid crystalline properties [49].
5. Luminescent Metallomesogens Based on Copper(I) Complexes
A major drawback in the study of the physical properties of metallomesogens is associated with issues relating to their high transition temperature and stability at elevated temperatures. It becomes increasingly difficult to study the emissions at such high temperatures due to strong tendencies of the excited electrons to undergo deactivation via non-radiative transitions [12]. Few attempts have been made in this regard, and interesting findings have been reported. The luminescence data of the copper(I) complexes discussed in this section are summarized in Table 1.

Table 1.
Summary of luminescence data for copper(I) metallomesogens reported in [57,58,59,60].
Kishimura et al. were the first to describe the emission properties of copper(I) complexes in their liquid crystalline phase [57] in 2005. They reported a number of dendritic copper(I) pyrazolate complexes 21 and 22 (Figure 7), which were used to produce some thermally rewritable phosphorescent papers useful for security purposes.
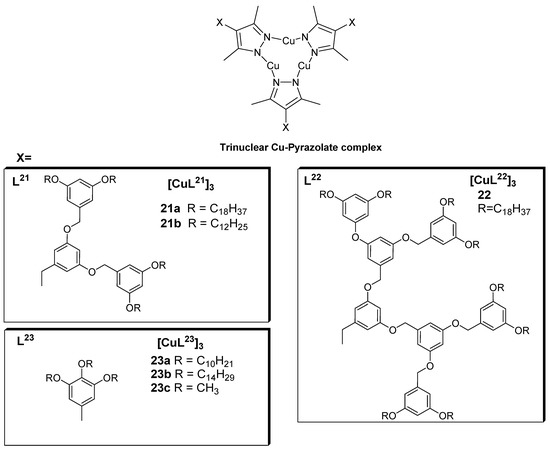
Figure 7.
Ligands and representative structure of the copper pyrazolate complexes reported in [57,58].
The luminescence investigation of complexes from the pyrazolate ligands L21 [57] revealed dichroism at room temperature for the solid form of complex 21a. Cooling of the hot melt (which emitted a red luminescence at λmax650 nm) naturally or by slow cooling led to blue-shift emission 640 and 610 nm. In essence, the red and yellow luminescence observed for 21a could be thermally changed from one form to the other, depending on the manner and rate of cooling, which was also found to be the case for its liquid crystalline properties. Both the analyze complex (21a), both in aged and non-aged form, appeared to be phosphorescent, which is thought to a result of Cu(I) to Cu(I) interactions [57]. The photoluminescent data are summarized in Table 1.
Furthermore, XRD analysis of the aged sample of complex 21a showed diffraction patterns synonymous with a one-dimensional columnar phase, and the same complex (21a) viewed under a polarized optical microscope showed a fan-shaped texture characteristic of discotic liquid crystals; accordingly, it was concluded that the aged complex (21a) was composed of long-range discotic columnar assembly. The XRD pattern of the non-aged complex after natural cooling also indicated the presence of a columnar structure. DSC measurements carried out on the aged and non-aged complex (21a) revealed patterns based on which it was concluded that the discotic columnar assembly, which is believed to involve metallophilic interactions of Cu(I) to Cu(I) units with long alkyl chains, is formed in the aging process at about 40–50 °C. The stability of the dichroic luminescence was therefore found to be dependent on the pyrazolate ligand structure. Complex 21b exhibited similar phosphorescent properties as complex 21b upon rapid and slow cooling of its hot melt; however, the red luminescence turned yellow spontaneously, even at very low temperatures. For complex 22 (Figure 7), which has more dendritic units than the other studied complexes, did not show a clear luminescence dichroism, and aging of the complex 22 by slow cooling of its hot melt resulted in only a 10 nm red shift of the luminescence [57].
Building on the aforementioned work by Kishimura and coworkers, Gimenez et al. (2020) [58] recently reported a series of liquid crystals achieved with cyclic trinuclear copper(I) complexes (23a and 23b) prepared using 3,5-dimethyl-4-(trialkoxyphenyl) pyrazolate ligands (Figure 7). The compounds displayed well-organized hexagonal columnar mesophases, which were found to be stable at room temperature or near room temperature (Figure 8).
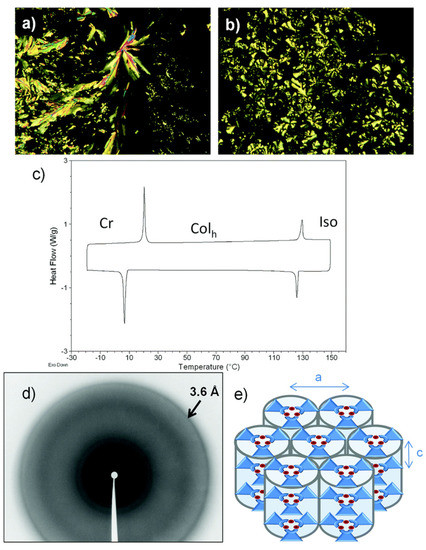
Figure 8.
(a) Microphotograph of the region between crossed polarizers observed for the texture of the Colh phase of 23a upon cooling of the isotropic liquid at 126 °C. (b) Microphotograph of the region between crossed polarizers observed for the texture of the Colh phase of 23b upon cooling of the isotropic liquid at 98 °C. (c) DSC thermogram of 23a. (d) XRD patterns of the Colh phase of 23a. The arrow indicates the halo corresponding to the stacking distance. (e) Self-assembly in the hexagonal columnar mesophase. (Reproduced from ref. [58] with permission from the Royal Society of Chemistry).
Both complexes 23a and 23b were reported to have orange–red-colored emissions at room temperature, and the photoluminescence exhibited a broad band centered around 661–664 nm (Figure 9). A landmark reported in this study [58] is the high quantum yield (QY) value of 42% measured in the liquid crystalline state, which is the highest recorded to date for any copper(I) metallomesogen. The QY obtained for 23a in its liquid crystalline state was higher than that obtained in the crystalline states of complexes 23b and 23c. Thus, the work carried out by Giminez et al. [58] showed that low-temperature phosphorescent metallomesogens can be obtained from the more affordable and abundant copper metal.
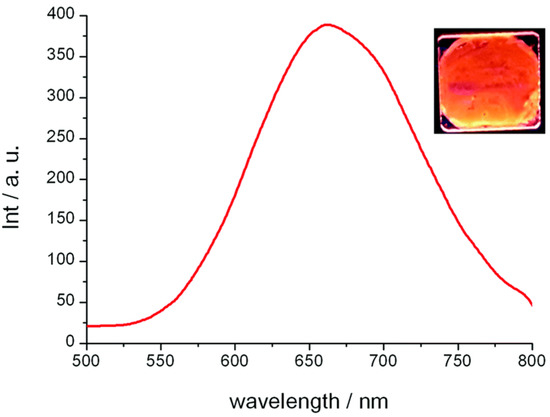
Figure 9.
Photoluminescence spectrum in the columnar mesophase for 23a at 25 °C (excitation wavelength, 290 nm) and emission of the film observed under irradiation with a 254 nm handheld lamp (reproduced from ref. [58] with permission from the Royal Society of Chemistry).
Camerel et al. (2016) [59] reported a new class of copper(I) liquid crystals with a cubane core and based on phosphine ligands functionalized with promesogenic gallate-based moieties bearing either long alkyl chains of C8, C12, and C16 or cyanobiphenyl (CBP) fragments (Figure 10). Copper(I) cubanes are known for their ability to display both luminescence mechanochromism and thermochromism behavior [61,62,63,64].
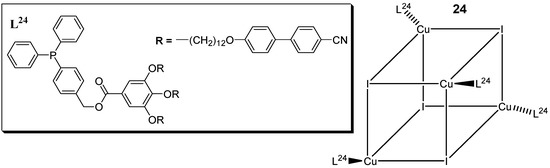
Figure 10.
General structure of the functionalized [Cu4I4(L24)4] copper iodide clusters with phosphine ligands [59].
This study is an inventive example of integrating luminescence characteristics of copper iodide clusters [Cu4I4(L24)4] with the flexible self-assembly of liquid crystals. Only the compound functionalized with a cyanobiphenyl group (CBP), i.e., 24, showed liquid crystalline behavior, displaying an SmA mesophase from room temperature to about 100 °C.
All the complexes reported in the work by Camerel et al. [59] revealed luminescence thermochromism for all the studied compounds; however, complex 24 displayed an unclassical behavior, which was attributed to the intrinsic luminescence properties of the cyanobiphenyl moiety itself, with a dual-emissive system that presented interesting emission properties. In addition to its liquid crystalline properties, compound 24 displayed luminescence mechanochromism with a modification of the emission wavelength in response to grinding (Figure 11).
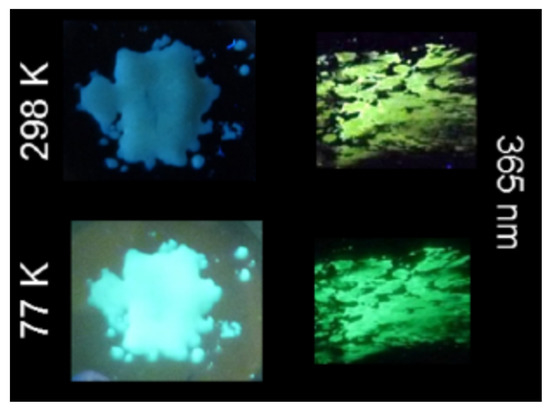
Figure 11.
Photos of 24 under 365 nm (UV lamp) before and after grinding at 298 and 77 K. Reprinted (adapted) with permission from Chem. Mater. 2016, 28, 22, 8190–8200 (ref. [59]). Copyright 2016 American Chemical Society.
Cretu et al. (2018) [60] reported a new class of Cu(I) coordination complexes (25a, 25b, 26a, and 26b) with 4,4’-bisubstituted-2,2’-biquinolines as depicted in Figure 12, which showed low-temperature lamello-columnar and columnar hexagonal thermotropic liquid crystalline phases. The highest deduction from the luminescence study is the presence of a medium–low-intensity band with a series of shoulders at 578 nm for 25a and 25b and at 560 nm for 26a and 26b, which is believed to be due to metal-to-ligand charge-transfer (MLCT) electronic transitions. When the solid samples were heated, they moved towards the liquid crystalline phases and retained the luminescence displayed in the solid state; however, with increased temperature, the intensity of the luminescence band decreased, and at temperatures over 120 °C, luminescence was completely quenched, although reversed with subsequent cooling of the samples. This behavior is attributed to the gain of the non-radiative kinetic constants when vibrational modes were enhanced by heating the samples [60].
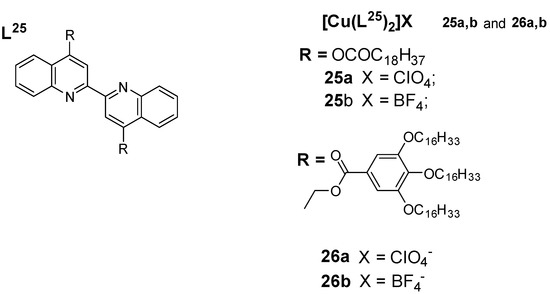
Figure 12.
Copper(I) complexes with bisubstituted biquinoline ligands [60].
6. Conclusions
This review provides an overview of the different strategies imagined by chemists to control the LC properties of copper(I) complexes, focusing on their luminescent properties. Examples of copper(I) metallomesogens based on isocyanide ligands are, by far, the most prevalent. By utilizing suitable mesogenic groups, their LC characteristics can be simply modified. While a few other isocyanides produced hexagonal and rectangular columnar phases, as well as a cubic phase for the dendritic isocyanide supermolecules, the bulk of these complexes exhibit calamitic behavior with either SmA or SmC phases. The reported copper(I) complexes of Schiff-base-type ligands of bipyridine imine-, 2-iminopyridin-, and picoline-substituted imino ligands and those of alkyl thiolates all displayed varying columnar mesophases with different transition temperature ranges. Both the ether type and benzoyl thiourea ligands displayed characteristics of a hexagonal columnar mesophase for the corresponding metallomesogens, while the copper(I) complexes from phenanthroline ligands had an oblique columnar mesophase. Furthermore, interesting luminescent properties in liquid crystalline states were observed for copper(I) complexes from ligands with pyrazolate derivatives, phosphine ligands functionalized with copper iodide, and those of bisubstituted biquinoline ligands. The complex from the functionalized phosphine ligand displayed interesting mechanochromic luminescence characteristics. A high quantum yield of 42% in the liquid crystalline phase of the copper(I) metallomesogens from a pyrazolate ligand was reported. This work provides a basis for the design and preparation of many new multifunctional materials based on more copper(I) complexes with liquid crystalline behavior and improved luminescent properties.
Author Contributions
M.A.—literature search and original draft; V.C.—conceptualization, review, and editing. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Data Availability Statement
Data sharing is not applicable to this article.
Acknowledgments
M.A. thanks the Romanian Ministry of Foreign Affairs and the University of Bucharest for funding and provision of other resources (project C1.2.PFE_CDI.2021-587/contract no.41PFE/30.12.2021).
Conflicts of Interest
There are no conflicting interest.
Abbreviations
LC—liquid crystal; DSC—differential scanning calorimetry; POM—polarizing optical microscopy; XRD—X-ray diffraction; TGA—thermogravimetric analysis; SmA/C—smectic A/C phase; Colrec—rectangular columnar phase; Colh—hexagonal columnar phase; Colhd—disordered hexagonal columnar phase; Cub—cubic phase; dec.—decomposition; g—glassy phase; Iso—isotropic liquid phase; Cr, Cr’—crystalline phases.
References
- Goodby, W.J.; Collins, P.; Gleeson, H.; Kato, T.; Tschierske, C.; Raynes, P. (Eds.) Handbook of Liquid Crystals; Wiley-VCH: Hoboken, NJ, USA, 2014. [Google Scholar]
- Donnio, B.; Guillon, D.; Deschenaux, R.; Bruce, D.W. Comprehensive Organometallic Chemistry III; Crabtree, R.H., Mingos, D.M.P., Eds.; Elsevier: Oxford, UK, 2006; Chapter 12.05; Volume 12, p. 195. [Google Scholar]
- Date, R.W.; Iglesias, E.F.; Rowe, K.E.; Elliott, J.M.; Bruce, D.W. Metallomesogens by ligand design. Dalton Trans. 2003, 10, 1914–1931. [Google Scholar] [CrossRef]
- Akiyoshi, R.; Zenno, H.; Sekine, Y.; Nakaya, M.; Akita, M.; Kosumi, D.; Lindoy, L.F.; Hayami, S. A Ferroelectric Metallomesogen Exhibiting Field-Induced Slow Magnetic Relaxation. Chem. Eur. J. 2022, 28, e202103367. [Google Scholar] [CrossRef] [PubMed]
- Micutz, M.; Iliş, M.; Staicu, T.; Dumitraşcu, F.; Pasuk, I.; Molard, Y.; Roisnel, T.; Cîrcu, V. Luminescent Liquid Crystalline Materials Based on Palladium(II) Imine Derivatives Containing the 2-Phenylpyridine Core. Dalton Trans. 2013, 43, 1151–1161. [Google Scholar] [CrossRef]
- Al-Karawi, A.J.M. From Mesogens to Metallomesogens. Synthesis, Characterisation, Liquid Crystal and Luminescent Properties. Liq. Cryst. 2017, 44, 2285–2300. [Google Scholar] [CrossRef]
- Cuerva, C.; Cano, M.; Lodeiro, C. Advanced Functional Luminescent Metallomesogens: The Key Role of the Metal Center. Chem. Rev. 2021, 121, 12966–13010. [Google Scholar] [CrossRef]
- Hakemi, H.-A.; Roviello, V.; Caruso, U. Optical and Thermal Investigations of Eutectic Metallomesogen Mixtures Based on Salicylaldiaminates Metal Complexes with a Large Nematic Stability Range. Inorganics 2023, 11, 32. [Google Scholar] [CrossRef]
- Zou, G.; Zhang, S.; Feng, S.; Li, Q.; Yang, B.; Zhao, Y.; Luo, K.; Wen, T.-B. Cyclometalated Platinum (II) Metallomesogens Based on Half-Disc-Shaped β-Diketonate Ligands with Hexacatenar: Crystal Structures, Mesophase Properties, and Semiconductor Devices. Inorg. Chem. 2022, 61, 11702–11714. [Google Scholar] [CrossRef]
- Wu, X.; Zhu, M.; Bruce, D.W.; Zhu, W.; Wang, Y. An overview of phosphorescent metallomesogens based on platinum and iridium. J. Mater. Chem. C 2018, 6, 9848–9860. [Google Scholar] [CrossRef]
- Wang, Y.; Shi, J.; Chen, J.; Zhu, W.; Baranoff, E. Recent progress in luminescent liquid crystal materials: Design, properties and application for linearly polarised emission. J. Mater. Chem. C 2015, 3, 7993–8005. [Google Scholar] [CrossRef]
- Binnemans, K. Luminescence of metallomesogens in the liquid crystal state. J. Mater. Chem. 2009, 19, 448–453. [Google Scholar] [CrossRef]
- Tudor, C.A.; Iliş, M.; Secu, M.; Ferbinteanu, M.; Cîrcu, V. Luminescent heteroleptic copper(I) complexes with phosphine and N-benzoyl thiourea ligands: Synthesis, structure and emission properties. Polyhedron 2022, 211, 115542. [Google Scholar] [CrossRef]
- Favarin, L.R.V.; Rosa, P.P.; Pizzuti, L.; Machulek, A.; Caires, A.R.L.; Bezerra, L.S.; Pinto, L.M.C.; Maia, G.; Gatto, C.C.; Back, D.F.; et al. Synthesis and structural characterization of new heteroleptic copper(I) complexes based on mixed phosphine/thiocarbamoyl-pyrazoline ligands. Polyhedron 2017, 121, 185–190. [Google Scholar] [CrossRef]
- Chan, K.C.; Cheng, S.C.; Lo, L.T.L.; Yiu, S.M.; Ko, C.C. Luminescent Charge-Neutral Copper(I) Phenanthroline Complexes with Isocyanoborate Ligand. Eur. J. Inorg. Chem. 2018, 2018, 897–903. [Google Scholar] [CrossRef]
- Bergmann, L.; Friedrichs, J.; Mydlak, M.; Baumann, T.; Nieger, M.; Bräse, S. Outstanding luminescence from neutral copper(i) complexes with pyridyl-tetrazolate and phosphine ligands. Chem. Commun. 2013, 49, 6501–6503. [Google Scholar] [CrossRef]
- Enikeeva, K.R.; Shamsieva, A.V.; Strelnik, A.G.; Fayzullin, R.R.; Zakharychev, D.V.; Kolesnikov, I.E.; Dayanova, I.R.; Gerasimova, T.P.; Strelnik, I.D.; Musina, E.I.; et al. Green Emissive Copper(I) Coordination Polymer Supported by the Diethylpyridylphosphine Ligand as a Luminescent Sensor for Overheating Processes. Molecules 2023, 28, 706. [Google Scholar] [CrossRef]
- Tsuge, K.; Chishina, Y.; Hashiguchi, H.; Sasaki, Y.; Kato, M.; Ishizaka, S.; Kitamura, N. Luminescent copper(I) complexes with halogenido-bridged dimeric core. Coord. Chem. Rev. 2016, 306, 636–651. [Google Scholar] [CrossRef]
- Sun, Y.; Lemaur, V.; Beltrán, J.I.; Cornil, J.; Huang, J.; Zhu, J.; Wang, Y.; Fröhlich, R.; Wang, H.; Jiang, L.; et al. Neutral Mononuclear Copper(I) Complexes: Synthesis, Crystal Structures, and Photophysical Properties. Inorg. Chem. 2016, 55, 5845–5852. [Google Scholar] [CrossRef]
- Borges, A.P.; Carneiro, Z.A.; Prado, F.S.; Souza, J.R.; Silva, L.H.F.E.; Oliveira, C.G.; Deflon, V.M.; de Albuquerque, S.; Leite, N.B.; Machado, A.E.H.; et al. Cu(I) complexes with thiosemicarbazides derived from p-toluenesulfohydrazide: Structural, luminescence and biological studies. Polyhedron 2018, 155, 170–179. [Google Scholar] [CrossRef]
- Brown, C.M.; Li, C.; Carta, V.; Li, W.; Xu, Z.; Stroppa, P.H.F.; Samuel, I.D.W.; Zysman-Colman, E.; Wolf, M.O. Influence of Sulfur Oxidation State and Substituents on Sulfur-Bridged Luminescent Copper(I) Complexes Showing Thermally Activated Delayed Fluorescence. Inorg. Chem. 2019, 58, 7156–7168. [Google Scholar] [CrossRef]
- Bergmann, L.; Braun, C.; Nieger, M.; Bräse, S. The coordination- and photochemistry of copper(i) complexes: Variation of N^N ligands from imidazole to tetrazole. Dalton Trans. 2018, 47, 608–621. [Google Scholar] [CrossRef]
- Li, X.; Xie, Y.; Li, Z. Diversity of Luminescent Metal Complexes in OLEDs: Beyond Traditional Precious Metals. Chem. Asian J. 2021, 16, 2817–2829. [Google Scholar] [CrossRef] [PubMed]
- Dumur, F. Recent advances in organic light-emitting devices comprising copper complexes: A realistic approach for low-cost and highly emissive devices? Org. Electron. 2015, 21, 27–39. [Google Scholar] [CrossRef]
- Yersin, H.; Czerwieniec, R.; Shafikov, M.Z.; Suleymanova, A.F. TADF Material Design: Photophysical Background and Case Studies Focusing on Cu(I) and Ag(I) Complexes in Highly Efficient OLEDs: Materials Based on Thermally Activated Delayed Fluorescence; Yersin, H., Ed.; Wiley-VCH: Hoboken, NJ, USA, 2018; pp. 1–60. [Google Scholar]
- Housecroft, C.E.; Constable, E.C. TADF: Enabling luminescent copper(i) coordination compounds for light-emitting electrochemical cells. J. Mater. Chem. C 2022, 10, 4456–4482. [Google Scholar] [CrossRef] [PubMed]
- Ravaro, L.P.; Zanoni, K.P.S.; de Camargo, A.S.S. Luminescent Copper(I) complexes as promising materials for the next generation of energy-saving OLED devices. Energy Rep. 2020, 6, 37–45. [Google Scholar] [CrossRef]
- Au, V.K.-M. Organic Light-Emitting Diodes Based on Luminescent Self-Assembled Materials of Copper(I). Energy Fuels 2021, 35, 18982–18999. [Google Scholar] [CrossRef]
- Czerwieniec, R.; Leitl, M.J.; Homeier, H.H.H.; Yersin, H. Cu(I) Complexes–Thermally Activated Delayed Fluorescence. Photophysical Approach and Material Design. Coord. Chem. Rev. 2016, 325, 2–28. [Google Scholar] [CrossRef]
- Mensah, A.; Shao, J.-J.; Ni, J.-L.; Li, G.-J.; Wang, F.-M.; Chen, L.-Z. Recent Progress in Luminescent Cu(I) Halide Complexes: A Mini-Review. Front. Chem. 2022, 9, 1127. [Google Scholar] [CrossRef]
- Liu, Y.; Yiu, S.-C.; Ho, C.-L.; Wong, W.-Y. Recent advances in copper complexes for electrical/light energy conversion. Coord. Chem. Rev. 2018, 375, 514–557. [Google Scholar] [CrossRef]
- Neve, F.; Ghedini, M.; Levelut, A.-M.; Francescangeli, O. Ionic metallomesogens. Lamellar mesophases in copper(I) azamacrocyclic complexes. Chem. Mater. 1994, 6, 70–76. [Google Scholar] [CrossRef]
- Espinet, P.; Carmen Lequerica, M.; Martín-Alvarez, J.M.Â. Synthesis, Structural Characterization and Mesogenic Behavior of Copper(I) n-Alkylthiolates. Chem. Eur. J. 1999, 5, 1982–1986. [Google Scholar] [CrossRef]
- Dance, I.G.; Fisher, K.J.; Banda, R.M.H.; Scudder, M.L. Layered Structure of Crystalline Compounds AgSR. Inorg. Chem. 1991, 30, 183–187. [Google Scholar] [CrossRef]
- Iliş, M.; Cîrcu, V. Discotic Liquid Crystals Based on Cu(I) Complexes with Benzoylthiourea Derivatives Containing a Perfluoroalkyl Chain. J. Chem. 2018, 2018, 7943763. [Google Scholar] [CrossRef]
- Lin, H.-D.; Lai, C.K. Ionic columnar metallomesogens formed by three-coordinated copper(I) complexes. J. Chem. Soc. Dalton Trans. 2001, 2383–2387. [Google Scholar] [CrossRef]
- Raczuk, E.; Dmochowska, B.; Samaszko-Fiertek, J.; Madaj, J. Different Schiff Bases—Structure, Importance and Classification. Molecules 2022, 27, 787. [Google Scholar] [CrossRef]
- Khalaji, A.D. Structural Diversity on Copper(I) Schiff Base Complexes, Current Trends in X-Ray Crystallography. Chandrasekaran, Q., Ed.; InTech: London, UK, 2011; pp. 161–190. ISBN 978-953-307-754-3. [Google Scholar]
- Jamain, Z.; Azman, A.N.A.; Razali, N.A.; Makmud, M.Z.H. A Review on Mesophase and Physical Properties of Cyclotriphosphazene Derivatives with Schiff Base Linkage. Crystals 2022, 12, 1174. [Google Scholar] [CrossRef]
- Rananavarem, S.B.; Pisipati, V.G.K.M. An Overview of Liquid Crystals Based on Schiff Base Compounds, Liquid Crystalline Organic Compounds and Polymers as Materials XXI Century: From Synthesis to Applications; Iwan, A., Schab-Balcerzak, E., Eds.; Transworld Research Network: Trivandrum, Kerala, 2011; ISBN 978-81-7895-496-7. [Google Scholar]
- Hoshino, N. Liquid crystal properties of metal–salicylaldimine complexes.: Chemical modifications towards lower symmetry. Coord. Chem. Rev. 1998, 174, 77–108. [Google Scholar] [CrossRef]
- Torroba, J.; Bruce, D.W. Comprehensive Inorganic Chemistry II: From Elements to Applications, 2nd ed.; Elsevier Ltd.: Amsterdam, The Netherlands, 2013; Volume 8, pp. 837–917. [Google Scholar]
- El-Ghayoury, A.; Douce, L.; Skoulios, A.; Ziessel, R. Cation-Induced Macroscopic Ordering of Non-Mesomorphic Modules—A New Application for Metallohelicates. Angew. Chem. Int. Ed. 1998, 37, 2205–2208. [Google Scholar] [CrossRef]
- Douce, L.; Diep, T.H.; Ziessel, R.; Skoulios, A.; Césario, M. Columnar liquid crystals from wedge-shaped tetrahedral copper(i) complexes. J. Mater. Chem. 2003, 13, 1533–1539. [Google Scholar] [CrossRef]
- Douce, L.; El-Ghayoury, A.; Ziessel, R.; Skoulios, A. Columnar mesophases from tetrahedral copper(I) cores and Schiff-base derived polycatenar ligands. Chem. Commun. 1999, 2033–2034. [Google Scholar] [CrossRef]
- Ziessel, R.; Pickaert, G.; Camerel, F.; Donnio, B.; Guillon, D.; Cesario, M.; Prangé, T. Tuning Organogels and Mesophases Will Phenanthroline Ligands and Their Copper Complexes by Inter- to Intramolecular Hydrogen Bonds. J. Am. Chem. Soc. 2004, 126, 12403–12413. [Google Scholar] [CrossRef]
- Hahn, F.E. The Coordination Chemistry of Multidentate Isocyanide Ligands. Angew. Chem. Int. Ed. 1993, 32, 650–665. [Google Scholar] [CrossRef]
- Singleton, E.; Oosthuizen, H.E. Metal Isocyanide Complexes. Adv. Organomet. Chem. 1983, 22, 209–310. [Google Scholar] [CrossRef]
- Dembinski, R.; Espinet, P.; Lentijo, S.; Markowicz, M.W.; Martín-Alvarez, J.M.; Rheingold, A.L.; Schmidt, D.J.; Sniady, A. Fluorophobic Effect in Metallomesogens–The Synthesis and Mesomorphism of Ag, Au, Cu, Fe, Pd, and Pt Fluorous Isocyanide Complexes. Eur. J. Inorg. Chem. 2008, 2008, 1565–1572. [Google Scholar] [CrossRef]
- Chico, R.; de Domingo, E.; Domínguez, C.; Donnio, B.; Heinrich, B.; Termine, R.; Golemme, A.; Coco, S.; Espinet, P. High One-Dimensional Charge Mobility in Semiconducting Columnar Mesophases of Isocyano-Triphenylene Metal Complexes. Chem. Mater. 2017, 29, 7587–7595. [Google Scholar] [CrossRef]
- Coco, S.; Cordovilla, C.; Donnio, B.; Espinet, P.; García-Casas, M.J.; Guillon, D. Self-Organization of Dendritic Supermolecules, Based on Isocyanide–Gold(I), –Copper(I), –Palladium(II), and –Platinum(II) Complexes, into Micellar Cubic Mesophases. Chem. Eur. J. 2008, 14, 3544–3552. [Google Scholar] [CrossRef]
- Benouazzane, M.; Coco, S.; Espinet, P.; Barberá, J. Supramolecular organization in copper(i) isocyanide complexes: Copper(i) liquid crystals from a simple molecular structure. J. Mater. Chem. 2001, 11, 1740–1744. [Google Scholar] [CrossRef]
- Benouazzane, M.; Coco, S.; Espinet, P.; Barberá, J. Binuclear Mesogenic Copper(I) Isocyanide Complexes with an Unusual Inorganic Core Formed by Two Tetrahedra Sharing an Edge. Inorg. Chem. 2002, 41, 5754–5759. [Google Scholar] [CrossRef]
- Rocaboy, C.; Hampel, F.; Gladysz, J.A. Syntheses and Reactivities of Disubstituted and Trisubstituted Fluorous Pyridines with High Fluorous Phase Affinities: Solid State, Liquid Crystal, and Ionic Liquid-Phase Properties. J. Org. Chem. 2002, 67, 6863–6870. [Google Scholar] [CrossRef]
- Johansson, G.; Percec, V.; Ungar, G.; Zhou, J.P. Fluorophobic Effect in the Self-Assembly of Polymers and Model Compounds Containing Tapered Groups into Supramolecular Columns. Macromolecules 1996, 29, 646–660. [Google Scholar] [CrossRef]
- Johansson, G.; Percec, V.; Ungar, G.; Smith, K. Fluorophobic Effect Generates a Systematic Approach to the Synthesis of the Simplest Class of Rodlike Liquid Crystals Containing a Single Benzene Unit. Chem. Mater. 1997, 9, 164–175. [Google Scholar] [CrossRef]
- Kishimura, A.; Yamashita, T.; Yamaguchi, K.; Aida, T. Rewritable phosphorescent paper by the control of competing kinetic and thermodynamic self-assembling events. Nat. Mater. 2005, 4, 546–549. [Google Scholar] [CrossRef]
- Giménez, R.; Crespo, O.; Diosdado, B.; Elduque, A. Liquid crystalline copper(i) complexes with bright room temperature phosphorescence. J. Mater. Chem. C 2020, 8, 6552–6557. [Google Scholar] [CrossRef]
- Huitorel, B.; Benito, Q.; Fargues, A.; Garcia, A.; Gacoin, T.; Boilot, J.-P.; Perruchas, S.; Camerel, F. Mechanochromic Luminescence and Liquid Crystallinity of Molecular Copper Clusters. Chem. Mater. 2016, 28, 8190–8200. [Google Scholar] [CrossRef]
- Cretu, C.; Andelescu, A.A.; Candreva, A.; Crispini, A.; Szerb, E.I.; La Deda, M. Bisubstituted-biquinoline Cu(i) complexes: Synthesis, mesomorphism and photophysical studies in solution and condensed states. J. Mater. Chem. C 2018, 6, 10073–10082. [Google Scholar] [CrossRef]
- Benito, Q.; Baptiste, B.; Polian, A.; Delbes, L.; Martinelli, L.; Gacoin, T.; Boilot, J.-P.; Perruchas, S. Pressure Control of Cuprophilic Interactions in a Luminescent Mechanochromic Copper Cluster. Inorg. Chem. 2015, 54, 9821–9825. [Google Scholar] [CrossRef]
- Volz, D.; Zink, D.M.; Bocksrocker, T.; Friedrichs, J.; Nieger, M.; Baumann, T.; Lemmer, U.; Bräse, S. Molecular Construction Kit for Tuning Solubility, Stability and Luminescence Properties: Heteroleptic MePyrPHOS-Copper Iodide-Complexes and Their Application in Organic Light-Emitting Diodes. Chem. Mater. 2013, 25, 3414–3426. [Google Scholar] [CrossRef]
- Liu, Z.; Qiu, J.; Wei, F.; Wang, J.; Liu, X.; Helander, M.G.; Rodney, S.; Wang, Z.; Bian, Z.; Lu, Z.; et al. Simple and High Efficiency Phosphorescence Organic Light-Emitting Diodes with Codeposited Copper(I) Emitter. Chem. Mater. 2014, 26, 2368–2373. [Google Scholar] [CrossRef]
- Cariati, E.; Lucenti, E.; Botta, C.; Giovanella, U.; Marinotto, D.; Righetto, S. Cu(I) hybrid inorganic–organic materials with intriguing stimuli responsive and optoelectronic properties. Coord. Chem. Rev. 2016, 306, 566–614. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).