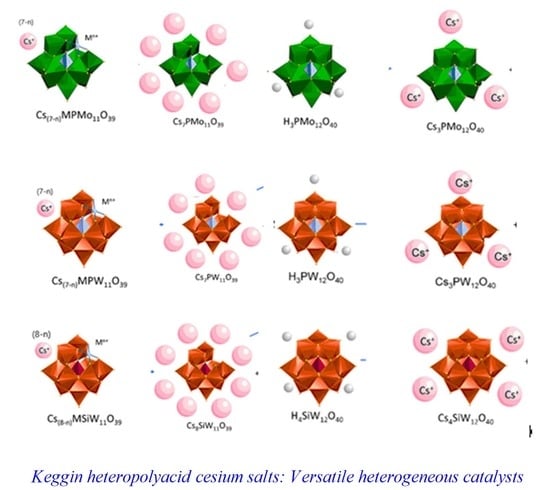
Cesium Heteropolyacid Salts: Synthesis, Characterization and Activity of the Solid and Versatile Heterogeneous Catalysts
Abstract
1. Introduction
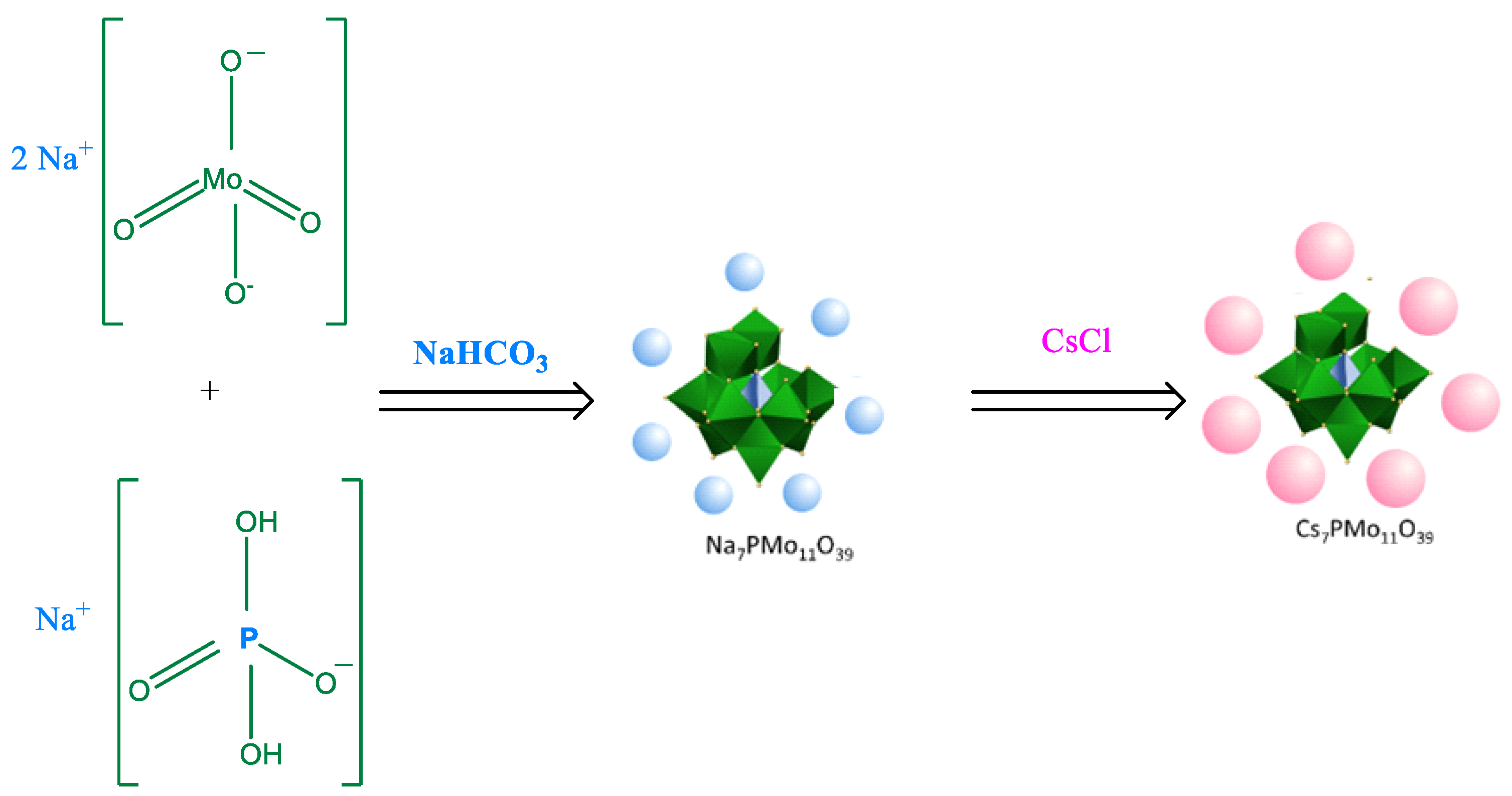
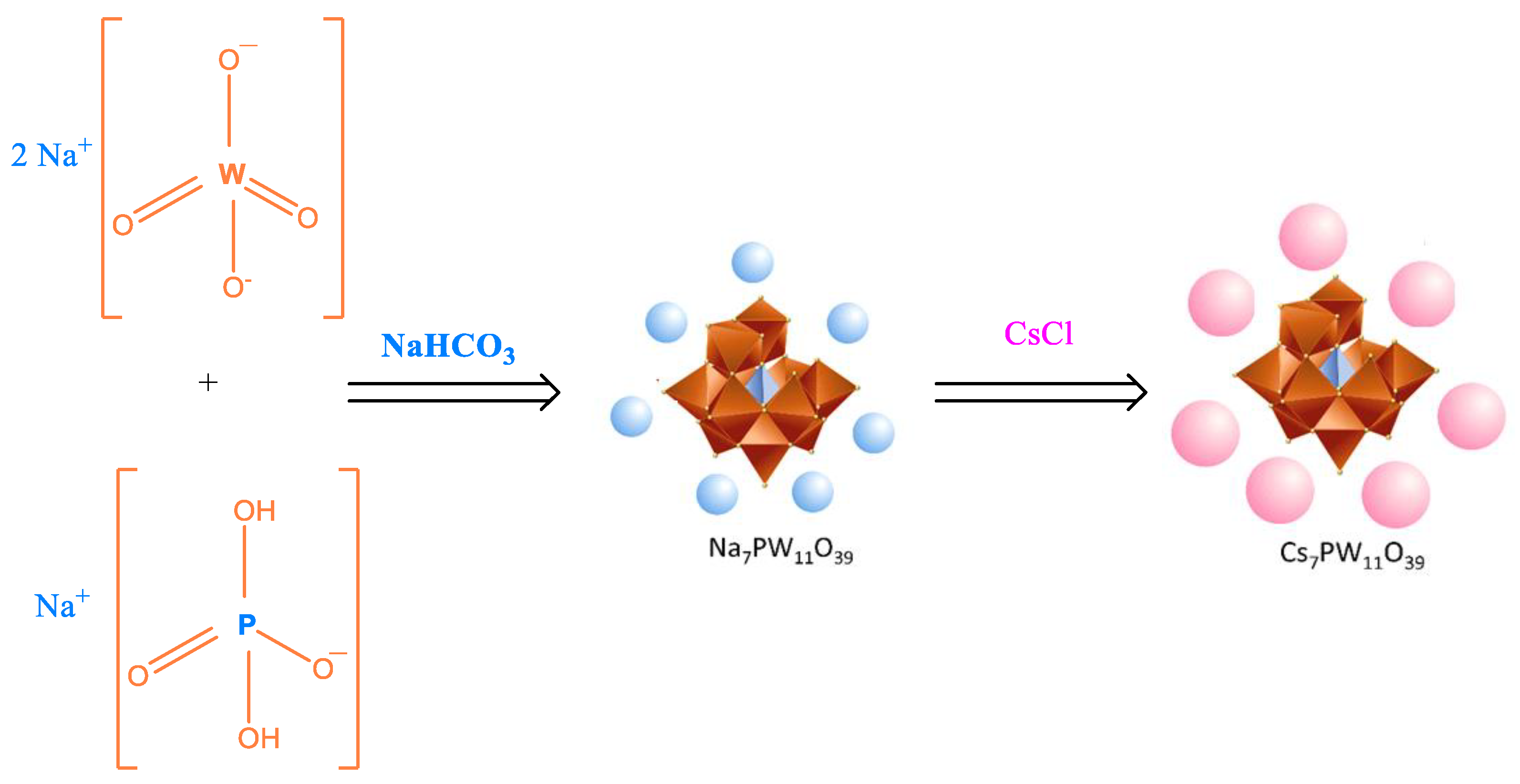
2. Main Routes to Synthesize POMs Salts
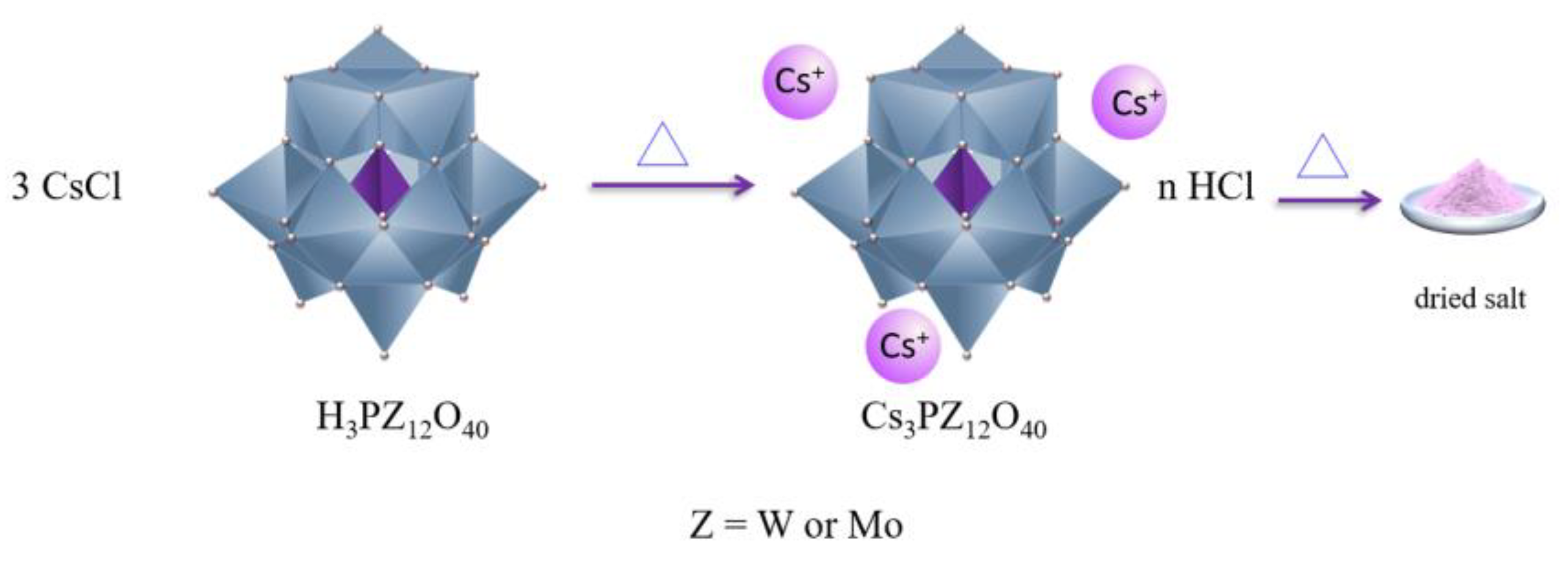
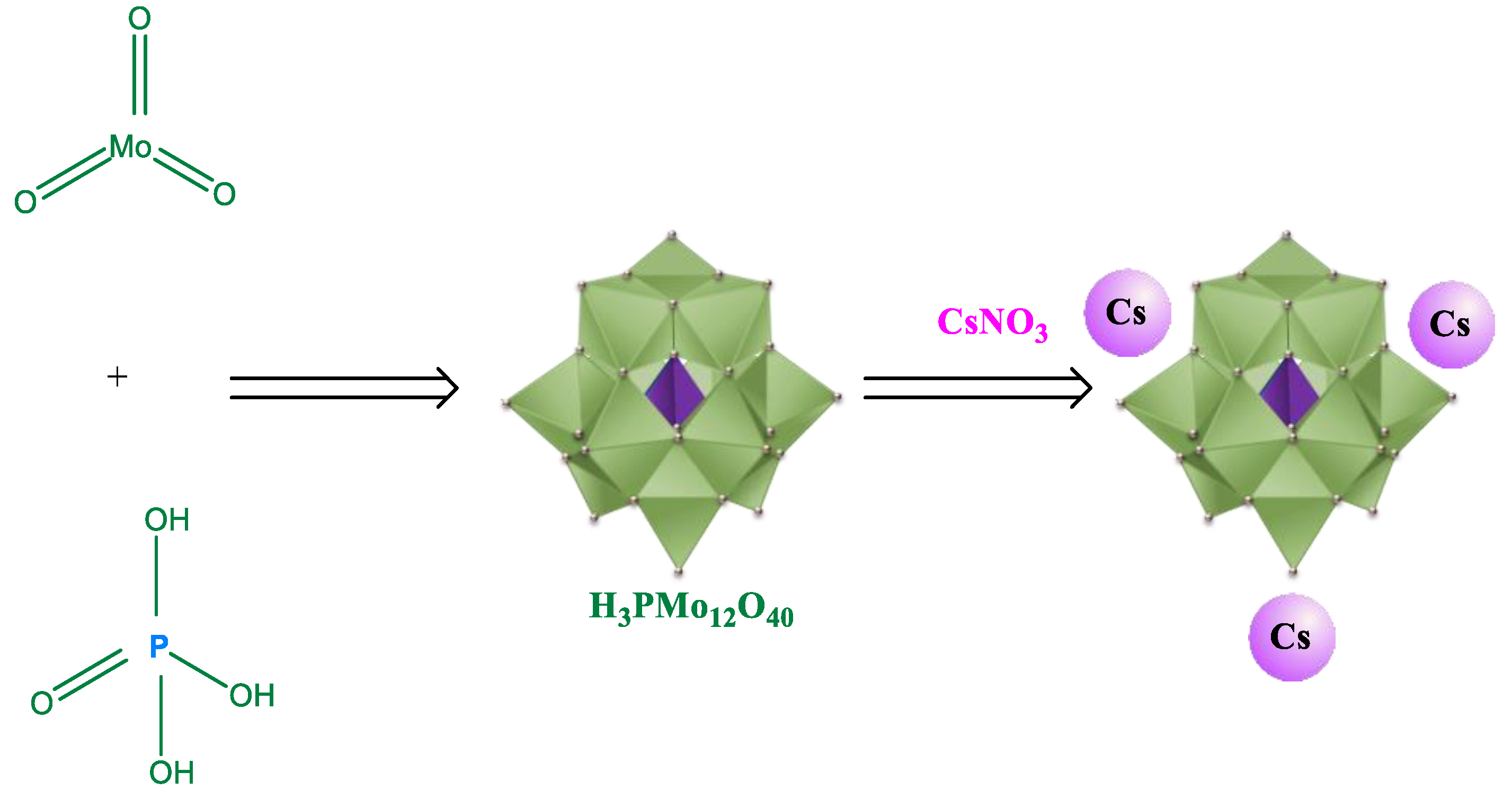
2.1. Synthesis of Keggin HPAs: Phosphotungstic, Phosphomolybdic and Silicotungstic Acid Cesium Salts
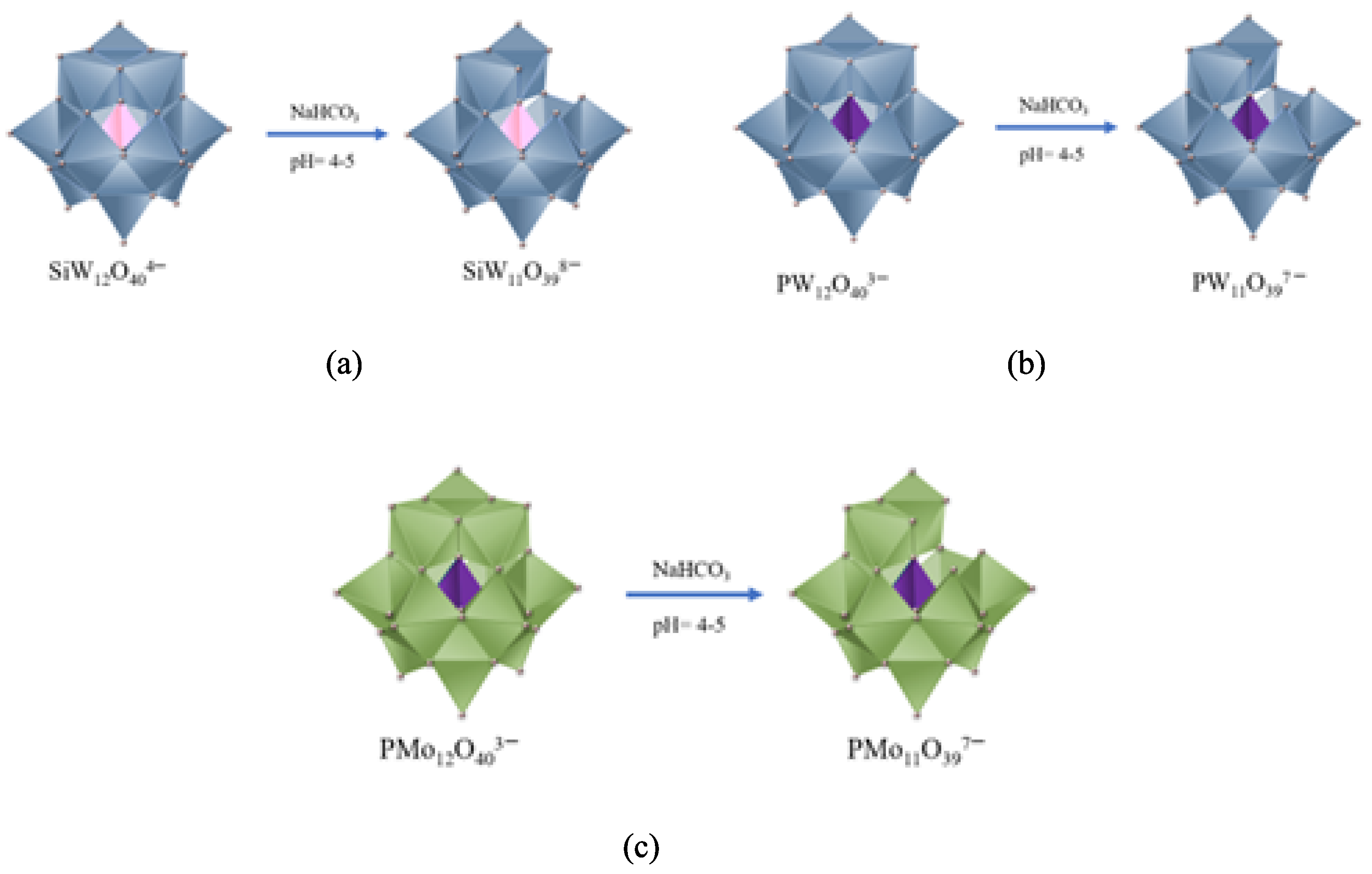
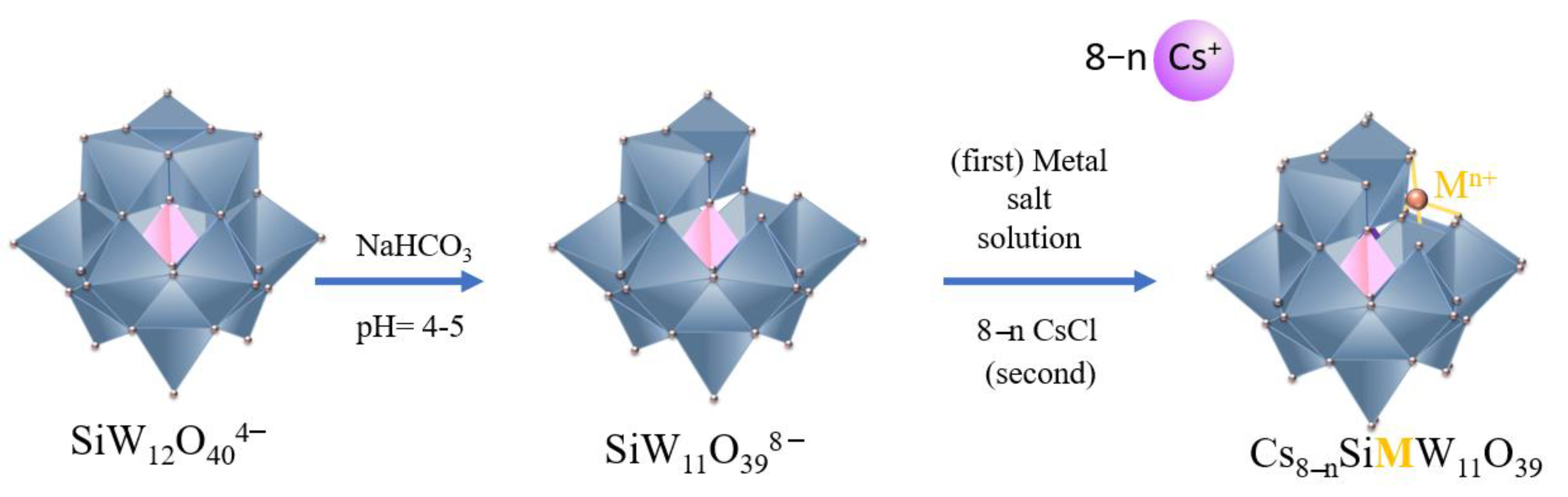
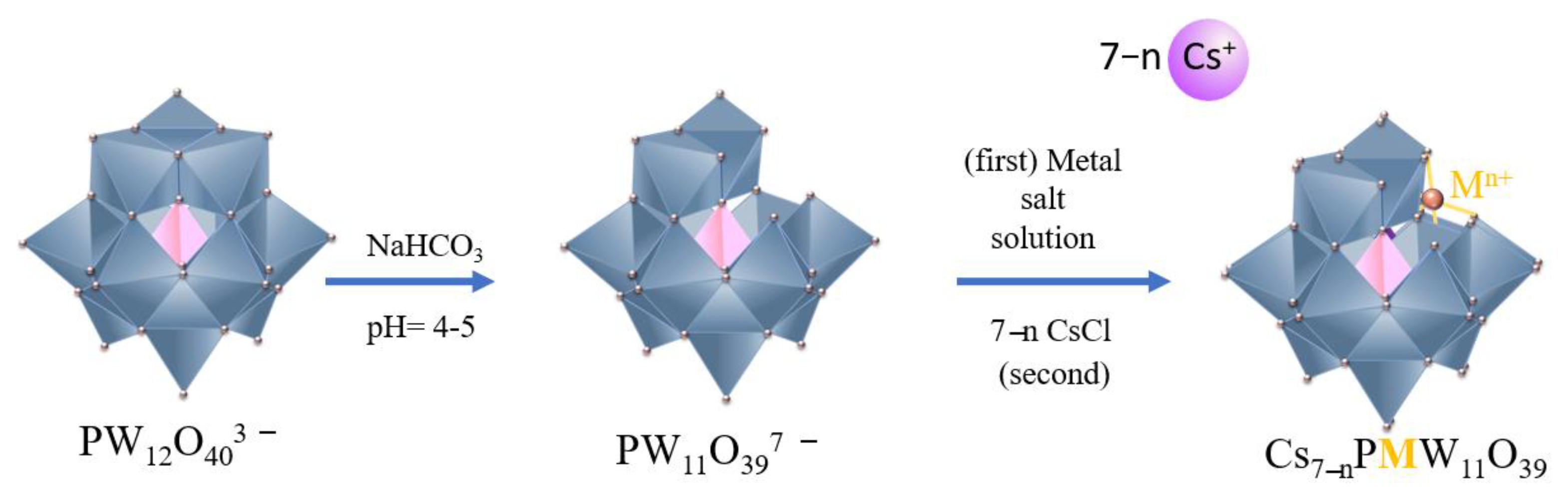
2.2. Synthesis of Lacunar Keggin HPA Cesium Salts
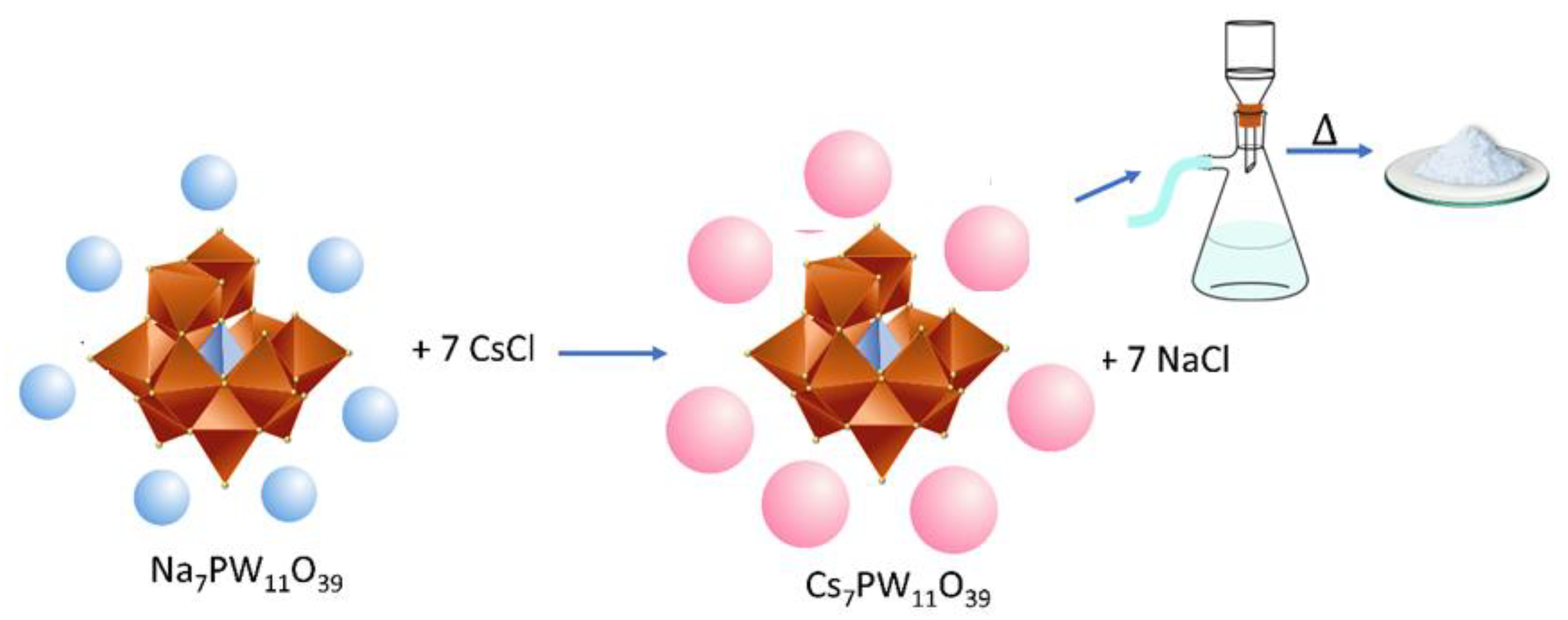
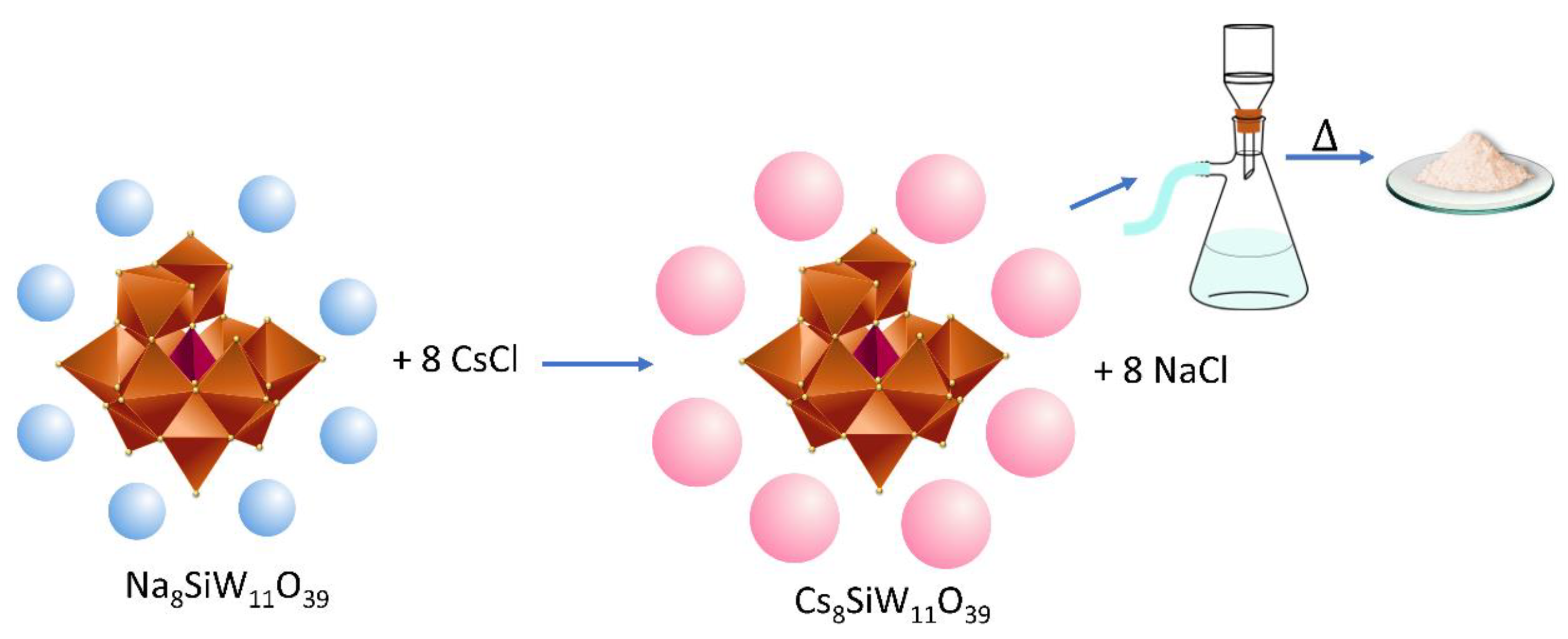
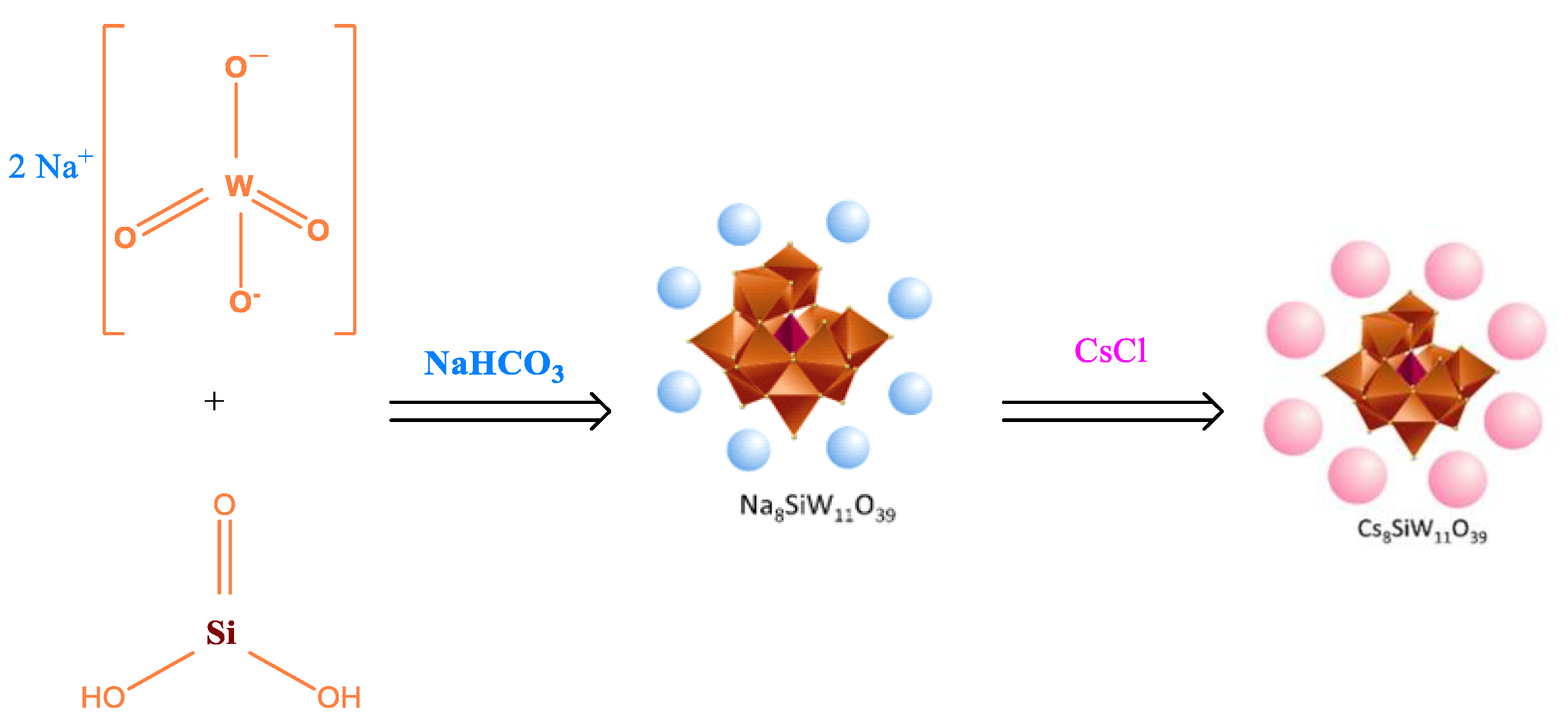
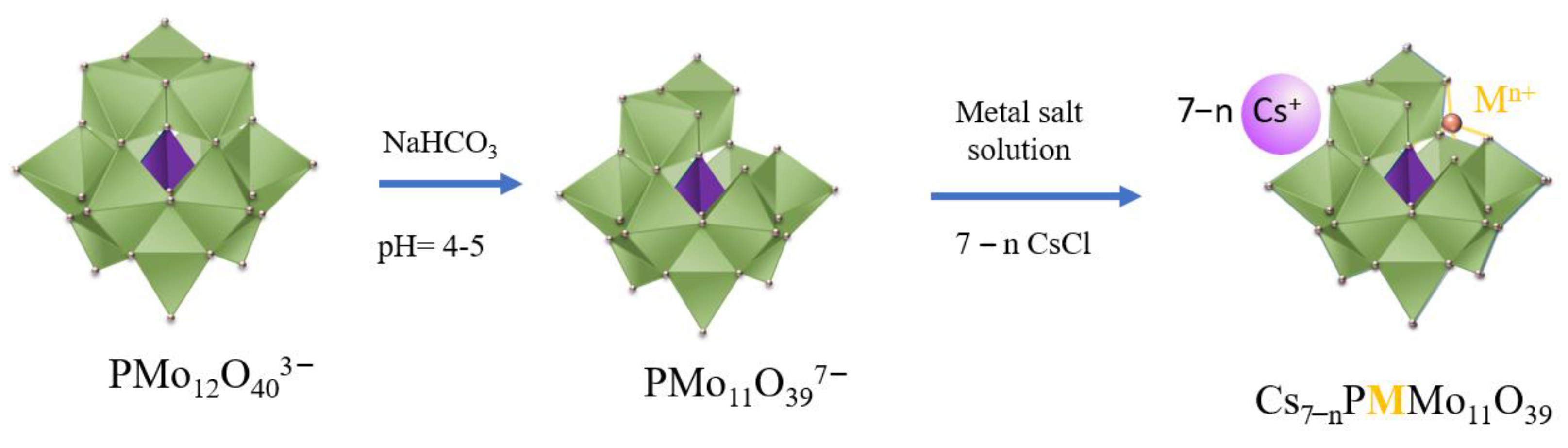
2.3. Synthesis of Metal-Substituted Lacunar Keggin HPA Cesium Salts
3. Characterization Techniques of Keggin HPA Salts
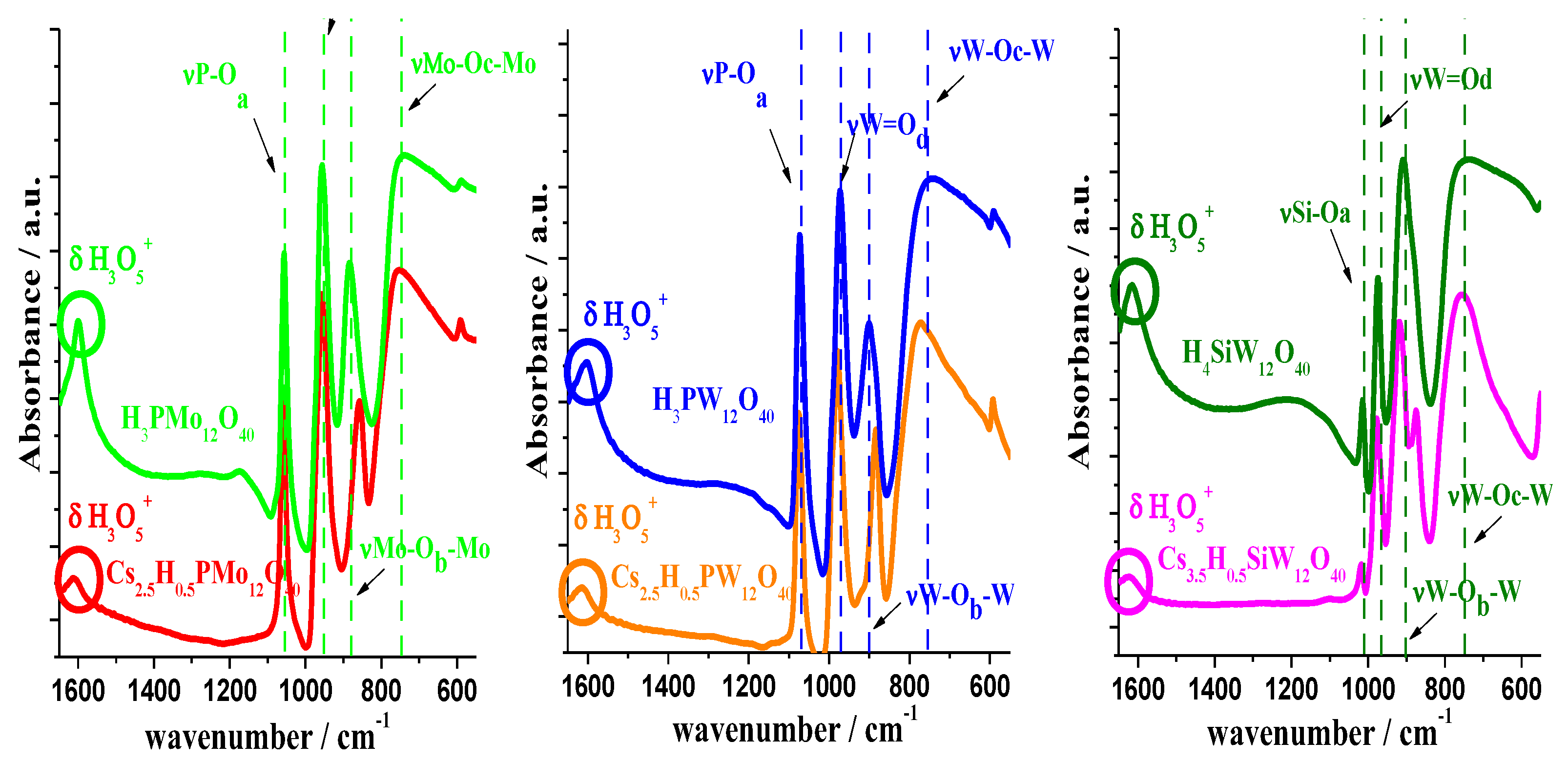
3.1. Infrared Spectroscopy
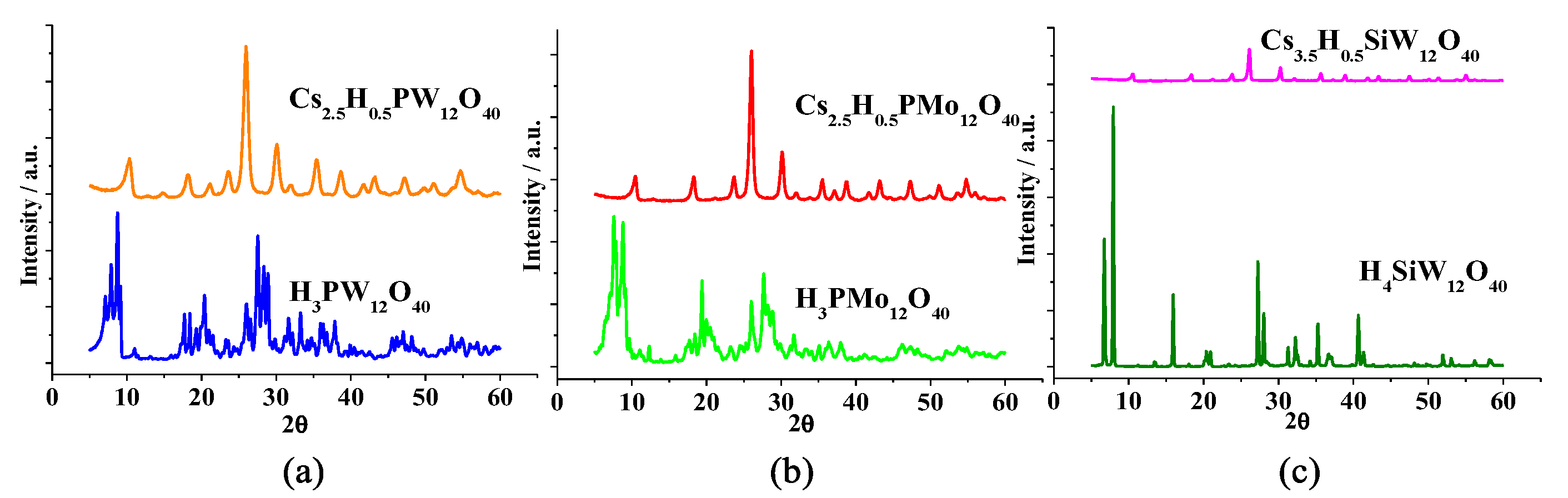
3.2. Powder XRD Patterns
3.3. Thermal Analyses
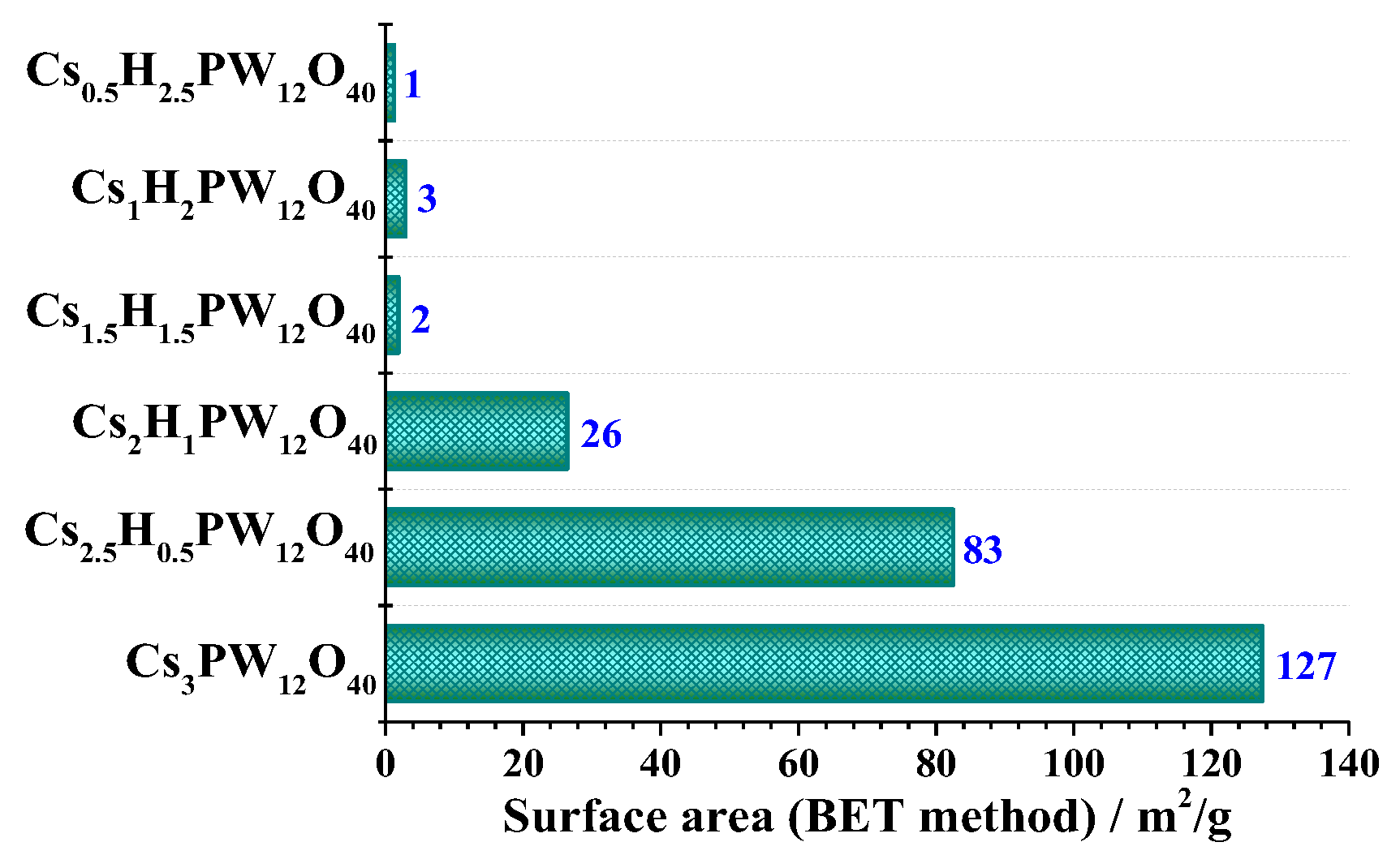
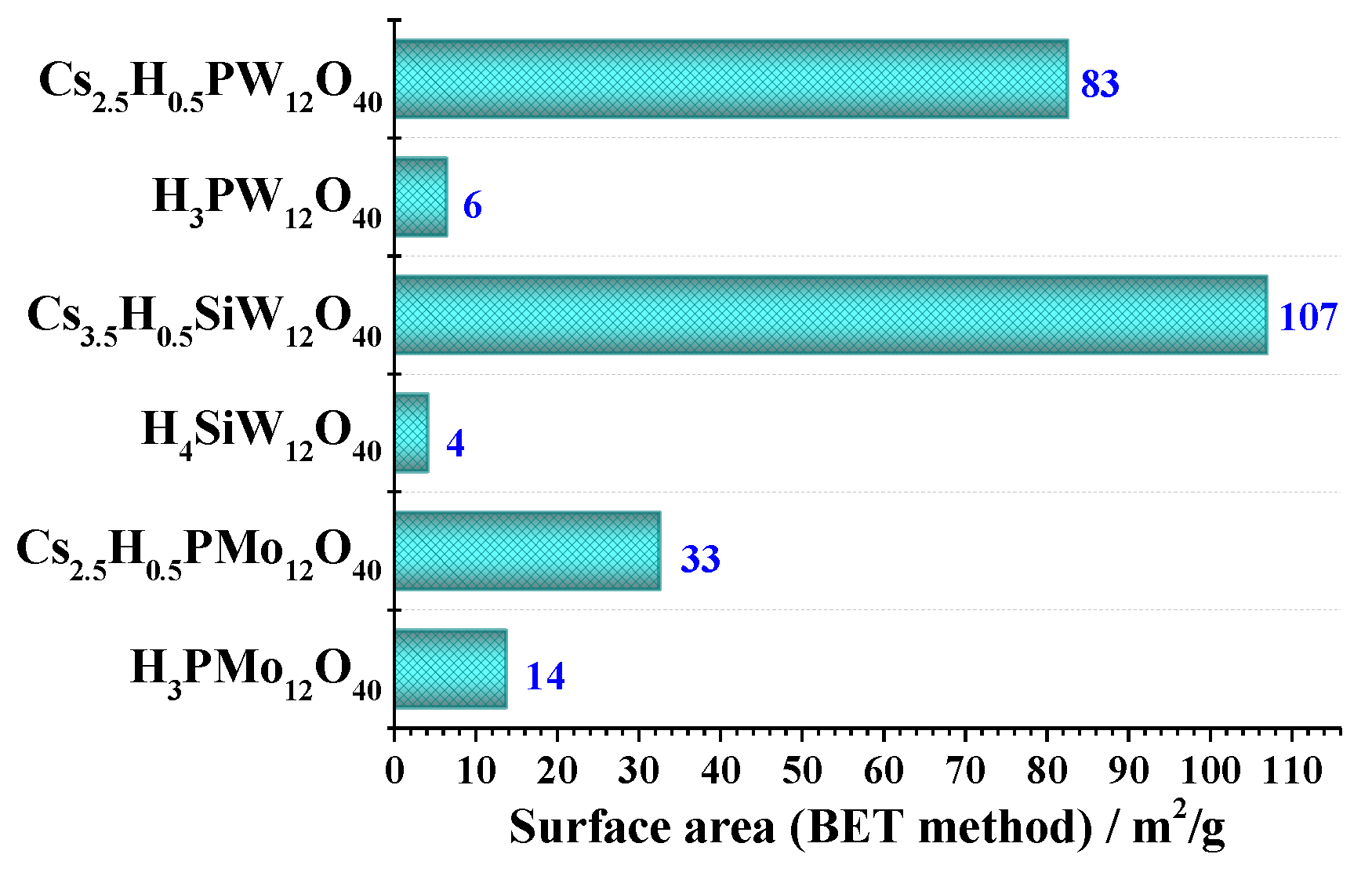
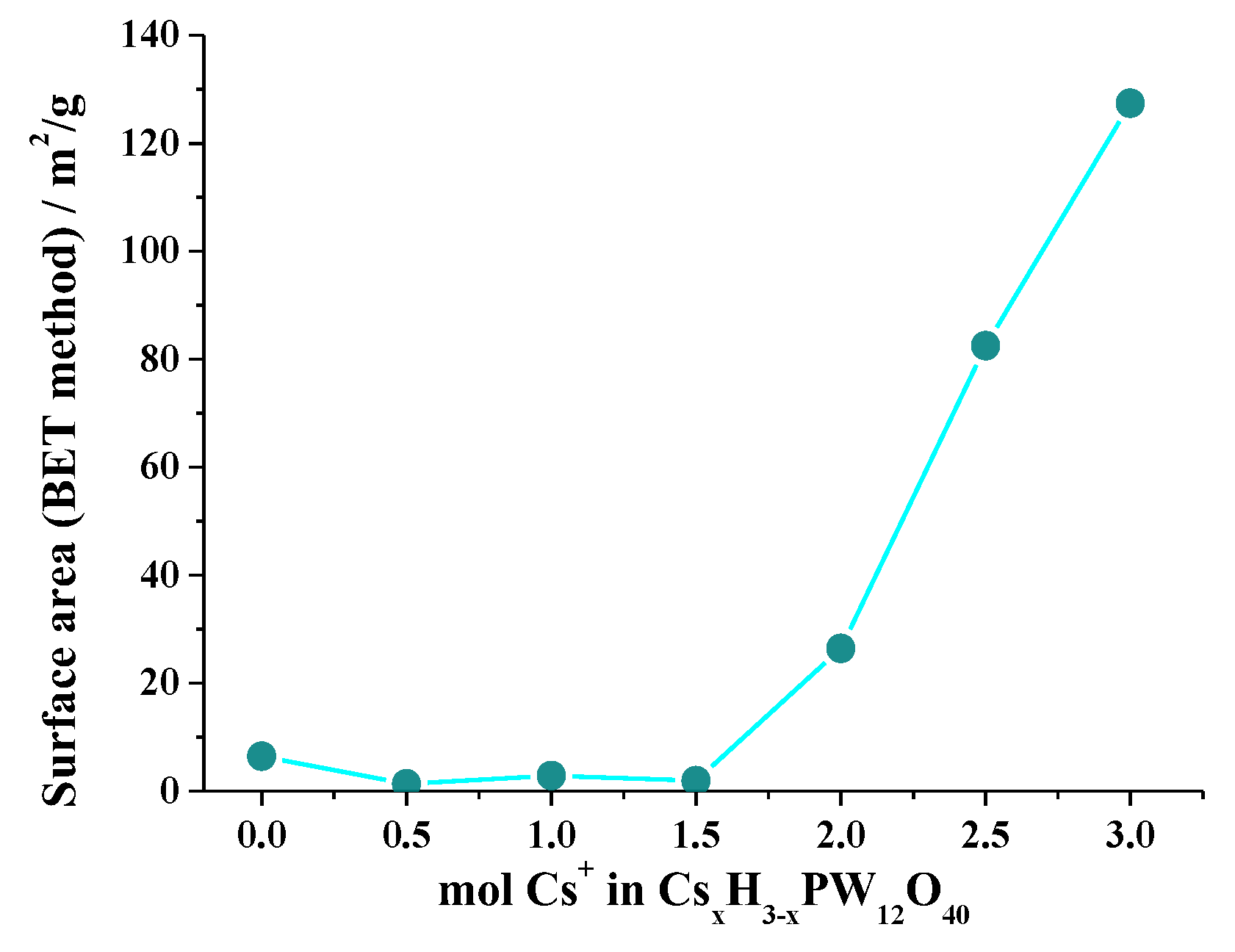
3.4. Surface Area: Brunauer–Emmett–Teller (BET) Method
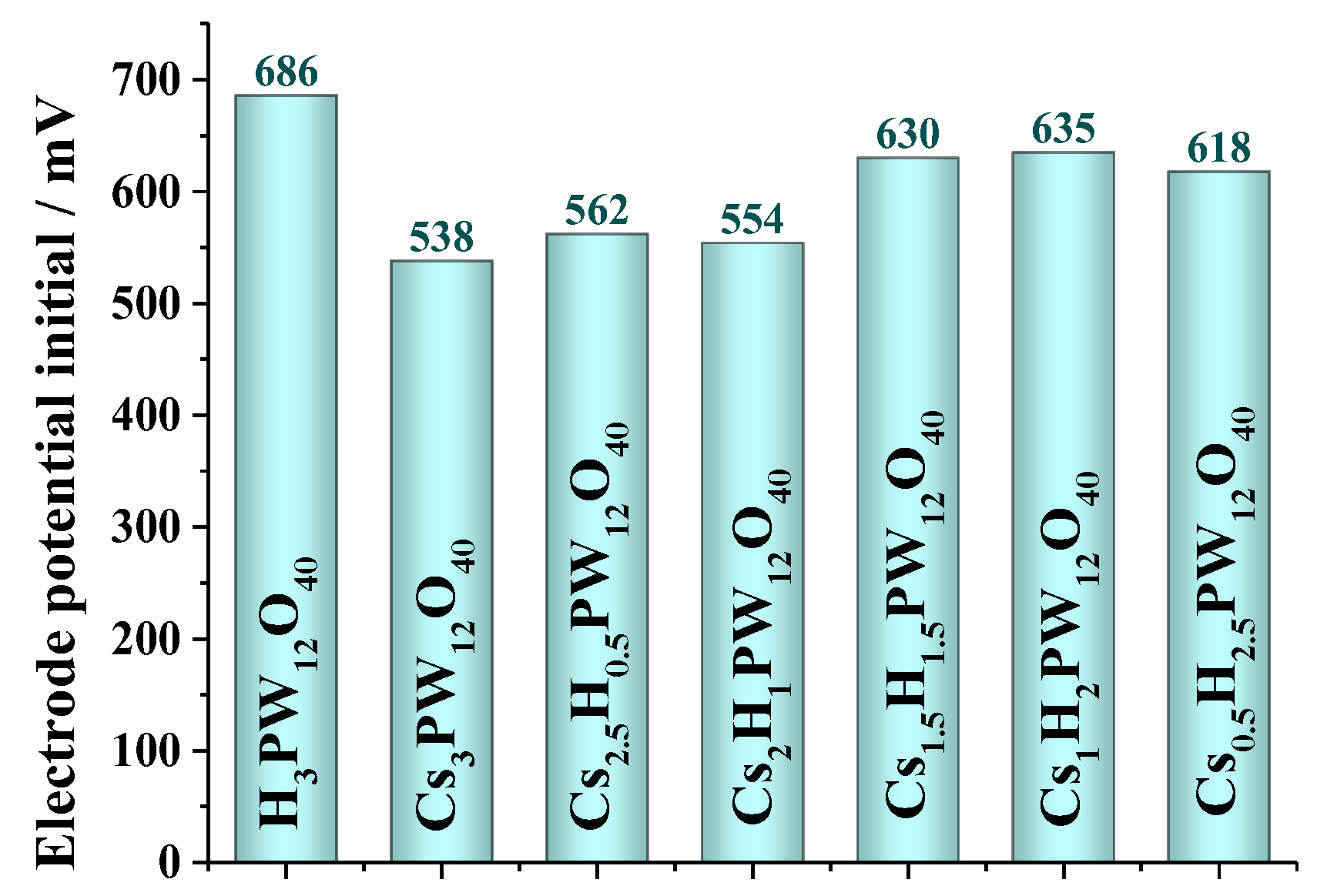
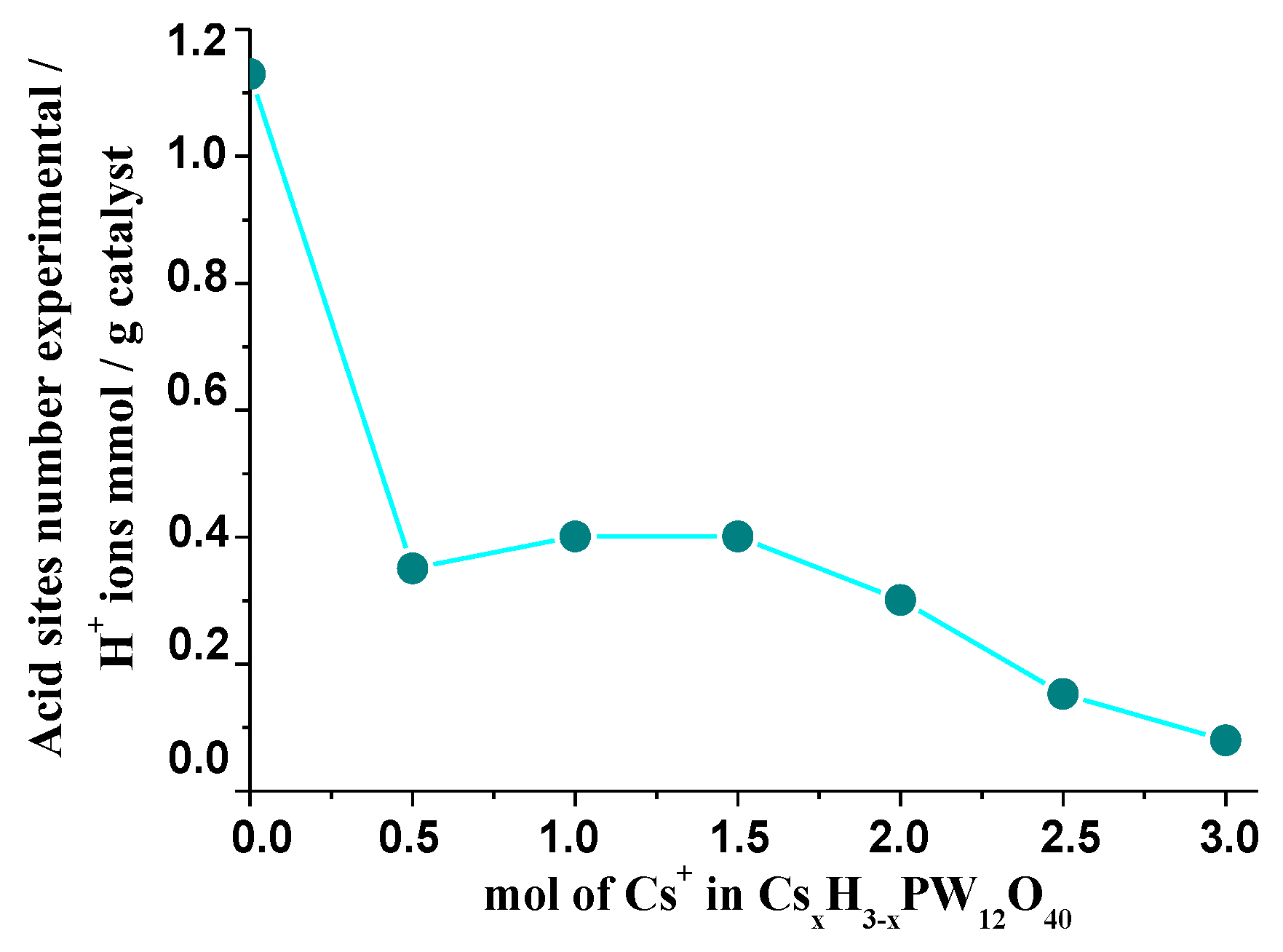
3.5. Measurements of Acidity Strength of Cesium Heteropoly Salts
4. Keggin HPA Cesium Salts-Catalyzed Reactions
4.1. Keggin HPA Cesium Salts: Acid-Catalyzed Reactions
4.2. Keggin HPA Cesium Salts as Catalysts in Oxidation Reactions
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Jambhulkar, D.K.; Ugwekar, R.P.; Bhanvase, B.A.; Barai, D.P. A review on solid base heterogeneous catalysts: Preparation, characterization, and applications. Chem. Eng. Commun. 2020, 209, 433–484. [Google Scholar] [CrossRef]
- Helwani, Z.; Othman, M.R.; Aziz, N.; Fernando, W.J.N.; Kim, J. Technologies for production of biodiesel focusing on green catalytic techniques: A review. Fuel Process. Technol. 2009, 90, 1502–1514. [Google Scholar] [CrossRef]
- Akinnawo, C.A.; Mosia, L.; Alimi, O.A.; Oseghale, C.O.; Fapojuwo, D.P.; Bingwa, N.; Meijboom, R. Eco-friendly synthesis of valuable fuel bio-additives from glycerol. Catal. Commun. 2021, 152, 106287–106293. [Google Scholar] [CrossRef]
- Nda-Umar, U.I.; Ramli, I.; Taufiq-Yap, Y.H.; Muhamad, E.N. An overview of recent research in the conversion of glycerol into biofuels, fuel additives and other biobased chemicals. Catalysts 2019, 9, 15. [Google Scholar] [CrossRef]
- Lin, L.; Han, X.; Han, B.; Yang, S. Emerging heterogeneous catalysts for biomass conversion: Studies of the reaction mechanism. Chem. Soc. Rev. 2021, 50, 11270–11292. [Google Scholar] [CrossRef]
- He, Q.; McNutt, J.; Yang, J. Utilization of the residual glycerol from biodiesel production for renewable energy generation. Renew. Sustain. Energy Rev. 2017, 71, 63–76. [Google Scholar] [CrossRef]
- Nanda, M.R.; Yuan, Z.; Qin, W.; Ghaziaskar, H.R.; Poirer, M.A.; Xu, C.C. Thermodynamic and kinetic studies of a catalytic process to convert glycerol into solketal as an oxygenated fuel additive. Fuel 2014, 117, 470–477. [Google Scholar] [CrossRef]
- Appaturi, J.N.; Ratti, R.; Phoon, B.L.; Batagarawa, S.M.; Din, I.U.; Selvaraj, M.; Ramalinga, R.J. A review of the recent progress on heterogeneous catalysts for Knoevenagel condensation. Dalton Trans. 2021, 50, 4445–4469. [Google Scholar] [CrossRef]
- Ruiz, V.E.; Velty, A.; Santos, L.L.; Perez, L.A.; Sabater, M.J.; Iborra, S.; Corma, A. Gold catalysts and solid catalysts for biomass transformations: Valorization of glycerol and glycerol—Water mixtures through formation of cyclic acetals. J. Catal. 2010, 271, 351–357. [Google Scholar] [CrossRef]
- Ballotin, F.C.; Da Silva, M.J.; Teixeira, A.P.C.; Lago, R.M. Amphiphilic acid carbon catalysts produced by bio-oil sulfonation for solvent-free glycerol ketalization. Fuel 2020, 274, 117779. [Google Scholar] [CrossRef]
- Yang, X.F.; Wang, A.; Qiao, B.; Li, J.; Liu, J.; Zhang, T. Single-atom catalysts: A new frontier in heterogeneous catalysis. Acc. Chem. Res. 2013, 46, 1740–1748. [Google Scholar] [CrossRef] [PubMed]
- Kozhevnikov, I.V. Heteropoly acids and related compounds as catalysts for fine chemical synthesis. Catal. Rev. 1995, 37, 311–352. [Google Scholar] [CrossRef]
- Lai, S.Y.; Ng, K.H.; Cheng, C.K.; Nur, H.; Nurhadi, M.; Arumugam, M. Photocatalytic remediation of organic waste over Keggin-based polyoxometalate materials: A review. Chemosphere 2021, 263, 128244. [Google Scholar] [CrossRef] [PubMed]
- Popa, A.; Sasca, V.; Kiš, E.E.; Radmila, J.; Marinkovi-Neducin, R.; Bokorov, M.T.; Halasz, M.T. Structure and texture of some Keggin type heteropolyacids supported on silica and titania. J. Optoelectron. Adv. Mat. 2005, 7, 3169–3177. [Google Scholar]
- Ruiz, D.M.; Pasquale, G.A.; Martínez, J.J.; Romanelli, G.P. Advances in novel activation methods to perform green organic synthesis using recyclable heteropolyacid catalysis. Green Process. Synth. 2022, 11, 766–809. [Google Scholar] [CrossRef]
- Amini, M.M.; Shaabani, A.; Bazgir, A. Tungstophosphoric acid (H3PW12O40): An efficient and eco-friendly catalyst for the one-pot synthesis of dihydropyrimidin-2(1H)-ones. Catal. Commun. 2006, 7, 843–847. [Google Scholar] [CrossRef]
- Patil, M.R.; Yelamaggad, A.; Keri, R.S. A mild, efficient, and reusable solid phosphotungstic acid catalyst mediated synthesis of benzoxazole derivatives: A grinding approach. Lett. Org. Chem. 2016, 13, 474–481. [Google Scholar] [CrossRef]
- Chaves, D.M.; Ferreira, S.O.; Chagas da Silva, R.; Natalino, R.; da Silva, M.J. Glycerol esterification over Sn(II)-exchanged Keggin heteropoly salt catalysts: Effect of thermal treatment temperature. Energy Fuels 2019, 33, 7705–7716. [Google Scholar] [CrossRef]
- Umbarkar, S.B.; Kotbagi, T.V.; Biradar, A.V.; Pasricha, R.; Chanale, J.; Dongare, M.K.; Mamede, A.S.; Lancelot, C.; Payen, E.J. Acetalization of glycerol using mesoporous MoO3/SiO2 solid acid catalyst. Mol. Catal. A 2009, 310, 150–158. [Google Scholar] [CrossRef]
- Serafim, H.; Fonseca, I.M.; Ramos, A.M.; Vital, J.; Castanheiro, J.E. Valorization of glycerol into fuel additives over zeolites catalysts. Chem. Eng. J. 2011, 178, 291–296. [Google Scholar] [CrossRef]
- Coronel, N.C.; Da Silva, M.J. Lacunar Keggin heteropolyacid salts: Soluble, solid, and solid-supported catalysts. J. Clust. Sci. 2018, 29, 195–205. [Google Scholar] [CrossRef]
- Da Silva, M.J.; Teixeira, M.G.; Natalino, R. Highly selective synthesis under benign reaction conditions of furfural dialkyl acetal using SnCl2 as a recyclable catalyst. New J. Chem. 2019, 43, 8606–8612. [Google Scholar] [CrossRef]
- Narkhede, N.; Patel, A. Sustainable valorisation of glycerol via acetalization as Well as carboxylation reactions over silicotungstates anchored to zeolite Hβ. Appl. Catal. A 2016, 515, 154–163. [Google Scholar] [CrossRef]
- Srikanth, A.; Viswanadham, B.; Kumar, V.P.; Anipindi, N.R.; Chary, K.V.R. Synthesis and characterization of Cs-exchanged heteropolyacid catalysts functionalized with Sn for carbonolysis of glycerol to glycerol carbonate. Appl. Petrochem. Res. 2016, 6, 145–153. [Google Scholar] [CrossRef]
- Ferreira, P.A.; Fonseca, I.M.; Ramos, A.M.; Vital, J.; Castanheiro, J.E. Valorization of glycerol by condensation with acetone over silica-included heteropolyacids. App. Catal. B Environ. 2010, 98, 94–99. [Google Scholar] [CrossRef]
- Charson, N.; Amnuaypanich, S.; Soontaranon, S.; Rugmai, S.; Amnuaypanich, S. Increasing solketal production from the solventless ketalization of glycerol catalyzed by nanodispersed phosphotungstic acid in poly(N-methyl-4-vinylpyridinium) grafted on silica nanoparticles. J. Indust. Eng. Chem. 2022, 112, 233–243. [Google Scholar] [CrossRef]
- Srinivas, M.; Raveendra, G.; Parameswaram, G.; Prasad, P.S.S.; Lingaiah, N. Cesium exchanged tungstophosphoric acid Supported on tin oxide: An efficient solid acid catalyst for etherification of glycerol with tert-butanol to synthesize biofuel additives. J. Mol. Catal. A 2016, 413, 7–14. [Google Scholar] [CrossRef]
- Narkhede, N.; Patel, A. Biodiesel production by esterification of oleic acid and transesterification of soybean oil using a new solid acid catalyst comprising 12-tungstosilicic acid and zeolite Hβ. Ind. Eng. Chem. Res. 2013, 52, 13637–13644. [Google Scholar] [CrossRef]
- Da Silva, M.J.; De Oliveira, C.M. Catalysis by Keggin Heteropolyacid Salts. Curr. Catal. 2018, 7, 26–34. [Google Scholar] [CrossRef]
- Da Silva, M.J.; Liberto, N.A. Soluble and solid supported Keggin heteropolyacids as catalysts in reactions for biodiesel production: Challenges and recent advances. Curr. Org. Chem. 2016, 20, 1263–1283. [Google Scholar] [CrossRef]
- Da Silva, M.J.; Ribeiro, C.J.A.; Vilanculo, C.B. How the content of protons and vanadium affects the activity of H3+nPMo12−nVnO40 (n = 0, 1, 2, or 3) catalysts on the oxidative esterification of benzaldehyde with hydrogen peroxide. Catal. Lett. 2022. [Google Scholar] [CrossRef]
- Narkhede, N.; Patel, A. Efficient synthesis of biodiesel over a recyclable catalyst comprising a monolacunary silicotungstate and zeolite Hβ. RSC Adv. 2014, 4, 64379–64387. [Google Scholar] [CrossRef]
- Vilanculo, C.B.; Da Silva, M.J.; Rodrigues, A.A.; Ferreira, S.O.; Da Silva, R.C. Vanadium-doped sodium phosphomolybdate salts as catalysts in the terpene alcohols oxidation with hydrogen peroxide. RSC Adv. 2021, 11, 24072–24085. [Google Scholar] [CrossRef] [PubMed]
- Da Silva, M.J.; Andrade, P.H.; Sampaio, V.F.C. Transition metal-substituted potassium silicotungstate salts as catalysts for oxidation of terpene alcohols with hydrogen peroxide. Catal. Lett. 2021, 151, 2094–2106. [Google Scholar] [CrossRef]
- Da Silva, M.J.; Lopes, N.P.G.; Bruziquesi, C.G.O. Furfural acetalization over Keggin heteropolyacid salts at room temperature: Effect of cesium doping. React. Kinet. Mech. Catal. 2021, 133, 913–931. [Google Scholar] [CrossRef]
- Da Silva, M.J.; Lopes, N.P.G.; Ferreira, S.O.; Da Silva, R.C.; Natalino, R.; Chaves, D.M.; Texeira, M.G. Monoterpenes etherification reactions with alkyl alcohols over cesium partially exchanged Keggin heteropoly salts: Effects of catalyst composition. Chem. Pap. 2021, 75, 153–168. [Google Scholar] [CrossRef]
- Lopes, N.P.G.; Da Silva, M.J. Cesium partially exchanged heteropolyacid salts: Efficient solid catalysts to produce bioadditives from the levulinic acid esterification with alkyl alcohols. React. Kinet. Mech. Catal. 2022, 135, 3173–3184. [Google Scholar] [CrossRef]
- Pizzio, L.R.; Blanco, M.N. A contribution to the physicochemical characterization of nonstoichiometric salts of tungstosilicic acid. Microporous Mesopor. Mater. 2007, 103, 40–47. [Google Scholar] [CrossRef]
- Park, H.W.; Park, S.; Park, D.R.; Choi, J.H.; Song, I.K. Decomposition of phenethyl phenyl ether to aromatics over CsxH3.0−xPW12O40 (X = 2.0–3.0) heteropolyacid catalysts. Catal. Commun. 2010, 12, 35. [Google Scholar] [CrossRef]
- Dias, J.A.; Caliman, E.; Dias, S.C.L. Effects of cesium ion exchange on the acidity of 12-tungstophosphoric acid. Micropor Mesopor. Mater. 2004, 76, 221–232. [Google Scholar] [CrossRef]
- Chai, F.; Cao, F.; Zhai, F.; Chen, Y.; Wang, X.; Su, Z. Transesterifcation of vegetable oil to biodiesel using a heteropolyacid solid catalyst. Adv. Synth. Catal. 2007, 349, 1057–1065. [Google Scholar] [CrossRef]
- Molchanov, V.V.; Maksimov, G.M.; Gojdin, V.V.; Kulikov, S.M.; Kulikova, O.M.; Kozhevnikov, I.V.; Bujanov, R.A.; Maksimovskaja, R.I.; Pljasova, L.M.; Lapina, O.B. Method of Preparing Phosphorus Heteropolyacids. RU 2076070 C1. 1997. Available online: https://www.elibrary.ru/item.asp?id=38057690 (accessed on 1 January 2023).
- Gu, Q.; Shi, S.; Liu, X.; Lian, H.; Wang, T.; Heng Zhang, H. Effects of preparation method on the catalytic properties and deactivation behaviors of acidic cesium phosphomolybdate for the alkylation of toluene with benzyl alcohol. React. Kinet. Mech. Catal. 2022, 135, 1819–1834. [Google Scholar] [CrossRef]
- Batalha, D.C.; Ferreira, S.O.; Da Silva, R.C.; Da Silva, M.J. Cesium-Exchanged Lacunar Keggin Heteropolyacid Salts: Efficient Solid Catalysts for the Green Oxidation of Terpenic Alcohols with Hydrogen Peroxide. Chem. Select 2020, 5, 1976–1986. [Google Scholar] [CrossRef]
- Da Silva, M.J.; Andrade, P.H.S.; Ferreira, S.O.; Vilanculo, C.B.; Oliveira, C.M. Monolacunary K8SiW11O39-catalyzed terpenic alcohols oxidation with hydrogen peroxide. Catal. Lett. 2018, 148, 2516–2527. [Google Scholar] [CrossRef]
- Patel, A.U.; Patel, J.R. Cesium salt of iron substituted phosphomolybdate: Synthesis, characterization, room temperature hydrogenation of styrene and its mechanistic evaluation. Mol. Catal. 2021, 513, 111827–111837. [Google Scholar] [CrossRef]
- Hiyoshi, N.; Kamiya, Y. Observation of microporous cesium salts of 12-tungstosilicic acid using scanning transmission electron microscopy. Chem. Commun. 2015, 51, 9975–9978. [Google Scholar] [CrossRef]
- Okuhara, T. Water-Tolerant Solid Acid Catalysts. Chem. Rev. 2002, 102, 3641–3666. [Google Scholar] [CrossRef]
- Drago, R.S.; Dias, J.A.; Maier, T.O. An Acidity Scale for Brönsted Acids Including H3PW12O40. J. Am. Chem. Soc. 1997, 119, 7702. [Google Scholar] [CrossRef]
- Deng, W.; Zhang, Q.; Wang, Y. Polyoxometalates as efficient catalysts for transformations of cellulose into platform chemicals. Dalton Trans. 2012, 41, 9817–9831. [Google Scholar] [CrossRef] [PubMed]
- Pathan, S.; Patel, A. Keggin type transition metal substituted phosphomolybdates: Heterogeneous catalysts for selective aerobic oxidation of alcohols and alkenes under solvent-free condition. Catal. Sci. Technol. 2014, 4, 648–656. [Google Scholar] [CrossRef]
- Karinen, R.S.; Krause, A.O.I. New Biocomponents from Glycerol. Appl Catal A 2006, 306, 128–133. [Google Scholar] [CrossRef]
- Subhash, M.; Pal, D.B.; Jana, S.K. Biofuels Additives Derived via Clay Supported Heteropoly Acid Catalyzed Etherification of Glycerol with tert-Butanol-Biomass to Liquid Oxygenates. Chem. Pap. 2022, 76, 775–784. [Google Scholar] [CrossRef]
- Veluturia, S.; Narula, A.; Shetty, S.P. Kinetic study of synthesis of bio-fuel additives from glycerol using a heteropolyacid. Resour. Technol. 2017, 3, 337–341. [Google Scholar] [CrossRef]
- Tsolakis, N.; Bam, W.; Srai, J.S.; Kumar, M. Renewable chemical feedstock supply network design: The case of terpenes. J. Clean Prod. 2019, 222, 802–822. [Google Scholar] [CrossRef]
- Corma, A.; Iborra, S.; Velty, A. Chemical Routes for the Transformation of Biomass into Chemicals. Chem. Rev. 2007, 107, 2411–2502. [Google Scholar] [CrossRef]
- Masyita, A.; Sari, R.M.; Astuti, A.D.; Yasir, B.; Rumata, N.R.; Emran, T.B.; Nainu, F.; Simal-Gandara, J. Terpenes and terpenoids as main bioactive compounds of essential oils, Terpenes and terpenoids as main bioactive compounds of essential oils, their roles in human health and potential application as natural, food preservatives. Food Chem. X 2022, 13, 100217–100231. [Google Scholar] [CrossRef]
- Montané, D.; Salvadó, J.; Torras, C.; Farriol, X. High-Temperature Dilute-Acid Hydrolysis of Olive Stones for Furfural Production. Biomass Bioenerg. 2002, 22, 295–304. [Google Scholar] [CrossRef]
- Zeitsch, K.J. The Chemistry and Technology of Furfural and Its Many By-Products, 1st ed.; Elsevier: Amsterdam, The Netherlands, 2000. [Google Scholar]
- Monteiro, J.L.F.; Veloso, C.O. Catalytic conversion of terpenes into fine chemicals. Top. Catal. 2004, 27, 169–180. [Google Scholar] [CrossRef]
- Bozell, J.J.; Petersen, G.R. Technology development for the production of biobased products from biorefinery carbohydrates–the US Department of Energy’s “Top 10” revisited. Green Chem. 2010, 12, 539–554. [Google Scholar] [CrossRef]
- Mascal, M.; Nikitin, E.B. High-yield conversion of plant biomass into the key value-added feedstocks 5- (hydroxymethyl)furfural, levulinic acid, and levulinate esters via 5-(chloromethyl)furfural. Green Chem. 2010, 12, 370–373. [Google Scholar] [CrossRef]
- Gurbuz, E.I.; Alonso, D.M.; Bond, J.Q.; Dumesic, J.A. Reactive extraction of levulinate esters and conversion to γ-valerolactone for production of liquid fuels. ChemSusChem 2011, 4, 357–361. [Google Scholar] [CrossRef] [PubMed]
- Xu, G.-Z.; Chang, C.; Zhu, W.-N.; Li, B.; Ma, X.-J.; Du, F.-G. A comparative study on direct production of ethyl levulinate from glucose in ethanol media catalysed by different acid catalysts. Chem. Pap. 2013, 67, 1355–1363. [Google Scholar] [CrossRef]
- Joshi, H.; Moser, B.R.; Toler, J.; Smith, W.F.; Walker, T. Ethyl levulinate: A potential bio-based diluent for biodiesel which improves cold flow properties. Biomass Bioenerg. 2011, 35, 3262–3266. [Google Scholar] [CrossRef]
- Saravanamurugan, S.; Riisager, A. Solid acid catalysed the formation of ethyl levulinate and ethyl glucopyranoside from mono- and disaccharides. Catal. Commun. 2012, 17, 71–75. [Google Scholar] [CrossRef]
- Fernandes, D.R.; Rocha, A.S.; Mai, E.F.; Mota, C.J.A.; Teixeira da Silva, V. Levulinic acid esterification with ethanol to ethyl levulinate production over solid acid catalysts. Appl. Catal. A 2012, 425–426, 199–204. [Google Scholar] [CrossRef]
- Vilanculo, C.B.; de Andrade Leles, L.C.; Da Silva, M.J. H4SiW12O40-Catalyzed Levulinic Acid Esterifcation at Room Temperature for Production of Fuel Bioadditives. Waste Biomass Valor 2020, 11, 1895–1904. [Google Scholar] [CrossRef]
- Patel, A.; Pathan, S. Keggin-type cesium salt of first series transition metal-substituted phosphomolybdates: One-pot easy synthesis, structural, and spectral analysis. J. Coord. Chem. 2012, 65, 3122–3132. [Google Scholar] [CrossRef]
- Pathan, S.; Patel, A. Transition-Metal-Substituted Phosphomolybdates: Catalytic and Kinetic Study for Liquid-Phase Oxidation of Styrene. Ind. Eng. Chem. Res. 2013, 52, 11913–11919. [Google Scholar] [CrossRef]
- Patel, K.; Shringarpure, P.; Patel, A. One-step synthesis of a Keggin-type manganese(II)-substituted phosphotungstate: Structural and spectroscopic characterization and non-solvent liquid phase oxidation of styrene. Transit. Met. Chem. 2011, 36, 171–177. [Google Scholar] [CrossRef]
- Li, X.; Zhang, Y. Oxidative Dehydration of Glycerol to Acrylic Acid over Vanadium-Substituted Cesium Salts of Keggin-Type Heteropolyacid. ACS Catal. 2016, 6, 2785–2791. [Google Scholar] [CrossRef]
- Pathan, S.; Patel, A. Solvent-free clean selective oxidation of alcohols catalyzed by mono transition metal (Co, Mn, Ni)-substituted Keggin-phosphomolybdates using hydrogen peroxide. Appl. Catal. A 2013, 459, 59–64. [Google Scholar] [CrossRef]
- Sandesh, S.; Manjunathan, P.; Halgeri, A.B.; Shanbha, G.V. Glycerol acetins: Fuel additive synthesis by acetylation and esterification of glycerol using cesium phosphotungstate catalyst. RSC Adv. 2015, 5, 104354–104362. [Google Scholar] [CrossRef]
- Iwase, Y.; Sano, S.; Mahardiani, L.; Abe, R.; Kamiy, Y. Bimodal cesium hydrogen salts of 12-tungstosilicic acid, CsxH4−xSiW12O40, as highly active solid acid catalysts for transesterification of glycerol tributyrate with methanol. J. Catal. 2014, 318, 34–42. [Google Scholar] [CrossRef]
- Xi, X.; Sun, D.; An, H.; Wang, Y. Cesium silicotungstate catalyzed solvent-free self-condensation of levulinic acid and its product identification. Biomass Conv. Bioref. 2022. [Google Scholar] [CrossRef]
- Gromov, N.V.; Medvedeva, T.B.; Taran, O.P.; Timofeeva, M.N.; Parmon, V.N. Hydrolysis of Cellulose in the Presence of Catalysts Based on Cesium Salts of Heteropoly Acids. Catal. Ind. 2021, 13, 73–80. [Google Scholar] [CrossRef]
- Akbay, E.; Demir, G. Nano-12-Tungstophosporic Acid Cesium Salt Synthesized by Ultrasound as Catalyst for Alkylation of Benzene with dec-1-ene. Int. J. Chem. React. Eng. 2017, 15, 20160189. [Google Scholar] [CrossRef]
- de Meireles, A.L.P.; Kelly, A.; da Silva Rocha, K.A.; Kozhevnikova, E.F.; Kozhevnikov, I.V.; EGusevskaya, E.V. Heteropoly acid catalysts in Prins cyclization for the synthesis of Florol®. Mol. Catal. 2021, 502, 111382. [Google Scholar] [CrossRef]
- Alotaibi, M.A.; Bakht, M.A.; Alharthi, A.I.; Geesi, M.; Alshammar, M.B.; Riadi, Y. Facile preparation of cesium salt of tungstophosphoric acid for catalytic synthesis of one-pot two-component 1,3,4-oxadiazole derivatives in water: A doubly green approach. Sustain. Chem. Pharm. 2020, 17, 100279–100288. [Google Scholar] [CrossRef]
- Gromov, N.V.; Medvedeva, T.B.; Rodikova, Y.A.; Babushkin, D.E.; Panchenko, V.N.; Timofeeva, M.N.; Zhizhina, E.G.; Taran, O.P.; Parmon, V.N. One-pot synthesis of formic acid via hydrolysis–oxidation of potato starch in the presence of cesium salts of heteropoly acid catalysts. RSC Adv. 2020, 10, 28856–28864. [Google Scholar] [CrossRef]





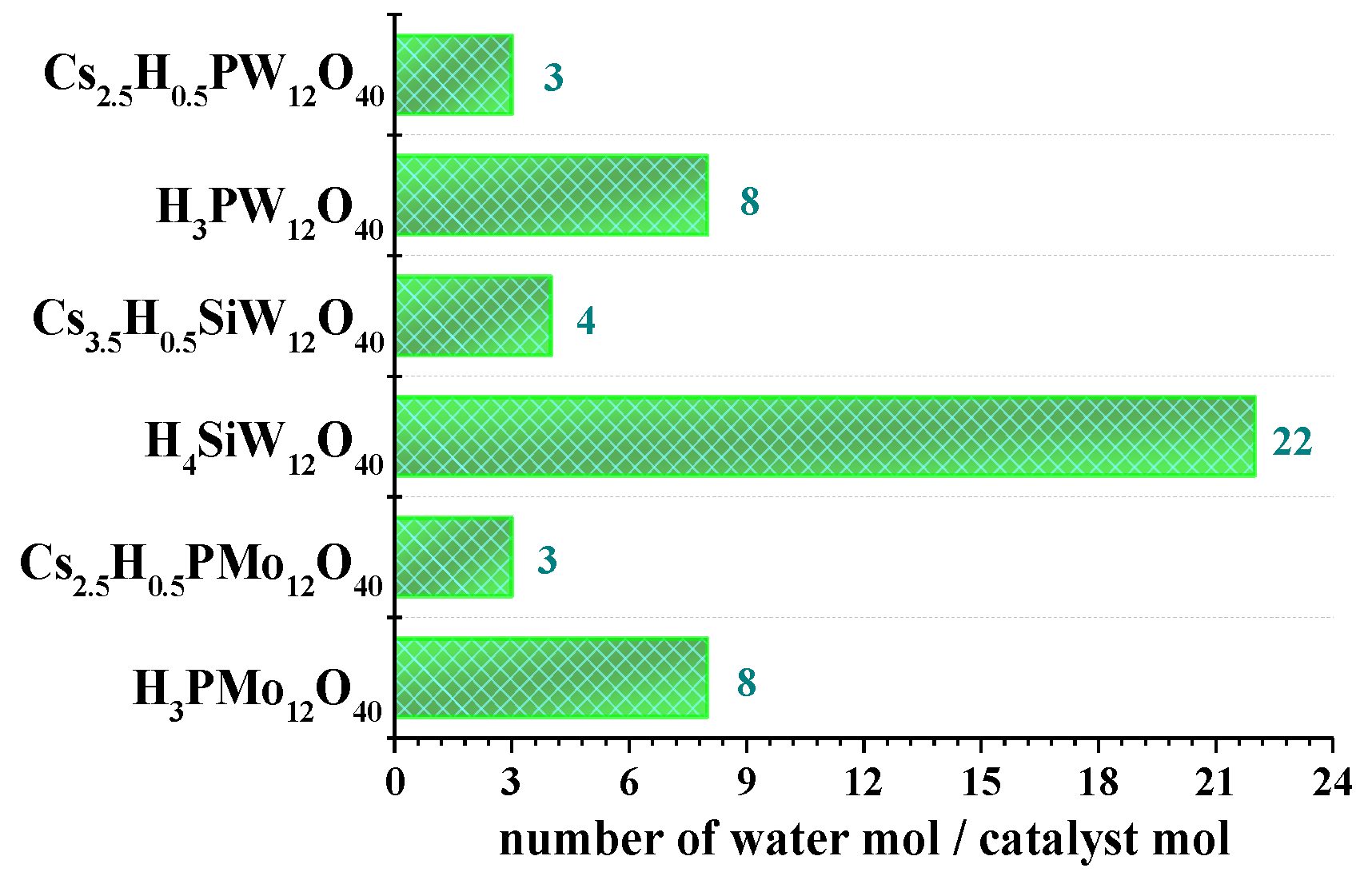

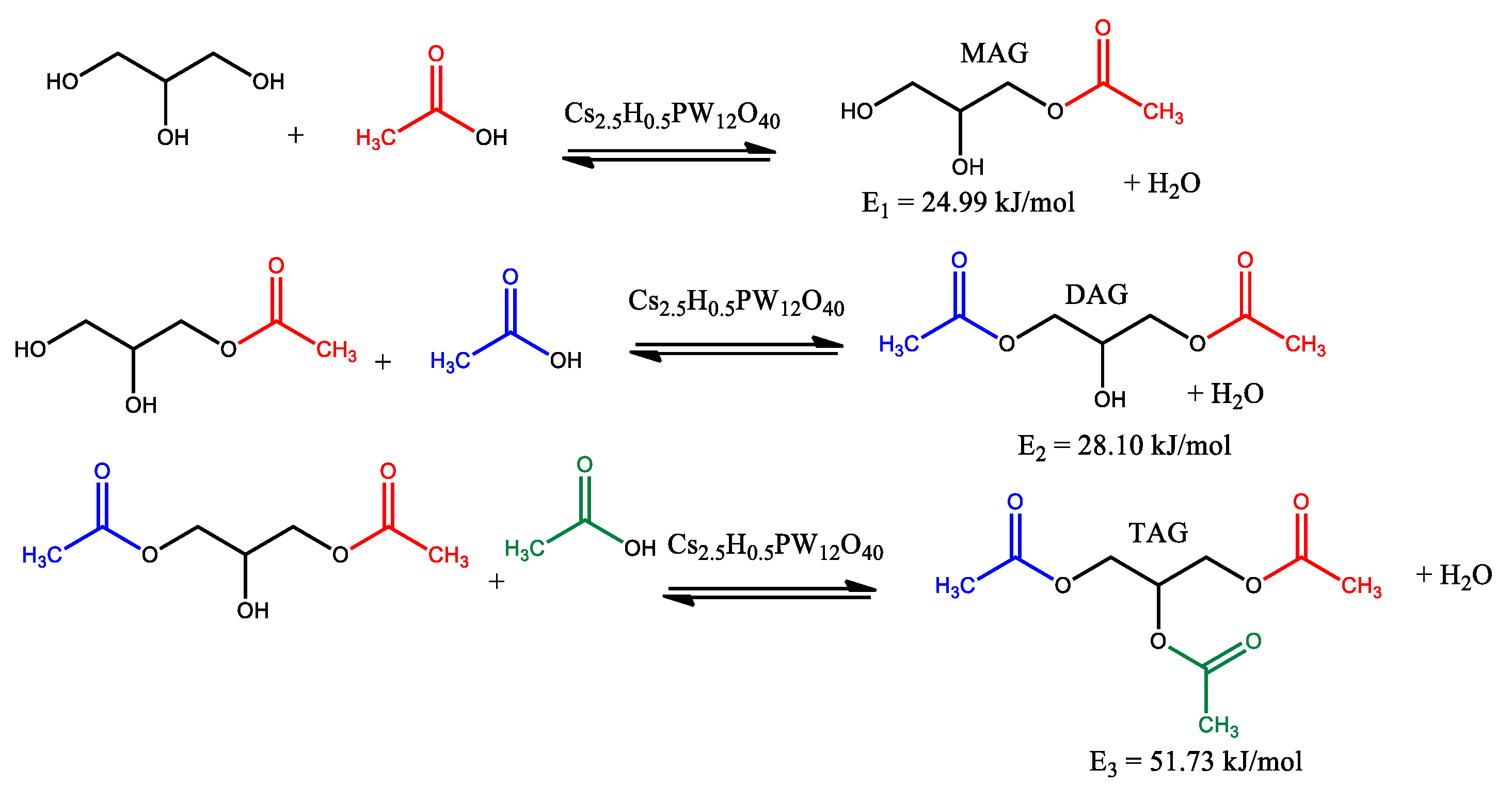
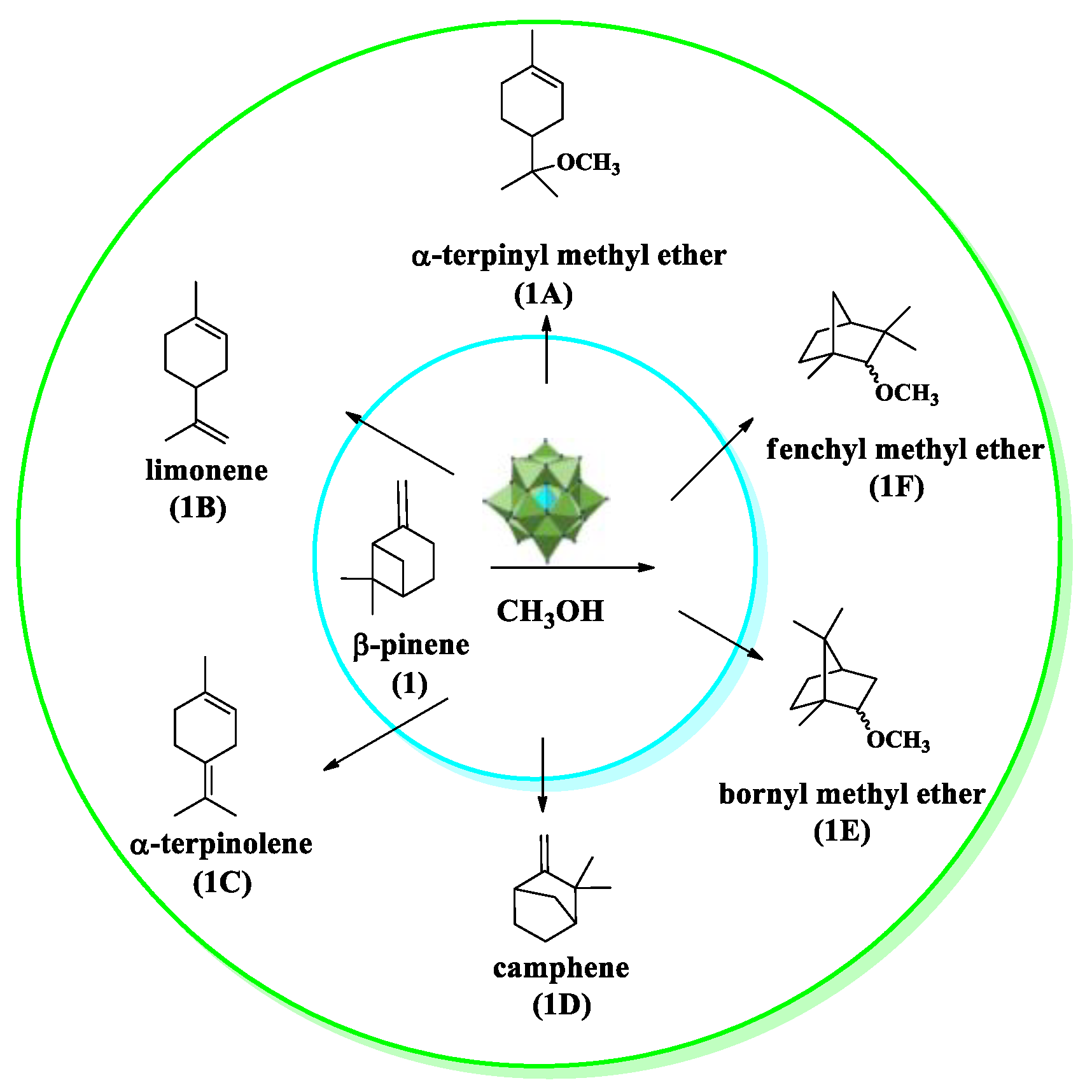
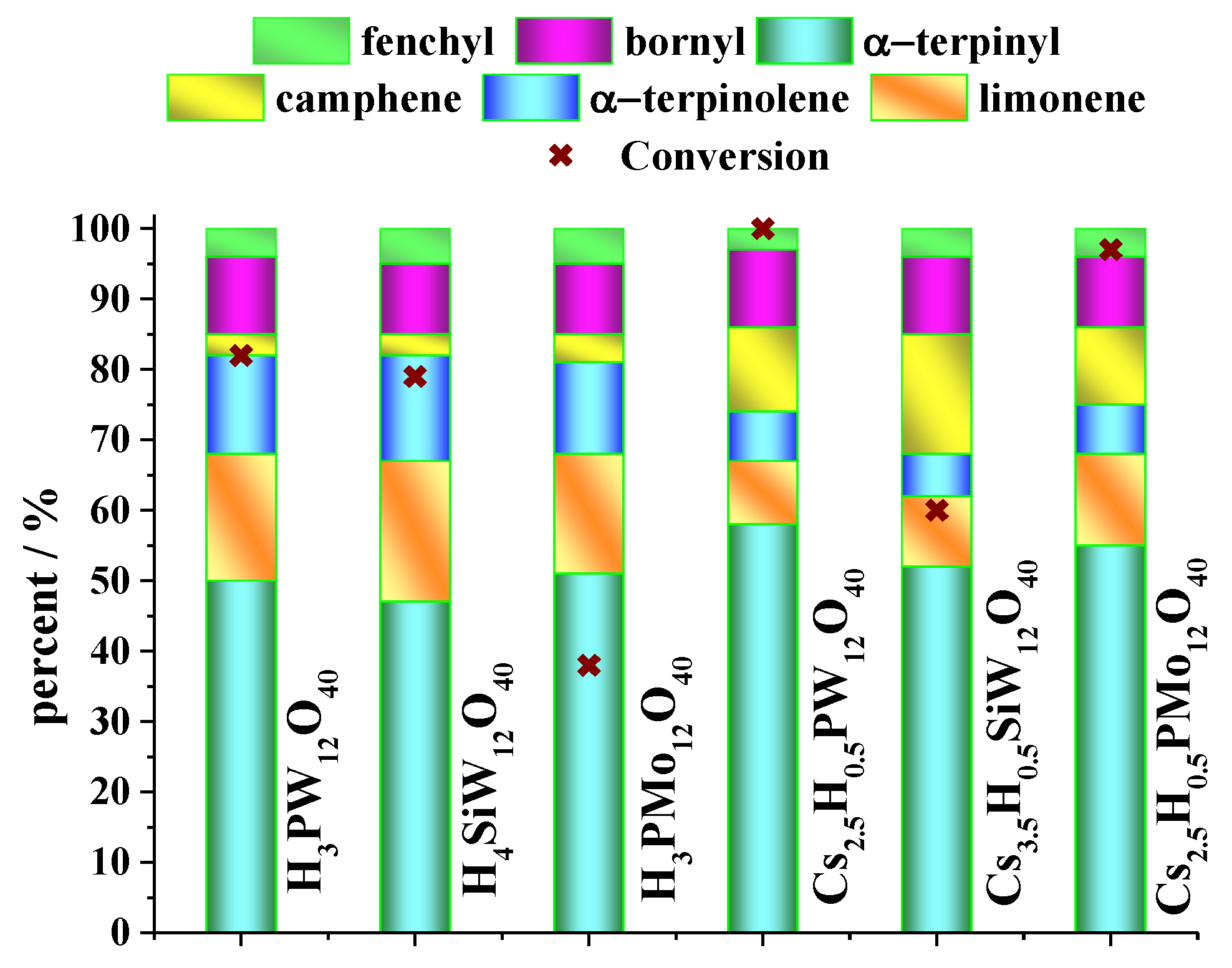
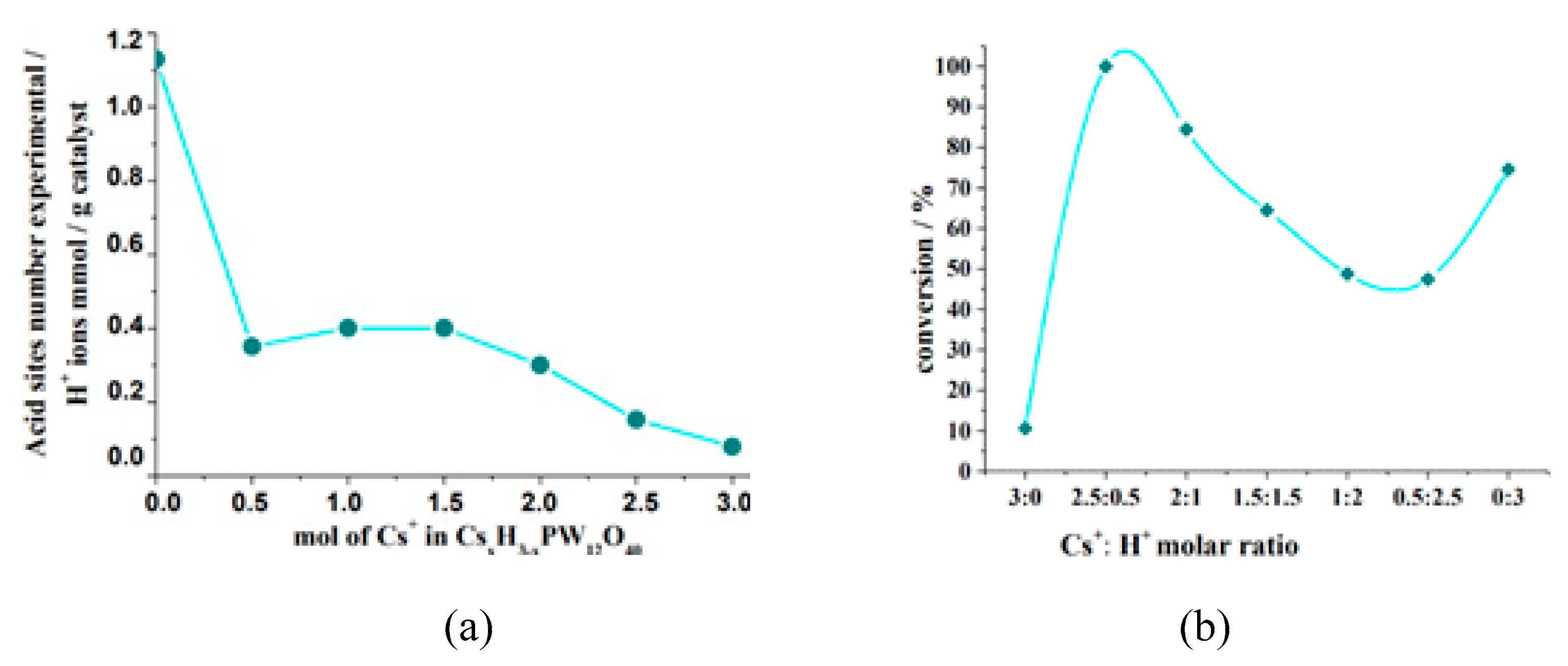
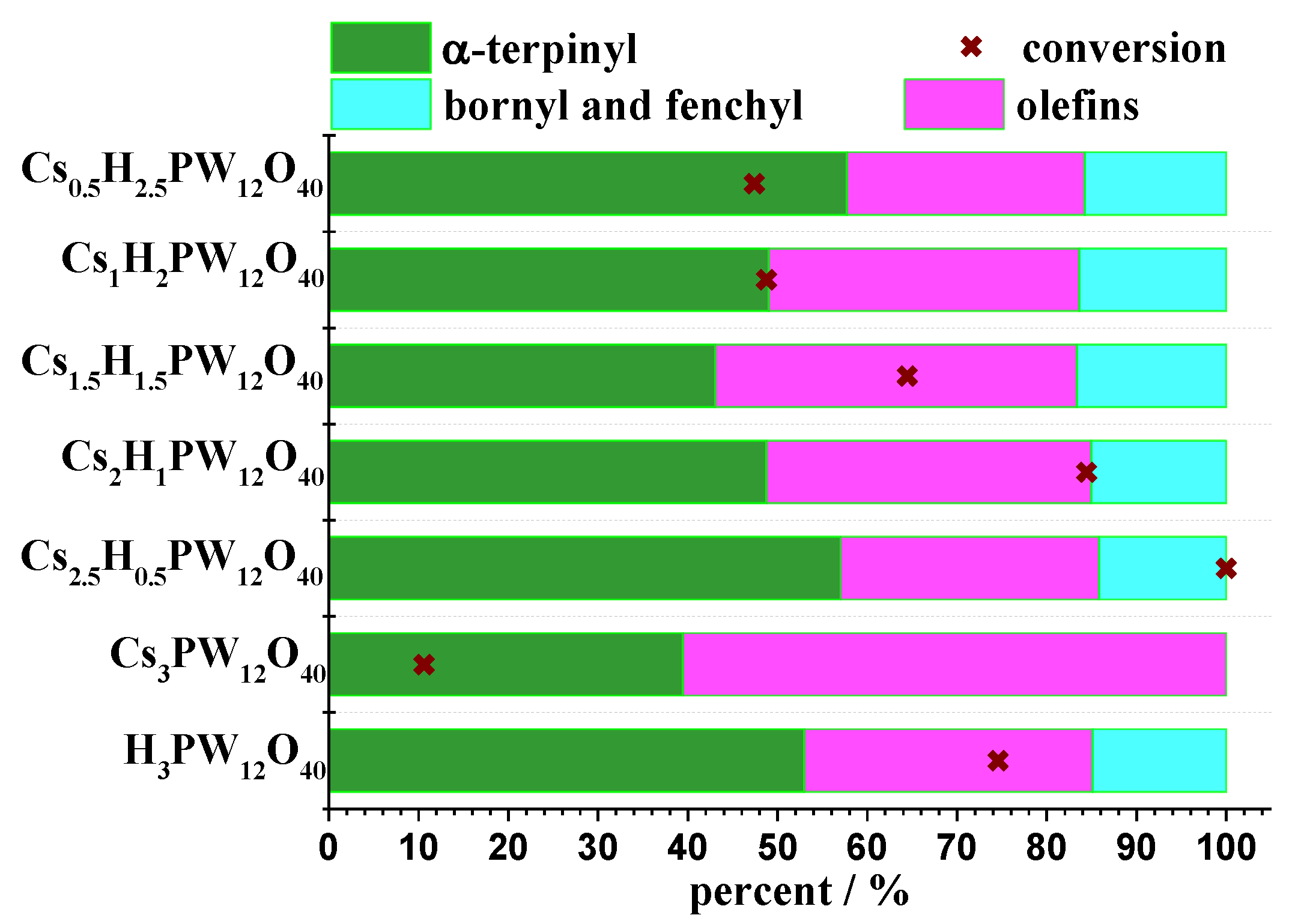
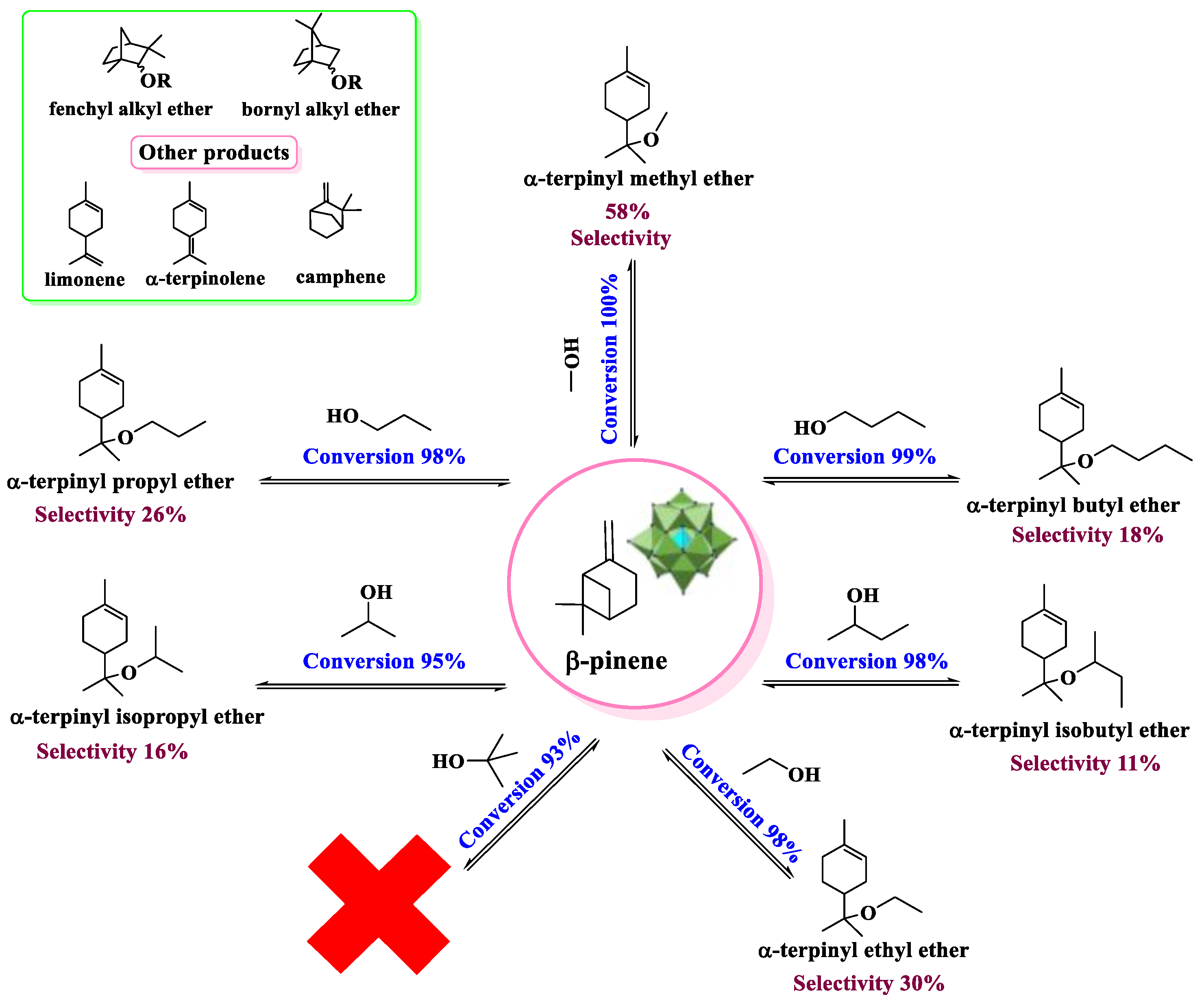
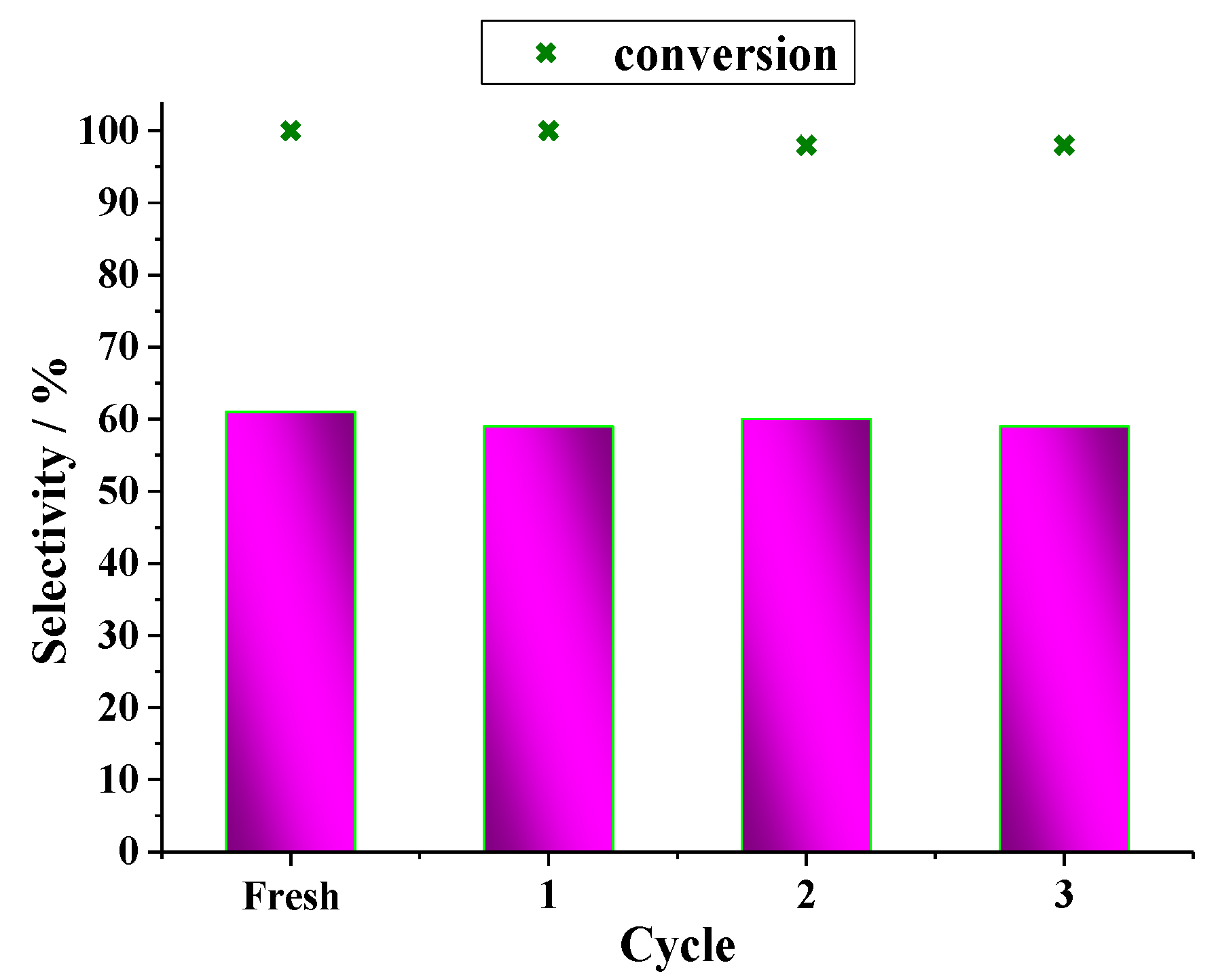
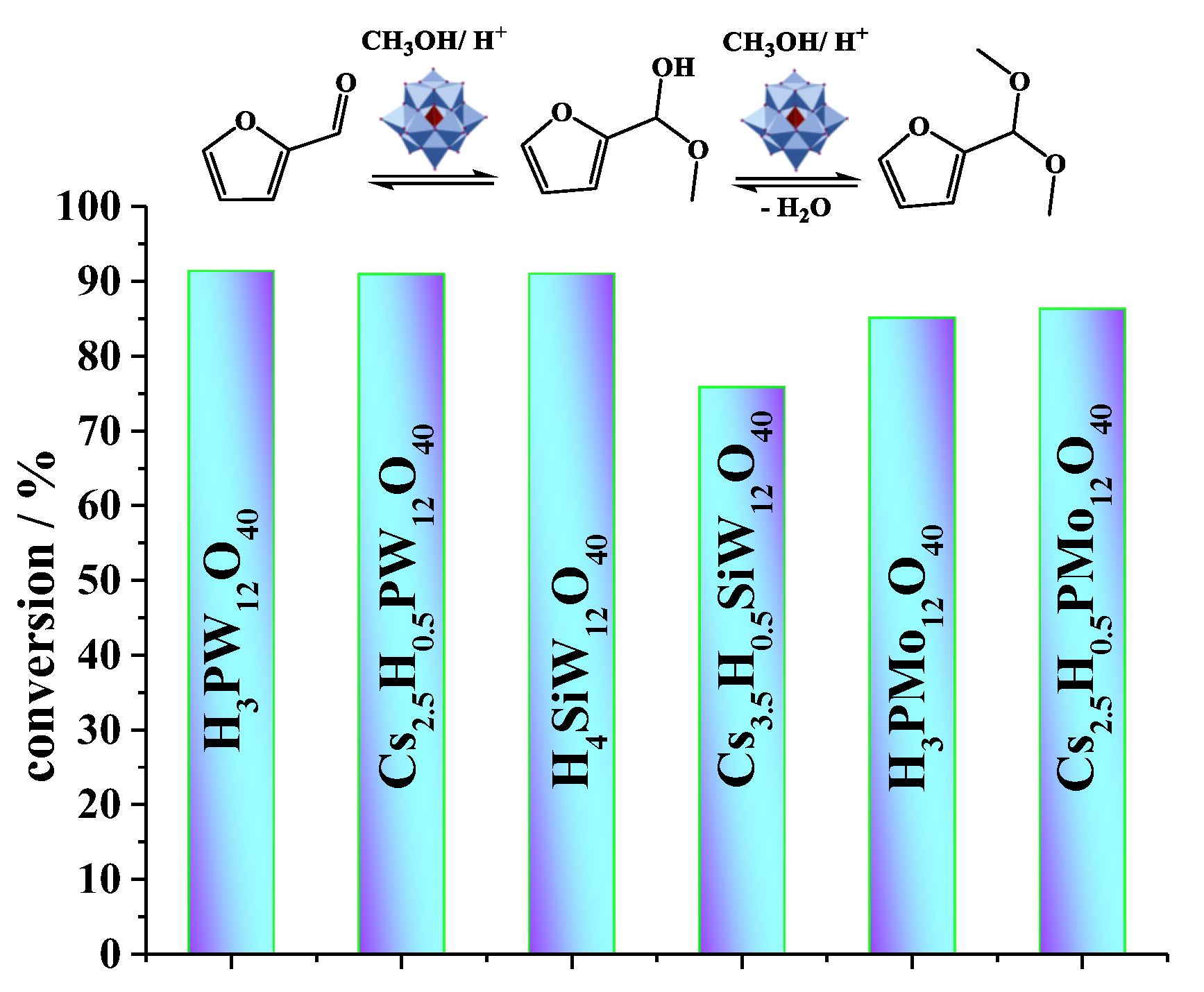
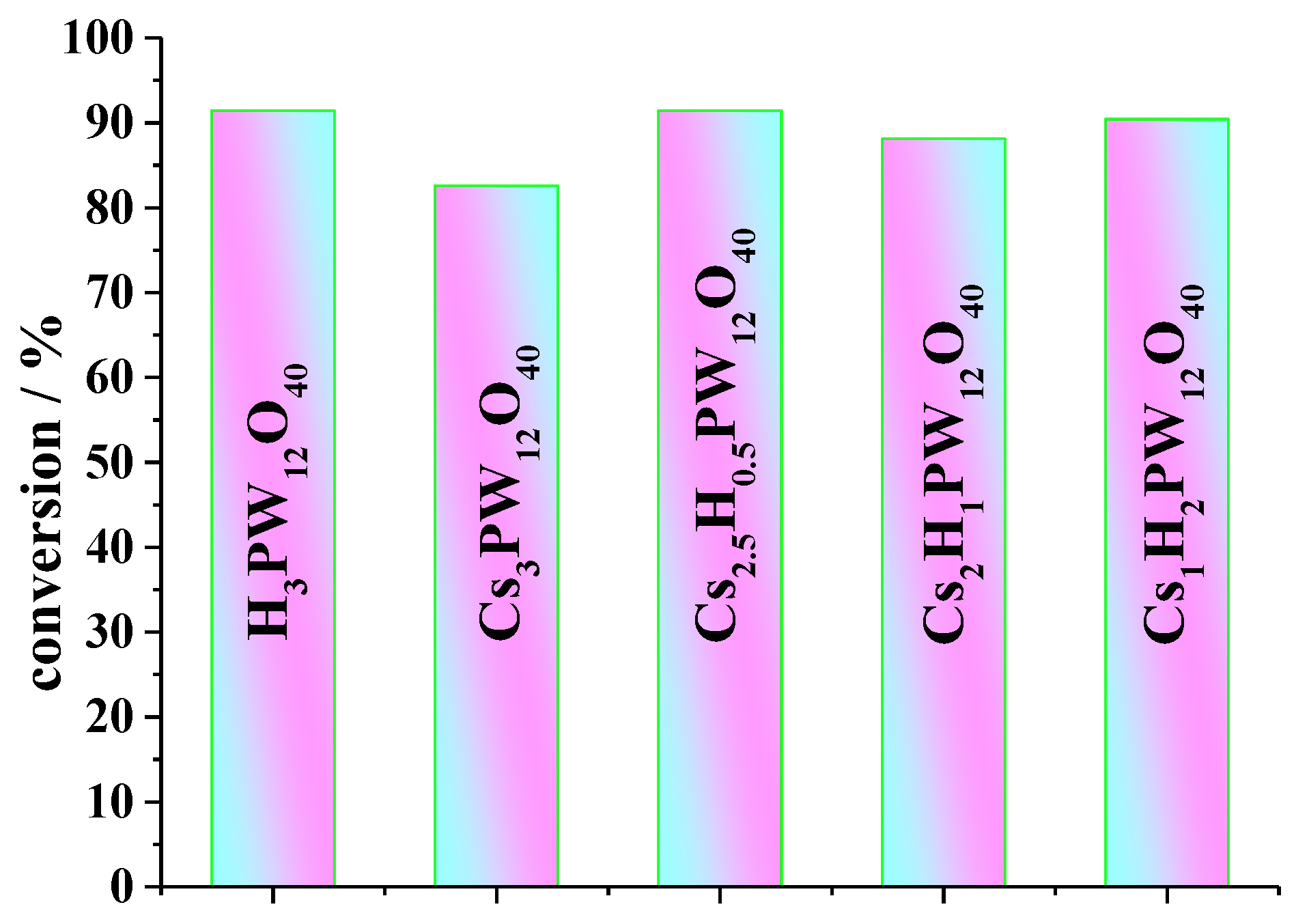
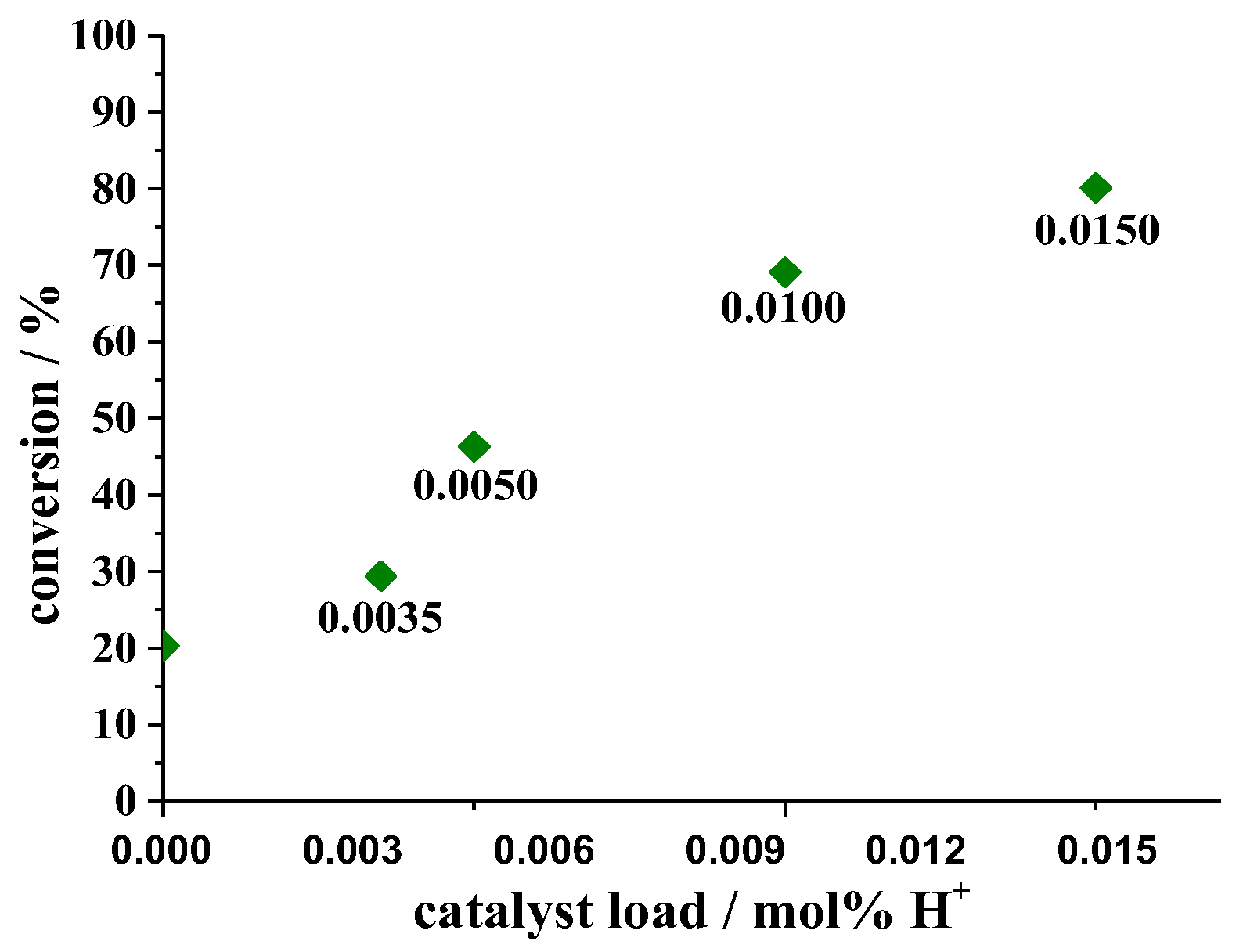
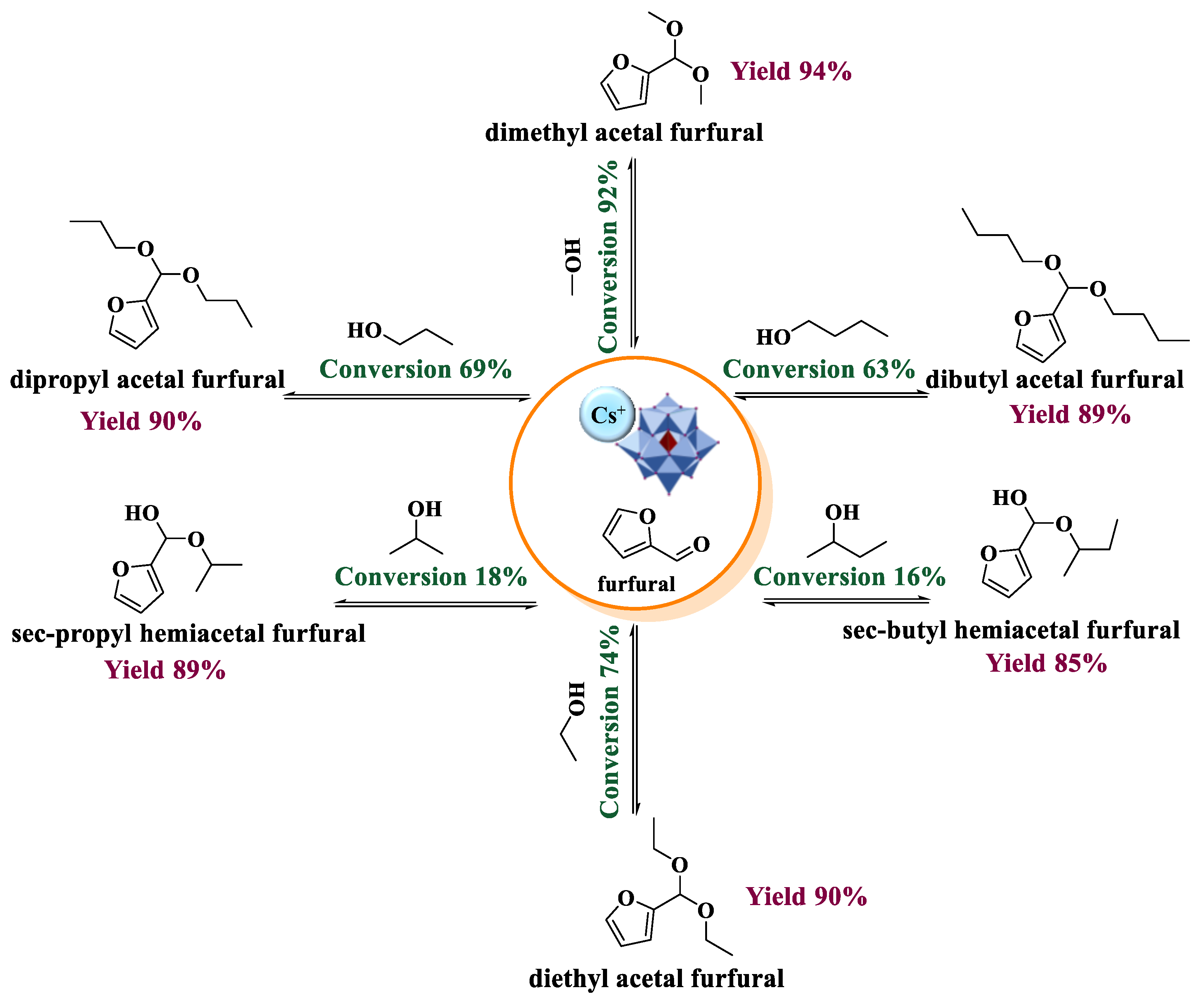
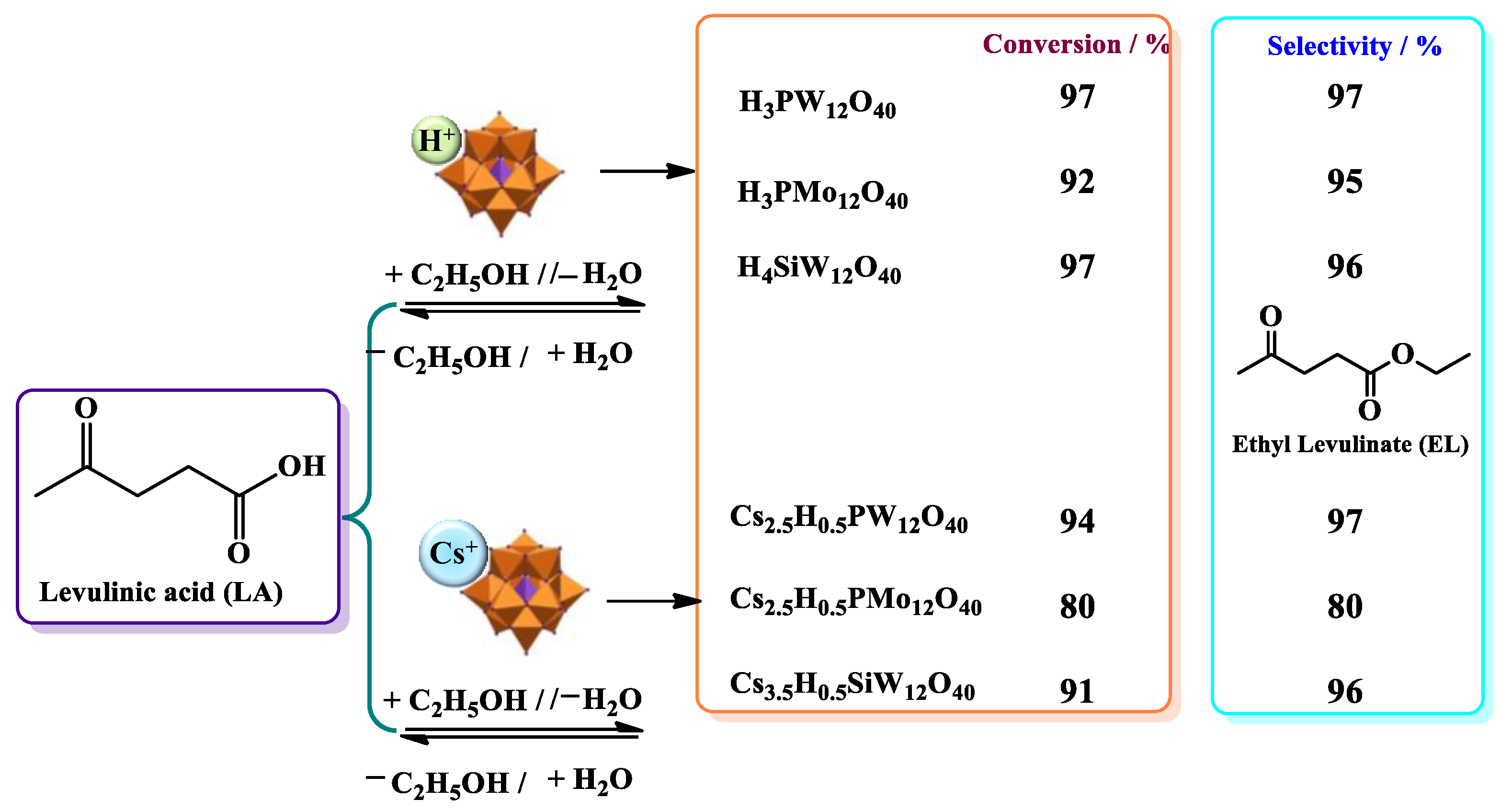
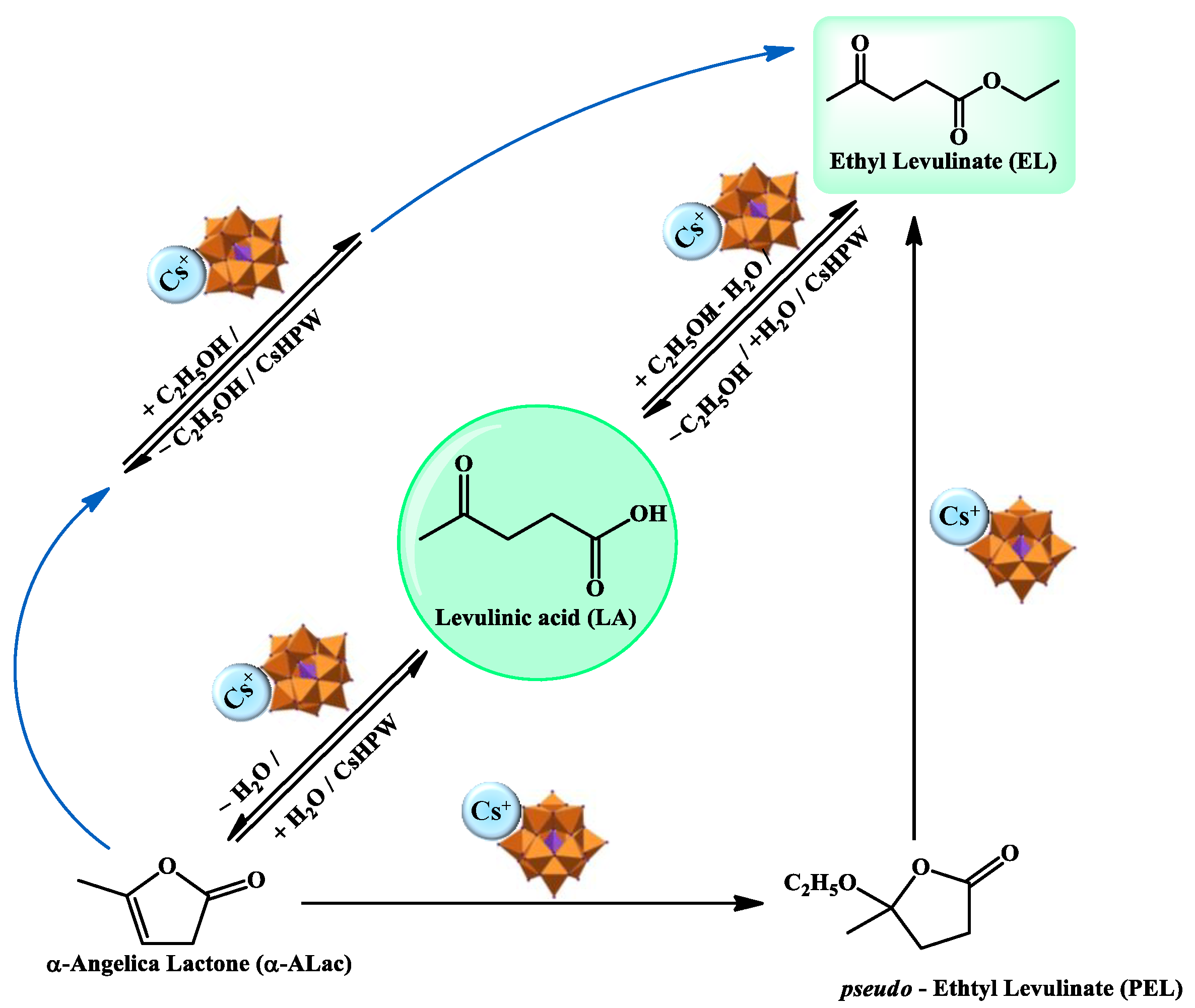
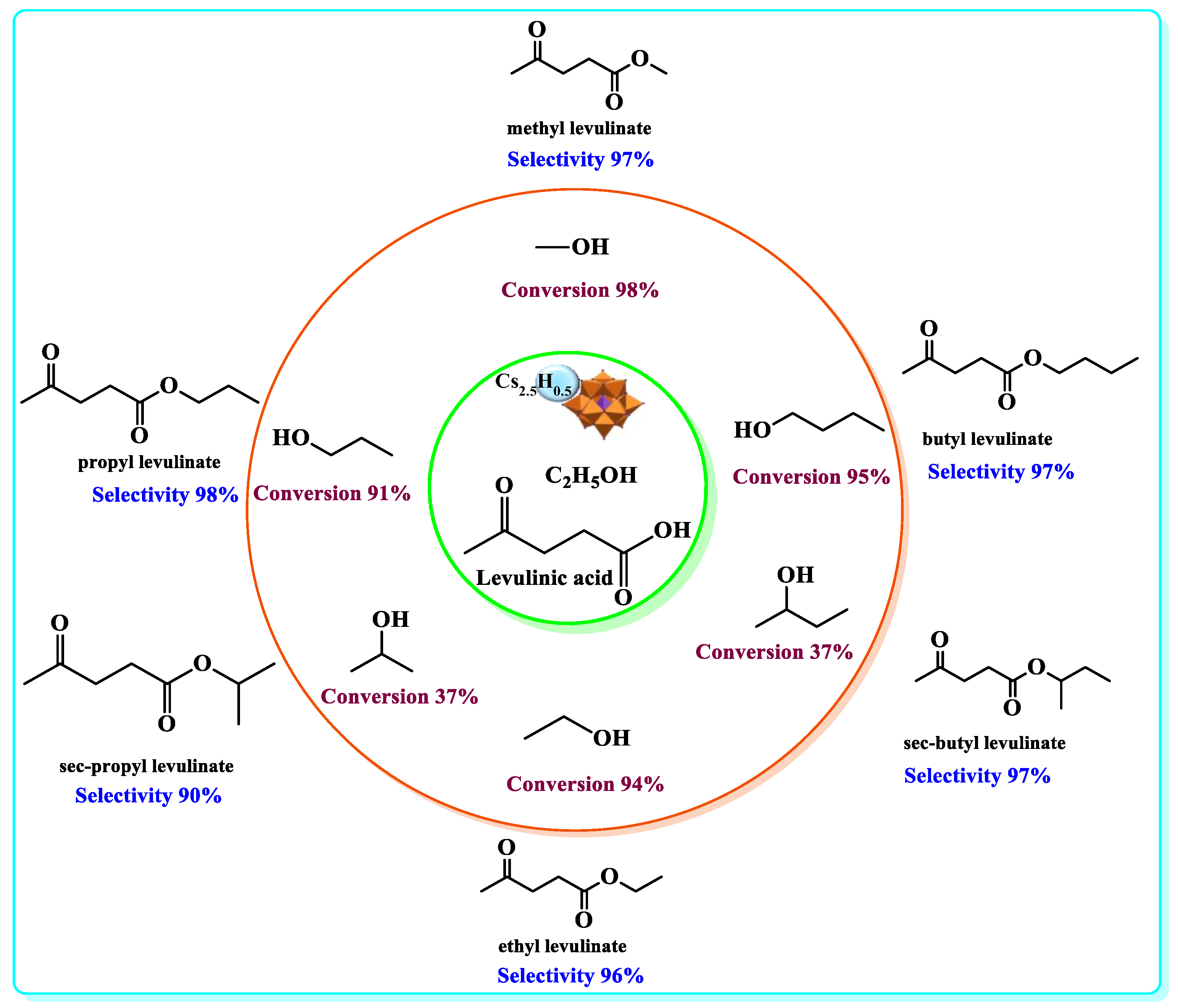
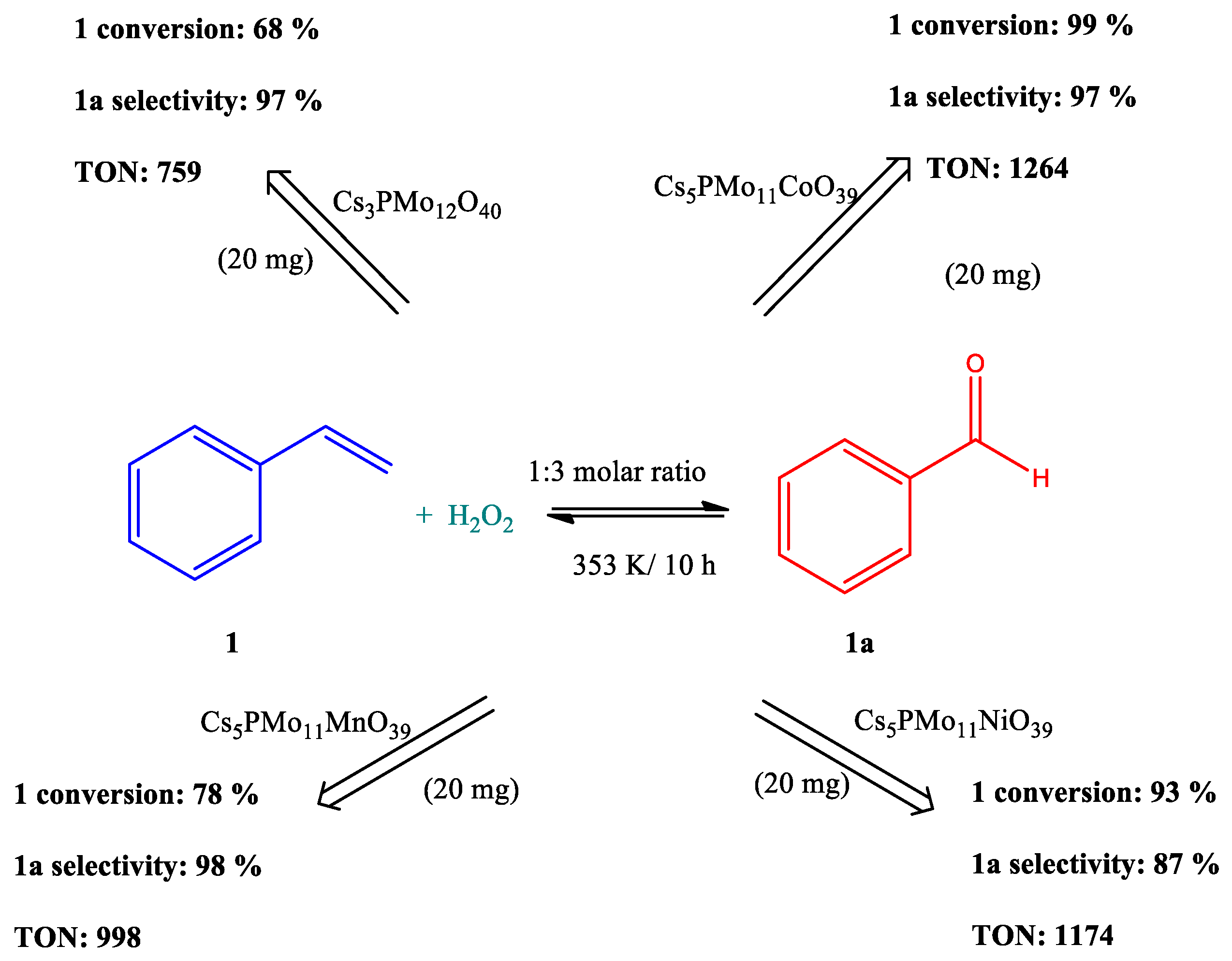
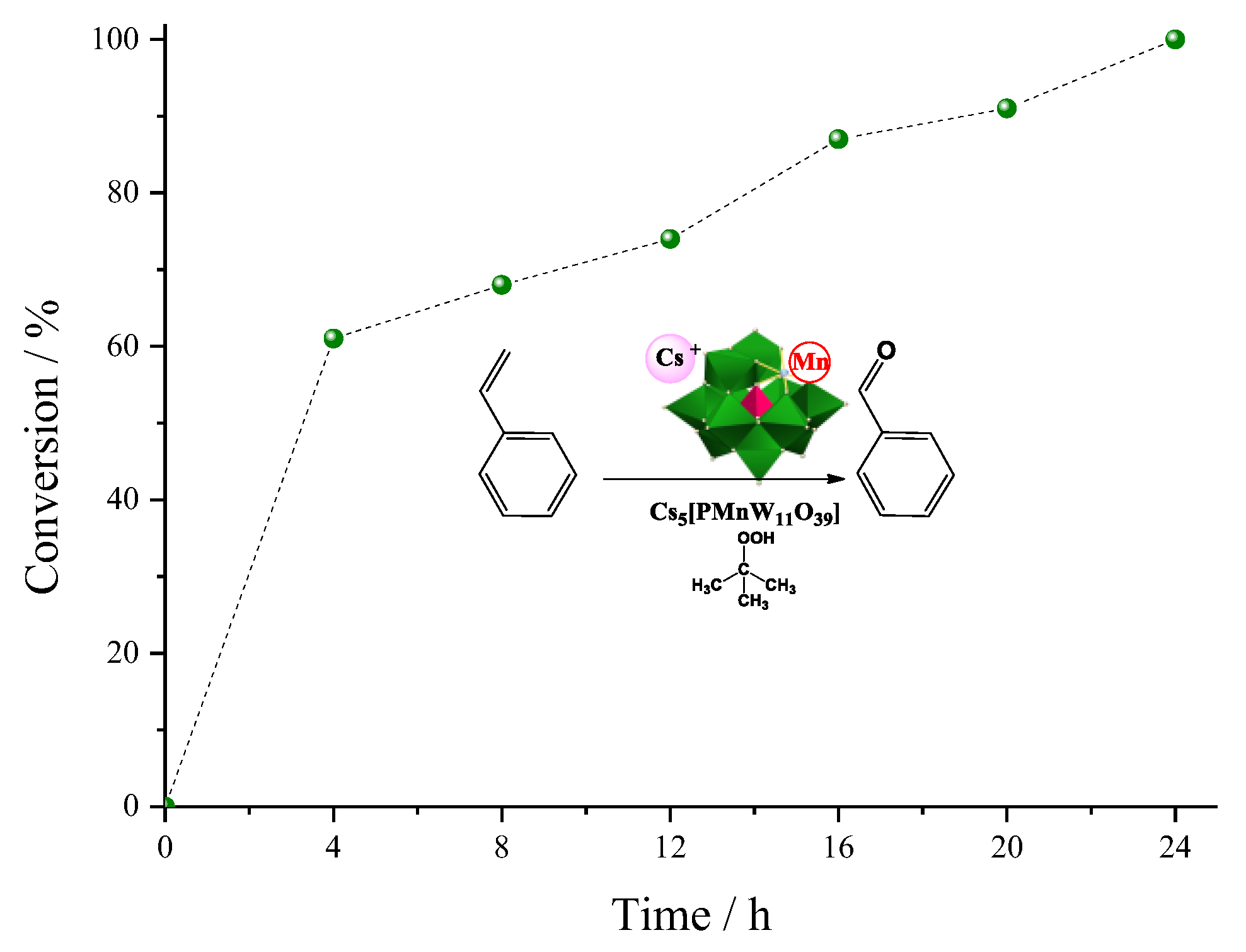


| Catalyst | Conversion of Levulinic Acid | Ethyl Levulinate Selectivity |
|---|---|---|
| H3PW12O40 | 97 | 97 |
| Cs0.5H2.5PW12O40 | 96 | 96 |
| CsH2PW12O40 | 96 | 95 |
| Cs1.5H1.5PW12O40 | 95 | 94 |
| Cs2HPW12O40 | 94 | 95 |
| Cs2.5H0.5PW12O40 | 94 | 97 |
| Cs3PW12O40 | 85 | 87 |
| Acidic Catalysis | ||
|---|---|---|
| Reaction | Catalysts | Reference |
| Furfural condensation reactions with alkyl alcohols | Cs2.5H0.5PW12O40 | [35] |
| β−pinene etherification with alkyl alcohols | Cs2.5H0.5PW12O40 | [36] |
| Levulinic acid esterification with alkyl alcohols | Cs2.5H0.5PW12O40 | [37] |
| Alkylation of toluene with benzyl alcohol | Cs3PMo12O40 | [43] |
| Hydrolysis of cellulose | Cs2.5H0.5PW12O40 | [48] |
| Glycerol esterification with acetic acid | CsnH3−nPX12O40 (n = 1.0–2.5) and CsnH4−nSiY12O40, where X = W6+, Mo6+ and Y = W6+ | [54] |
| Glycerol acetylation and esterification reactions with acetic anhydride and acetic acid | Cs2.5H0.5PW12O40 | [74] |
| Transesterification of glycerol tributyrate with methanol | Bimodal cesium hydrogen salts of 12-tungstosilicic acid CsxH4−xSiW12O40 | [75] |
| Solvent-free self-condensation of levulinic acid | CsxH4−xSiW12O40 | [76] |
| Hydrolysis of cellulose | Cs4−xHxSiW12O40 (x = 3 and 3.5), Cs3−xHxPMo12O40 and Cs3−xHxPW12O40 (x = 2 and 2.5) | [77] |
| Alkylation of toluene with benzyl alcohol | Nano Cs2.5H0.5PW12O40 | [78] |
| Prins cyclization of isoprenol and isovaleraldehyde to Florol®. | Cs2.5H0.5PW12O40/SiO2, Cs2.5H0.5PW12O40 | [79] |
| Oxidative reactions | ||
| Styrene oxidation | Mn-doped cesium phosphomolybdate salts | [71] |
| Alcohols oxidation with hydrogen peroxide | (Co, Mn, Ni)-substituted Keggin-phosphomolybdates | [73] |
| Hydrogenation reactions | ||
| Styrene hydrogenation | Cesium salt of iron substituted phosphomolybdate | [46] |
| One-pot reactions | ||
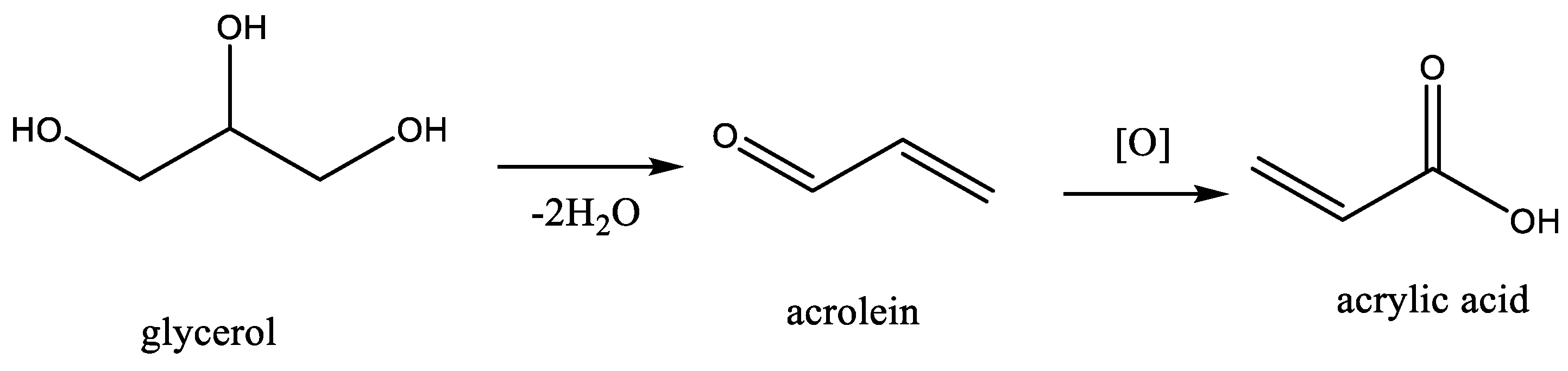
| Oxidative dehydration of glycerol to acrylic acid | vanadium-doped cesium phosphotungstate or phosphomolybdate | [72] |
| Synthesis of one-pot two-component 1,3,4-oxadiazole derivatives | Cs3PW12O40 | [80] |
| One-pot synthesis of formic acid via hydrolysis–oxidation of potato starch | Cs3.5H0.5PW11VO40, Cs4.5H0.5SiW11VO40, Cs3.5H0.5PMo11VO40, Cs2.5H0.5PMo12O40 | [81] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
da Silva, M.J.; Rodrigues, A.A.; Lopes, N.P.G. Cesium Heteropolyacid Salts: Synthesis, Characterization and Activity of the Solid and Versatile Heterogeneous Catalysts. Chemistry 2023, 5, 662-690. https://doi.org/10.3390/chemistry5010047
da Silva MJ, Rodrigues AA, Lopes NPG. Cesium Heteropolyacid Salts: Synthesis, Characterization and Activity of the Solid and Versatile Heterogeneous Catalysts. Chemistry. 2023; 5(1):662-690. https://doi.org/10.3390/chemistry5010047
Chicago/Turabian Styleda Silva, Marcio Jose, Alana Alves Rodrigues, and Neide Paloma Gonçalves Lopes. 2023. "Cesium Heteropolyacid Salts: Synthesis, Characterization and Activity of the Solid and Versatile Heterogeneous Catalysts" Chemistry 5, no. 1: 662-690. https://doi.org/10.3390/chemistry5010047
APA Styleda Silva, M. J., Rodrigues, A. A., & Lopes, N. P. G. (2023). Cesium Heteropolyacid Salts: Synthesis, Characterization and Activity of the Solid and Versatile Heterogeneous Catalysts. Chemistry, 5(1), 662-690. https://doi.org/10.3390/chemistry5010047