Abstract
In heritage conservation, paper deterioration can be slowed down by controlling the environmental conditions surrounding heritage objects and stabilizing the materials these objects are made of. As conservation materials can also cause optical, chemical, and physical changes in the object, their application should be safe, minimalistic, and purposeful. This paper aimed to observe the functional applications of three biopolymers used in paper conservation. For that purpose, a model paper was coated with methylcellulose (MC), cellulose nanocrystals (CNCs), and wheat starch (WSP) using different wet film deposits. The prepared samples were characterized by determining their physical, optical, and surface properties. The results show that changes in the wet film deposit thickness influenced paper properties. With CNCs, the increase has caused a drastic change of colour properties, with MC hydrophobicity increased and with WSP grammage and thickness increased. All coatings (except CNC24) have contributed to the preservation of the colour properties of the paper from the damage caused by thermal ageing.
1. Introduction
Although historic handmade paper, primarily from the medieval period, is considered one of the most durable information carriers [1], it is still prone to ongoing and unavoidable process of deterioration. There are two methods for slowing down the rate of ageing: controlling the environmental conditions surrounding paper-based heritage objects and stabilizing the materials these objects are made of. Some parts of the stabilizing processes are carried out using adhesives in liquid form, which are applied to the object’s surface overall or locally, depending on the problem at hand. Although they are usually applied for a specific purpose, such as surface cleaning, joining, consolidation, strengthening, sizing, or similar, adhesives can sometimes serve multiple roles [2].
As newly introduced material can cause optical, chemical, and physical changes in the paper-based object, its application should be safe, minimalistic, purposeful [2], and, preferably, easy to reverse. Ideal materials in the conservation of cultural-heritage objects are of spectrographic grade (highest purity), whereas industrial-grade products are acceptable, provided there are no subsequent unwanted reactions [3]. In addition to the purity of the applied materials, the long-term success of most interventions depends on the physicochemical compatibility with the original materials, as incompatibility can lead to more degradation.
The commonly used adhesives in paper conservation are starch pastes, carboxymethylcellulose, hydroxypropylcellulose, and methylcellulose [4,5]. Although most traditional adhesives have been studied or modified throughout the past 50 years [4,6,7,8,9,10,11,12,13,14], their performance remains a subject of interest, as well as a framework to explore and valorize noncommon and innovative materials [15,16,17].
Recently, nanocellulose in its highly crystalline form [18], also known as cellulose nanocrystals (CNCs), has become one of the most researched materials by both the scientific sector and the industrial sector. CNCs are low-cost and renewable nanomaterials produced from native cellulosic fibres, with properties differing from cellulose. Their low density, shear thinning behaviour, high tensile strength [19], optical transparency, thermal stability [20], and the ability to adhere to paper by establishing a hydrogen-bond network with paper fibres [21], make CNCs a material suitable for surface coatings.
Previous studies have found CNC-based coatings more or less appropriate for use in the consolidation of paper [22,23] and other plant-derived writing material [24] as the results have been shown to depend on the type of substrate used, the materials preparation, and the application technique [25]. In our previous study [26], we observed how different amounts of CNCs and variations in the application order of the layers (single and multiple layers) have affected paper properties in the short term. However, changes that may occur under the influence of air, heat, light, or time were not investigated, as the study was conducted to assess only the primary effects of the coating application. For these reasons, this study aimed to investigate the secondary effects of applications, including both the physical and the chemical changes in a coated paper after thermal ageing. To broaden the perspective of the study, two additional biopolymers commonly used in paper conservation (wheat starch and methylcellulose) were investigated using the same experimental setup. An analysis of the obtained wet film thicknesses (both short- and long-term) acts as a tool to emphasize possible treatment options.
The results of the treatments were assessed by determining physical, optical, and surface properties, as well as the degradation degree of the samples, prior to and post-thermal ageing. The main goal of the paper was to observe the functional applications of the three coatings (cellulose nanocrystals, wheat starch, and methylcellulose) used in the paper conservation.
2. Materials and Methods
In this research, Japanese handmade paper Takogami B (43 gm−2, 70% Kozu fibre + 30% pulp, and purchased from Japico-Feinpapier-Vertriebs GmbH, Vienna, Austria) was used as a model paper for analytical investigations, representing historic handmade paper. The model paper samples (felt side [27]) were immersed in distilled water for 15 min to remove oxidation products and foreign matter from their surface, as it enables better wetting [28] and consequently, improves adhesion. Wet samples were placed on blotting paper and left to air dry.
In addition to CNCs, we have selected two common conservation adhesives (wheat starch and methylcellulose) with different properties and application ranges.
The characteristics and preparation of a ready-made 4 wt.% CNCs suspension (trade name NCC/CNC, purchased from Nanocrystacell/Navitas d.o.o., Stari trg pri Ložu, Slovenia) used in the experiment are described in detail elsewhere [26].
The wheat-starch suspension (WSP) was prepared by adding 7 g of precooked starch (trade name Wheat paste No. 301, item # TAD002001, purchased from Talas, New York, NY, USA) to 100 mL of demineralized water, followed by magnetic stirring for 15 min at 1000 rpm. Precooked starch was chosen for analytical investigations to eliminate the time needed to prepare a traditional starch paste for conservation purposes.
Methylcellulose (MC) solution was prepared by adding 1 g of MC powder (trade name Methyl Cellulose, item # TAD016003, purchased from Talas, New York, NY, USA) to 100 mL of demineralized water. The solution was left to settle for 24 h. The methylcellulose used was of technical-grade quality [29]. As previously mentioned, technical grade products are acceptable for conservation purposes, as long as no unwanted reactions occur in the long term [3].
Prepared colloid suspensions were applied onto the paper surface with an automatic coating unit K Control Coater, model 202 (RK PrintCoat Instruments Ltd., Litlington, UK) at the speed of 4 m/min. Different wet film deposits were obtained using coating bars #2 (12 µm), #3 (24 µm), #5 (50 µm), and #8 (100 µm) in a single pass. As coatings are expected to have uniform thickness and spread over the entire surface [28], we utilized a range of thicknesses to ensure the proper amount of wet film is being applied and to provide more insight into the influence of the wet film thickness on paper properties.
The samples were denominated as a combination of letters MC (paper coated with methylcellulose), CNCs (paper coated with CNCs), and WSP (paper coated with wheat starch) and numbers representing coating thicknesses expressed in µm (12, 24, 50, and 100). Additionally, the uncoated paper was denominated as RP.
After coating, the samples of Japanese paper were left to air dry at room temperature for 24 h and then analyzed. As paper deterioration can be caused by many factors, such as temperature, humidity, acids, oxygen, microorganisms, pollutants, etc., predicting the changes caused by some of these factors can be achieved through accelerated ageing tests. Four ageing procedures are prescribed by ISO and many others are standardized by other organizations [30]. As heat is one of the few exogenous factors influencing paper durability [1], we employed the thermal ageing test at 100 °C for 50 h in a Memmert UNB400 heating chamber (Memmert GmbH, Schwabach, Germany), as previously described by Sayed Darwish [31].
2.1. Suspension Properties
To characterize suspension properties, the coatings’ pH value and dynamic viscosity were measured using a WTW 340 pH metre and RheolabQC rotational rheometre (Anton Paar GmbH, Graz, Austria) in a constant shear rate mode with the value of 50 s−1 and at 22 °C.
2.2. Visual Assessment
The film-forming property of coatings was assessed by observing micrographs obtained using an Olympus BX51 microscope with a DP72 digital camera attached (Olympus, Tokio, Japan). The micrographs were recorded at the magnification of 100×. The camera resolution was 4140 × 3096 pixels.
2.3. Physical Properties
As coatings are expected to be only a fraction of a millimetre thin [28], changes in thickness and weight of the coated samples have been monitored throughout the study with a digital analytical electronic balance (Mettler Toledo XS205, Greifensee, Switzerland) and a digital micrometre DGTB01 (Enrico Toniolo S.r.l., Milano, Italy) with a weight pressure of 49.03 kPa and with 0.01 mm resolution. In weight measurements, all samples were cut to the size of 38 × 80 mm.
2.4. Optical Properties
The CIE LAB colour parameters, opacity, yellowness (ASTM E313-20), and gloss were measured to determine the changes in the visual appearance of samples caused by thermal ageing.
CIE LAB parameters, opacity, and yellowness were measured with a Techkon SpectroDens device (Techkon, Königstein, Germany). CIE LAB values were determined using the following settings: illuminant D50, a standard observer angle of 2°, M1 measuring conditions (ISO 13655:2017), and the sample placed on a white backing. These settings are commonly used when performing colour measurements in the printing industry as described by ISO 13655:2017. The backing was additionally introduced since the investigated paper sample has relatively low opacity and the background colour influences the colour being measured. The CIE LAB parameters were used to calculate the ∆Eab colour difference [32]. Both yellowness and opacity were determined using the measuring geometry of 0°:45° and the following settings: standard illuminant D50, a standard observer angle of 10°, and M1 measuring conditions. Yellowness was measured on a white backing, while opacity was measured using SpectroDens’ opacity function, which includes spectrophotometric measurements of the sample on a black-and-white backing. Before the tests, the measuring unit was calibrated to absolute white.
Gloss levels were determined using an Elcometer 407 Statistical Glossmeter (Elcometer, Manchester, UK) with the measurement angle of 60°.
2.5. Surface Properties
In order to observe changes in the surface properties throughout the study, we investigated the contact angle of redistilled water. The contact angle measurements were performed using Dataphysics’ OCA30 device (DataPhysics Instruments GmbH, Filderstadt, Germany) and its controlling software SCA 20. The applied water droplets had the volume of 2 µL, and the measurements were conducted using the sessile drop method. The contact-angle measurements were performed 0.2 s after the initial water–substrate contact using the Laplace–Young fit.
Furthermore, we investigated the absorption of the water into the surface by measuring the time needed for the droplet to be fully absorbed by the sample. For that test, the camera (IDS Imaging Development Systems GmbH, Obersulm, Germany) had a framerate of 25 fps, and the water droplet was the same volume as for the contact-angle measurement (2 µL).
2.6. Determination of Sample’s Molecular Structure Change
Fourier transform infrared (FTIR) spectroscopy offers insight into a degradation process on the molecular level [30]. FTIR spectra were obtained to investigate the chemical changes due to thermal ageing. FTIR spectra were measured using a Shimadzu FTIR IRAffinity-21 spectrometer (Shimadzu Corporation, Kyoto, Japan) with the Specac Silver Gate Evolution as a single reflection ATR sampling accessory with a ZnSe flat crystal plate (refraction index 2.4). The observed spectral range was 4000–600 cm−1.
3. Results and Discussions
The pH value and viscosity of the prepared coatings are shown in Table 1. The pH value of the CNCs and the MC were measured as neutral (slightly above 7), unlike that of the wheat starch (<7). In paper conservation, the pH value of the added material is expected to be acid free, as acidity is the most damaging factor responsible for the breakdown of cellulose molecules [33]. The pH value of the WSP suspension used in the experiment was slightly acidic, which has not been corrected for observation purposes. However, this value should be set to slightly alkaline during the actual treatment of the historic paper.

Table 1.
The pH value and viscosity of the prepared coatings.
Viscosity is an important property to consider when performing conservation treatments. Low-viscosity coatings are preferable as they can more successfully flow into the substrate’s pores [2]. However, viscosity is influenced by several factors, including temperature, the viscosity of the solvent, the concentration and molecular weight of the solid, etc. [2]. The results for dynamic viscosity varied among prepared coatings, with CNCs having the highest viscosity of the three.
Figure 1 shows microscopic images of the prepared samples. The RP and MC100 samples have fibres visible on the surface. Due to the paper being handmade, there is no visible grain direction, which can usually be observed in machine-made paper. Based on the micrographs of the samples (Figure 1), it seems that the MC impregnates the paper surface instead of forming a film, regardless of the wet film deposit applied (the given figure shows the highest MC wet film deposit). Nevertheless, it should be noted that the MC appears transparent, making it invisible in the micrographs.
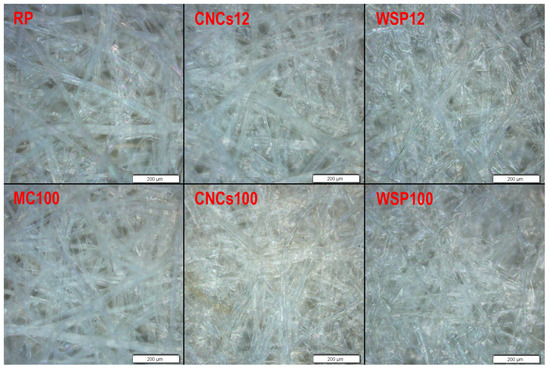
Figure 1.
Microscopic images of the samples at 100× magnification.
Conversely, CNCs form an adhesive film on the paper surface. In addition, there is also a visible difference in the surface structure of the samples treated with different amounts of coating (different wet film deposits). CNCs100 fills most of the voids between the fibres, closing the paper surface. The film-forming ability of CNCs, characterized by the absence of voids, has previously been highlighted by researchers [21].
WSP film exhibits an effect similar to the CNCs film by filling most of the voids between fibres resulting in a closed surface (Figure 1). It should also be noted that even the lowest WSP wet film deposit results in a surface similar to the surface containing the highest CNCs wet film deposit, which could be attributed to the filmogenic properties of the starches [34,35].
Since thermal ageing did not cause any notable changes in the samples’ surfaces, the microscopic images resulting from thermal ageing tests were excluded from this paper.
Application of all three coatings resulted in an increased thickness of the RP (Figure 2b). Although the MC film could not be seen on micrographs (Figure 1), the increase in grammage and thickness indicates that a film may have formed but remained invisible due to its high transparency [36].
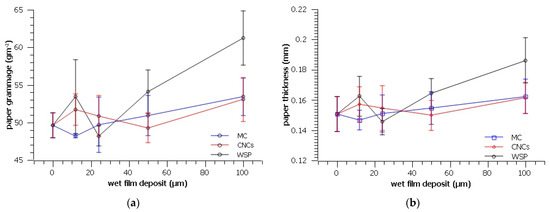
Figure 2.
Grammage (a) and thickness (b) of the prepared samples.
Furthermore, although the CNCs film was visible on micrographs, its application did not increase the RP grammage significantly, while the application of WSP did. The highest increase in both the thickness and grammage is observed in paper coated with WSP, which could have resulted from suspension being prepared with the highest solids weight concentration among the three coatings (1%, 7%, and 4% w/w for MC, WSP, and CNCs, respectively). Large SD values showing variations in the thicknesses of the coated samples could be explained by the work of surface tension, which is known to prevent liquid films from retaining uniform thickness when spread over a rough solid surface [28].
In terms of conservation, it is required that the application of new materials does not alter the appearance of the objects being treated. For this reason, we investigated the optical properties of paper (colour parameters, yellowness, opacity, and gloss) before and after thermal ageing.
As can be seen in Table 2, MC and WSP do not affect the colour appearance of the RP significantly, which is consistent with previous studies [4,37]. The application of CNCs, however, results in a colour difference that increases with the amount of wet film.

Table 2.
ΔEab between the coated and the RP.
The most notable difference between the CNC-coated paper and the RP could be seen by observing the L* value (CNCs cause darkening of the paper, L* value of the RP was measured at 89.36, while CNCs100 measured 88.10) and the b* value (b* value of the RP was measured at 11.85, while CNCs100 measured 15.29).
To determine how thermal ageing had influenced the samples, we calculated the colour difference between unaged and aged samples (Table 3).

Table 3.
ΔEab between the unaged and the thermally aged samples.
The results for thermally aged samples show that the coated paper samples have changed colour to a smaller extent than the uncoated ones (the RP), except for WSP24, which indicates the protection provided by the coatings.
The paper yellowing could be attributed to the oxidation of cellulose [38] or the behaviour of the acid compounds formed during the ageing process [39]. In addition, in degraded cellulose, the formation of carbonyls on aldehydic groups (CHO) and conjugated diketons are responsible for the paper’s optical degradation in the UV and visible range. They can be indicated as one of the main chromophores responsible for paper yellowing [40]. The discolouration is also known to be caused by the oxidation of impurities present in the coating [3].
It could be noted that coatings have not drastically influenced the opacity of the RP (Table 4).

Table 4.
Opacity of the prepared samples.
The opacity of paper is related to the volume of air in a cellulose-based paper, i.e., the distribution of numerous small air pores with a large total surface area. Increased opacity of paper means a larger quantity of light scattered from the surfaces of numerous small pores [41]. Heat treatment results in higher porosity and opacity of paper because of the greater number of pores left after drying due to the smaller amount of hydrogen bonds formed between the cellulose fibres [42]. Due to the heterogeneous structure and surface of the RP and coated samples, the opacity changes after the thermal ageing process are insignificant, as confirmed by large SD values. Moreover, the changes in FTIR spectra around 1307–1340 cm−1 confirm the rearrangement in hydrogen bonding, as shown below.
CNCs are significantly increasing the yellowness of RP, which is related to the wet film deposit increase (Table 5). On the other hand, the yellowness of paper coated by MC and WSP is almost unaffected (Table 5). However, thermal ageing has increased the yellowness of all samples.

Table 5.
Yellowness of the prepared samples.
The increased yellowness is the consequence of oxidation products as detected by the FTIR analysis (Figures 5–8).
The gloss value of the RP used in this research is low and mostly unaffected by the treatment with MC. However, the gloss value is increased by 1 GU when applying CNCs or WSP with the highest wet film deposit (Table 6). The increase of the surface gloss with the amount of CNCs added was also confirmed by previous studies [21,43].

Table 6.
Gloss (GU) of the prepared samples.
It should be noted that the increase of the wet film deposit raises the gloss value of the samples coated by both CNCs and WSP.
The thermal ageing of the samples has not influenced gloss, whereas coating has resulted in a slightly increased gloss value. This behaviour can be observed in the micrographic images, which show a film formed by CNCs and WSP (Figure 3 and Figure 4).
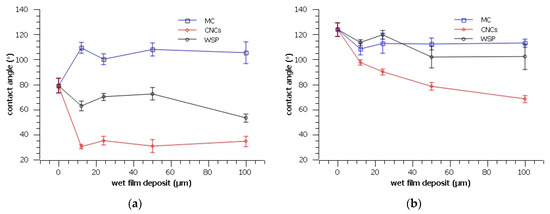
Figure 3.
Contact angle of water of the prepared samples before (a) and after (b) thermal ageing.
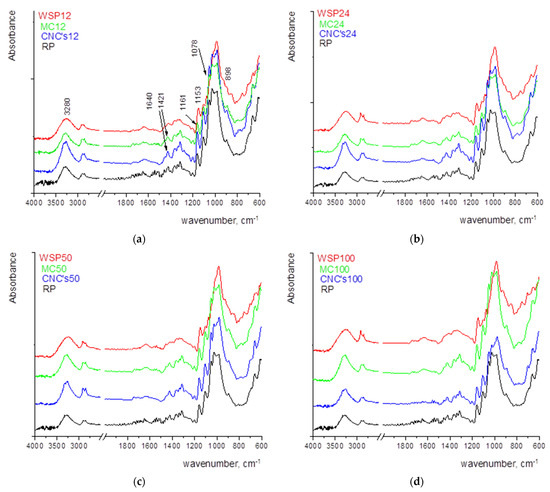
Figure 4.
FTIR spectra of the reference paper (RP) and the wet film deposit of (a) 12 µm, (b) 24 µm, (c) 50 µm, and (d) 100 µm.
The importance of surface phenomena in the determination of nanosystems behaviour has been extensively highlighted by researchers [44]. As mentioned earlier, the maximum contact of a coating with the surface depends on the cleanliness and structure of the surface, as well as its wetting [28]. However, wetting itself is influenced by the forces within the liquid and between the liquid and the surface [2].
Since the presence of water could lead to swelling of the fibres and weakening of the paper, we performed contact-angle measurements to determine the influence of the three coatings on the surface properties of the paper.
As exemplified in Figure 3a, MC has increased the contact angle of paper prior to thermal ageing. CNCs have significantly decreased the contact angle, making the surface more hydrophilic. The wet film deposit change shows no significant influence on the interaction between the surface and water, which can be explained by the formation of oxidation products as confirmed by the FTIR analysis.
Thermal ageing increases hydrophobicity and leads to a contact angle higher than 120° in the RP. Furthermore, it increases the contact angle for all samples, with the change being the smallest in the samples coated with MC. In contrast with unaged samples, thermally aged samples interact differently with water, depending on the amount of wet film applied. Namely, an increase in the wet film deposit results in a decrease in the contact angle.
In addition to the interaction between the paper surface and water, we determined the water droplet absorption, which is defined as the time in which the paper structure fully absorbs the constant volume of water.
The results of the water absorption are shown in Table 7. It should be noted that thermal ageing may contribute to surface sealing.

Table 7.
Time (s) for the absorption of a 2 µL water droplet.
The most resistance to the penetration by the droplet is shown in the thermally aged samples coated with WSP, where samples WSP50 and WSP100 failed to finish the test in 12 min.
Furthermore, although CNCs have shown the lowest contact angles (i.e., they have proven to be the most hydrophilic), the formed film provided a better barrier than the film formed by MC.
Figure 4 shows the FTIR spectra of the samples where the strong bands of cellulose, namely 1159 cm−1 (antisymmetric bridge stretching of C-O-C groups) and C-O stretching in cellulose/hemicellulose molecules at 1105 and 1024 cm−1. The strongest band observed in the spectrum at 1024 cm−1 is accompanied by two characteristic peaks at 1051 and 981 cm−1 [13,45]. The vibrational band assigned to C–O–C stretching at β-(1-4)-glycosidic linkages at 893 cm−1 is called an “amorphous” absorption band, while the vibrational band assigned to a symmetric CH2 bending vibration observed around 1429 ± 1 cm−1, is known as the “crystallinity” band [13,46,47]. The characteristic vibrational bands mentioned above are observed in RF and in papers coated with MC and CNCs. The paper coated with WSP shows somewhat different vibrational bands but the contribution of paper vibrational bands can be noticed as well, especially in the fingerprint region. A vibrational band located around 1639 ± 1 cm−1 can be assigned to the bending vibration of water molecules (adsorbed water) [48]. However, considering the large noise that appears in the FTIR spectrum of the RF, it can be assumed that there was a slight oxidation of the surface. With the increase in thickness of wet film deposits, the vibrational bands are sharper. In addition, the amount of adsorbed water increases, as seen from the broadening and the increase of the vibrational band located around 1640 cm−1, especially in the case of MC and WSP.
In the FTIR spectra of paper coated with WSP, the vibrational band at 1630 cm−1 can be attributed to the presence of bound water and the band at 3420 cm−1 was due to the presence of hydroxyl groups (O–H), while the stretching vibrational bands of the C–O in the C–O–H groups result in a formation of a band at 1540 cm−1. The band at 1470 cm−1 can be assigned to the stretching vibration of C–O [49]. The vibrational band at 895 cm−1 is characteristic of β-glycosidic linkage [13].
With the application of CNCs and MC, the change in bands at 1370, 1340, and 1317 cm−1 in the spectrum of used samples indicates a rearrangement of the hydrogen-bonded network [13].
The FTIR spectrum does not show significant changes in the aged and unaged paper spectra. The most significant changes are visible in the range of 1500–1600 cm−1. The loss of adsorbed water in the sample is also visible. This indicates that thermal ageing affected the paper’s residual water content (i.e., desorption of water molecules), as confirmed by previous research [48,50].
With the thermal ageing process, the broadening of the vibrational band at 893 cm−1 reflects a higher amount of disordered structure of the RP (Figure 5).
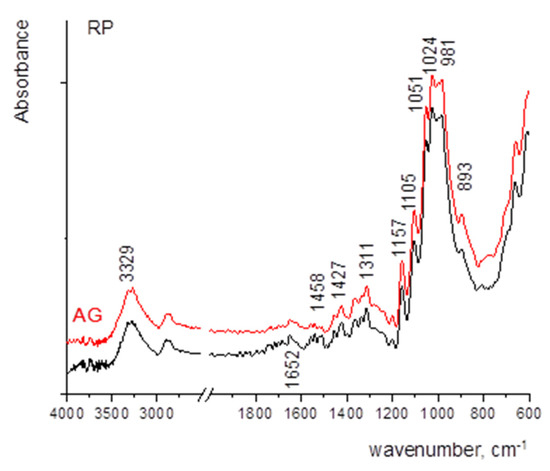
Figure 5.
FTIR spectra of the RP before and after thermal ageing.
An increase in the amount of CNCs raises the stability of the film. The greatest degradation is visible at a wet film deposit of 24 µm. With the application of smaller wet film deposit, it is assumed that the FTIR spectrum is largely masked by the cellulose spectrum from the paper and overlaps, while with a coating of 24 µm, oxidation and the formation of oxidation products are visible in the spectral range of 1745–1710 cm−1 (Figure 6).
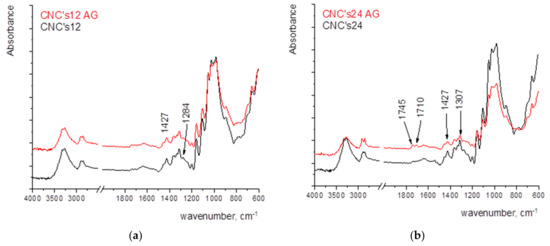
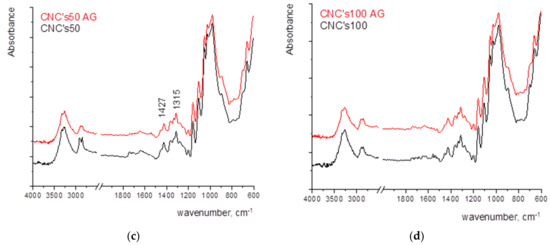
Figure 6.
FTIR spectra of paper coated with CNCs with the wet film deposit of (a) 12 µm, (b) 24 µm, (c) 50 µm, and (d) 100 µm.
The larger changes after ageing in the MC12 may be attributed to the penetration of the coating into the paper structure, the oxidative pathways of both the MC and paper, and the overlapping of their vibrational bands (Figure 7a).
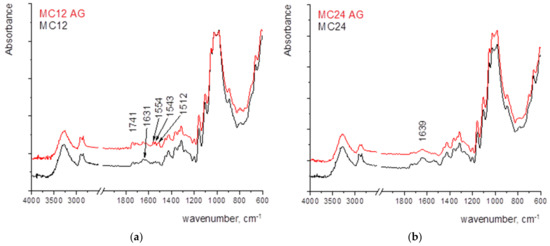
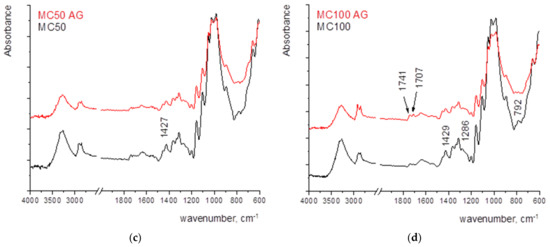
Figure 7.
FTIR spectra of paper coated with MC with the wet film deposit of (a) 12 µm, (b) 24 µm, (c) 50 µm, and (d) 100 µm.
The most notable change in the sample MC24 is the loss of adsorbed water which can be monitored by the change of the vibrational band present at 1639 cm−1 (its decrease) (Figure 7b).
In sample MC24, the peak of crystalline cellulose and adsorbed water decreases.
The most significant changes occur in the spectrum of the sample coated with 100 µm (MC100, Figure 7d), where the peak of crystalline cellulose and adsorbed water decreases. New vibrational bands appear in the range of 1800–1500 cm−1, indicating the formation of oxidation products. The formation of vibrational bands 1741–1707 cm−1 can be explained by the hydrolysis of hemiacetal bonds, which may eventually generate aldehyde groups on opening the terminal rings. Those bands represent the final oxidation stage of carbon atoms in glucopyranose rings [50,51].
The changes in the oxidation state of the samples with propagating degradation are observed in the FTIR spectra in the range of 1500–1850 cm−1, where the carbonyl groups in various chemical environments appear. However, the problem can occur due to the water molecules appearing at 1640 cm−1 masking the products of cellulose oxidation (Figure 8).
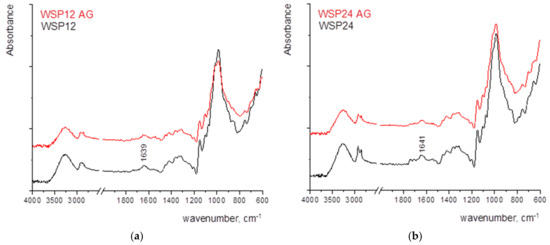
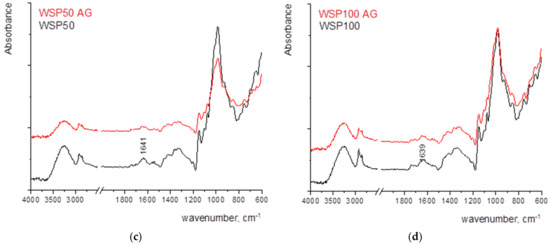
Figure 8.
FTIR spectra of paper coated with WSP and wet film deposit of (a) 12 µm, (b) 24 µm, (c) 50 µm, and (d) 100 µm.
4. Conclusions
This research aimed to analyse the influence of different wet film deposits of three coatings (CNCs, MC, and WSP) on the properties of Japanese paper, which was used as a representation of historic paper. The results show that CNCs cause the highest colour difference and increase the yellowness of paper, while the other two coatings have not altered the colour noticeably (ΔE < 1). All coatings, except for CNC24, have contributed to preserving colour properties from the damage caused by thermal ageing.
Data also show that MC has increased the hydrophobicity of the paper surface. WSP and MC have increased the paper’s water-droplet absorption time, which was additionally prolonged in both coated and uncoated samples after thermal ageing.
Higher amounts of the wet film have influenced the colour properties of paper coated with CNCs the most.
Since the FTIR spectrum does not show significant changes in the spectra of aged and unaged paper, further study on thermal ageing at other temperatures and time intervals needs to be conducted.
To conclude, wet film deposit has proven to be an important parameter that should be considered when applying the coatings mentioned above. Therefore, CNCs should be applied sparingly (up to 24 µm). Furthermore, the increase in the amount of MC will not affect the appearance of the paper significantly, while in WSP, it will raise gloss.
Author Contributions
Conceptualization, G.A. and T.C.; methodology, G.A. and T.C.; validation, G.A. and T.C.; formal analysis, G.A., T.C., M.V. and K.I.I.; investigation, G.A., T.C. and M.V.; resources, G.A., T.C. and K.I.I.; writing—original draft preparation, G.A., T.C. and M.V.; writing—review and editing, G.A., T.C., M.V. and K.I.I.; visualization, T.C. and M.V.; supervision, T.C. All authors have read and agreed to the published version of the manuscript.
Funding
The APC was funded by University of Zagreb Faculty of Graphic Arts.
Data Availability Statement
Data is contained within the article.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Strlič, M.; Kolar, J.; Scholten, S. Paper and Durability. In Ageing and Stabilisation of Paper; Strlič, M., Kolar, J., Eds.; National and University Library: Ljubljana, Slovenia, 2005; pp. 3–8. [Google Scholar]
- Horie, C.V. Materials for Conservation: Organic Consolidants, Adhesives and Coatings. In Butterworth-Heinemann Series in Conservation and Museology, 2nd ed.; Rees-Jones, S.G., Linstrum, D., Eds.; Butterworth & Co, Ltd.: London, UK, 1987. [Google Scholar]
- The Conservation Unit Museums and Galleries Commission. The Science For Conservators Series Volume 1: An Introduction to Materials, 2nd ed.; Ashley-Smith, J., Wilks, H., Eds.; Routledge: London, UK, 1992; Volume 1. [Google Scholar]
- da Silva Borges, I.; Casimiro, M.H.; Macedo, M.F.; Sequeira, S.O. Adhesives Used in Paper Conservation: Chemical Stability and Fungal Bioreceptivity. J. Cult. Herit. 2018, 34, 53–60. [Google Scholar] [CrossRef]
- Indictor, N.; Baer, N.S.; Phelan, W.H. An Evaluation of Pastes for Use in Paper Conservation. Restaurator 1975, 2, 139–150. [Google Scholar] [CrossRef]
- Hassan, R.R.A.; Mahmoud, S.M.A.; Nessem, M.A.; Aty, R.T.A.; Ramzy, M.G.; Dessoky, E.S.; Abdelkhalek, A.; Salem, M.Z.M. Hydroxypropyl Cellulose Loaded with ZnO Nanoparticles for Enhancing the Mechanical Properties of Papyrus (Cyperuspapyrus L.) Strips. Bioresources 2021, 16, 2607–2625. [Google Scholar] [CrossRef]
- Ali Hassan, R.R.; Amer Mahmoud, S.M.; Karam, Y.A.; Salah, S.M.; Ebrahim, S.Y.; Hassan Ahmed, A.H.M.; Ali, H.M.; Böhm, M.; Salem, M.Z. Application of Frankincense and Rice Starch as Eco-Friendly Substances for the Resizing of Paper as a Conservation Practice. Bioresources 2021, 16, 7180–7204. [Google Scholar] [CrossRef]
- Schönbohm, D.; Blüher, A.; Banik, G. Enzymes in Solvent Conditioned Poultices for the Removal of Starch-Based Adhesives from Iron Gall Ink Corroded Manuscripts. Restaurator 2004, 25, 267–281. [Google Scholar] [CrossRef]
- Fairbrass, S. Sticky Problems for Conservators of Works of Art on Paper. Int. J. Adhes. Adhes. 1995, 15, 115–120. [Google Scholar] [CrossRef]
- Psilodimitrakopoulos, S.; Gavgiotaki, E.; Melessanaki, K.; Tsafas, V.; Filippidis, G. Polarization Second Harmonic Generation Discriminates between Fresh and Aged Starch-Based Adhesives Used in Cultural Heritage. Microsc. Microanal. 2016, 22, 1072–1083. [Google Scholar] [CrossRef]
- Van Steene, G.; Masschelein-Kleiner, L. Modified Starch for Conservation Purposes. Stud. Conserv. 1980, 25, 64–70. [Google Scholar] [CrossRef]
- Maitland, C. Microscopy for Paper Conservation: Comparing Various Adhesives and Examining Wheat Starch Paste Preparation Methods. Book Pap. Group Annu. 2010, 29, 129–138. [Google Scholar]
- Tkalčec, M.M.; Bistričić, L.; Leskovac, M. Influence of Adhesive Layer on the Stability of Kozo Paper. Cellulose 2016, 23, 853–872. [Google Scholar] [CrossRef]
- Hayakawa, N. Scientific Approaches for Adhesives in the Conservation of Japanese Paintings. In Proceedings of the Adapt & Evolve 2015: Proceedings from the Icon Book & Paper Group Conference; The Institute of Conservation: London, UK, 2017; pp. 59–68. [Google Scholar]
- Lama, E.; Veneranda, M.; Prieto-Taboada, N.; Hernando, F.L.; Rodríguez Laso, M.D.; Madariaga, J.M. A First Evaluation of the Usefulness of Kudzu Starch in Cultural Heritage Restoration. Sci. Rep. 2020, 10, 15598. [Google Scholar] [CrossRef] [PubMed]
- Noshy, W.; Ali Hassan, R.R.; Mohammed, N. Using Biopolymers to Strengthen the Historical Printed Paper: Mechanical and Optical Characters. Pigment. Resin Technol. 2022, 51, 212–226. [Google Scholar] [CrossRef]
- Rushdy, A.M.; Wahba, W.N.; Abd-Aziz, M.S.; El Samahy, M.; Kamel, S. A Comparative Study of Consolidation Materials for Paper Conservation. Int. J. Conserv. Sci. 2017, 8, 441–452. [Google Scholar]
- Li, Q.; McGinnis, S.; Sydnor, C.; Wong, A.; Renneckar, S. Nanocellulose Life Cycle Assessment. ACS Sustain. Chem. Eng. 2013, 1, 919–928. [Google Scholar] [CrossRef]
- Ngo, T.-D.; Danumah, C.; Ahvazi, B. Production of Cellulose Nanocrystals at InnoTech Alberta. In Nanocellulose and Sustainability: Production, Properties, Applications and Case Studies; Lee, K.-Y., Ed.; CRC Press: Boca Raton, FL, USA, 2018; pp. 269–287. [Google Scholar]
- Xu, C.; Huang, C.; Huang, H. Recent Advances in Structural Color Display of Cellulose Nanocrystal Materials. Appl. Mater. Today 2021, 22, 100912. [Google Scholar] [CrossRef]
- Spagnuolo, L.; D’Orsi, R.; Operamolla, A. Nanocellulose for Paper and Textile Coating: The Importance of Surface Chemistry. Chempluschem 2022, 87, e202200204. [Google Scholar] [CrossRef]
- Operamolla, A.; Mazzuca, C.; Capodieci, L.; Di Benedetto, F.; Severini, L.; Titubante, M.; Martinelli, A.; Castelvetro, V.; Micheli, L. Toward a Reversible Consolidation of Paper Materials Using Cellulose Nanocrystals. ACS Appl. Mater. Interfaces 2021, 13, 44972–44982. [Google Scholar] [CrossRef]
- Elmetwaly, T.E.; Darwish, S.S.; Attia, N.F.; Hassan, R.R.A.; El Ebissy, A.A.; Eltaweil, A.S.; Omer, A.M.; El-Seedi, H.R.; Elashery, S.E.A. Cellulose Nanocrystals and Its Hybrid Composite with Inorganic Nanotubes as Green Tool for Historical Paper Conservation. Prog. Org. Coat. 2022, 168, 106890. [Google Scholar] [CrossRef]
- Ahmed, R.H.; Wahba, W.N.; Abouzeid, R.E.; Ali, K.A. Evaluation of Starch and Cellulose Based Consolidation Materials on the Mechanical Properties of Papyrus. Cellul. Chem. Technol. 2022, 56, 391–401. [Google Scholar] [CrossRef]
- Völkel, L.; Ahn, K.; Hähner, U.; Gindl-Altmutter, W.; Potthast, A. Nano Meets the Sheet: Adhesive-Free Application of Nanocellulosic Suspensions in Paper Conservation. Herit. Sci. 2017, 5, 23. [Google Scholar] [CrossRef]
- Aleksić, G.; Cigula, T.; Itrić Ivanda, K. Influence of Multilayered Films Containing Cellulose Nanocrystals on the Properties of Japanese Paper. In Proceedings of the Eleventh International Symposium GRID 2022; Vladić, G., Ed.; University of Novi Sad, Faculty of Technical Sciences, Department of Graphic Engineering and Design: Novi Sad, Serbia, 2022; pp. 459–466. [Google Scholar]
- Farnsworth, D. Sidedness: Wire and Felt Side of Handmade Paper; Stone, N., Ed.; Magnolia Editions: Oakland, CA, USA, 2018. [Google Scholar]
- Conservation Unit of the Museums and Galleries Commission. The Science for Conservators Series: Volume 3: Adhesives and Coatings, 2nd ed.; Ashley-Smith, J., Ed.; Routledge: London, UK, 1992; Volume 3. [Google Scholar]
- Talas Methyl Cellulose. Available online: https://www.talasonline.com/Methyl-Cellulose (accessed on 5 March 2023).
- Strlič, M.; Kolar, J.; Pihlar, B. Methodology and Analytical Techniques in Paper Stability Studies. In Ageing and Stabilisation of Paper; Strlič, M., Kolar, J., Eds.; National and University Library: Ljubljana, Slovenia, 2005; pp. 27–47. [Google Scholar]
- Sayed Darwish, S. Evaluation of the Effectiveness of Some Consolidants Used for the Treatment of the XIXth Century Egyptian Cemetery Wall Paintings. Int. J. Conserv. Sci. 2013, 4, 413–422. [Google Scholar]
- Mokrzycki, W.; Tatol, M. Color Difference Delta E—A Survey. Mach. Graph. Vis. 2011, 20, 383–411. [Google Scholar] [CrossRef]
- An Investigation into the Use of PH Indicators on Paper. Available online: https://cool.culturalheritage.org/byorg/abbey/ap/ap03/ap03-3/ap03-314.html (accessed on 22 February 2023).
- Thakur, R.; Pristijono, P.; Scarlett, C.J.; Bowyer, M.; Singh, S.P.; Vuong, Q.V. Starch-Based Films: Major Factors Affecting Their Properties. Int. J. Biol. Macromol. 2019, 132, 1079–1089. [Google Scholar] [CrossRef] [PubMed]
- Żołek-Tryznowska, Z.; Kałuża, A. The Influence of Starch Origin on the Properties of Starch Films: Packaging Performance. Materials 2021, 14, 1146. [Google Scholar] [CrossRef]
- Hynninen, V.; Patrakka, J. Nonappa Methylcellulose–Cellulose Nanocrystal Composites for Optomechanically Tunable Hydrogels and Fibers. Materials 2021, 14, 5137. [Google Scholar] [CrossRef] [PubMed]
- Feller, R.L.; Wilt, M. Evaluation of Cellulose Ethers for Conservation; The Getty Conservation Institute: Los Angeles, CA, USA, 1990; Volume 3. [Google Scholar]
- Carter, H.A. Chemistry Everyday for Everyone The Chemistry of Paper Preservation Part 2. The Yellowing of Paper and Conservation Bleaching. J. Chem. Educ. 1996, 73, 1068. [Google Scholar] [CrossRef]
- Sevastyanova, O.; Li, J.; Geilerstedt, G. On the Reaction Mechanism of the Thermal Yellowing of Bleached Chemical Pulps. Nord. Pulp. Paper Res. J. 2006, 21, 188–192. [Google Scholar] [CrossRef]
- Mosca Conte, A.; Pulci, O.; Knapik, A.; Bagniuk, J.; Del Sole, R.; Lojewska, J.; Missori, M. Role of Cellulose Oxidation in the Yellowing of Ancient Paper. Phys. Rev. Lett. 2012, 108, 158301. [Google Scholar] [CrossRef]
- Pauler, N. Paper Optics—Optical and Colour Science Related to the Pulp and Paper Industry; ABB Lorentzen & Wettre: Stockholms Lan, Sweden, 2012. [Google Scholar]
- Evans, J.; Youngquist, J.A. Papermaking. In Encyclopedia of Forest Sciences; Elsevier Ltd.: Oxford, UK, 2004; pp. 477–619. [Google Scholar]
- Puceković, N.; Hooimeijer, A.; Lozo, B. Cellulose Nanocrystals Coating—A Novel Paper Coating for Use in the Graphic Industry. Acta Graph. 2015, 26, 21–26. [Google Scholar]
- Caminati, G. Cultural Heritage Artefacts and Conservation: Surfaces and Interfaces. In Nanoscience for the Conservation of Works of Art; Baglioni, P., Chelazzi, D., Eds.; RSC Publishing: Cambridge, UK, 2013; pp. 1–48. [Google Scholar]
- Ferreira, P.J.; Gamelas, J.A.; Moutinho, I.M.; Ferreira, A.G.; Gómez, N.; Molleda, C.; Figueiredo, M.M. Application of FT-IR-ATR Spectroscopy to Evaluate the Penetration of Surface Sizing Agents into the Paper Structure. Ind. Eng. Chem. Res. 2009, 48, 3867–3872. [Google Scholar] [CrossRef]
- Ciolacu, D.; Ciolacu, F.; Popa, V.I. Amorphous Cellulose-Structure and Characterization. Cellul. Chem. Technol. 2011, 45, 13–21. [Google Scholar]
- Proniewicz, L.M.; Paluszkiewicz, C.; Wesełucha-Birczyńska, A.; Majcherczyk, H.; Barański, A.; Konieczna, A. FT-IR and FT-Raman Study of Hydrothermally Degradated Cellulose. J. Mol. Struct. 2001, 596, 163–169. [Google Scholar] [CrossRef]
- Łojewski, T.; Miśkowiec, P.; Molenda, M.; Lubańska, A.; Łojewska, J. Artificial versus Natural Ageing of Paper. Water Role in Degradation Mechanisms. Appl. Phys. A 2010, 100, 625–633. [Google Scholar] [CrossRef]
- Gao, L.; Zhu, T.; He, F.; Ou, Z.; Xu, J.; Ren, L. Preparation and Characterization of Functional Films Based on Chitosan and Corn Starch Incorporated Tea Polyphenols. Coatings 2021, 11, 817. [Google Scholar] [CrossRef]
- Lojewska, J.; Lubanska, A.; Miśkowiec, P.; Łojewski, T.; Proniewicz, L.M. FTIR in Situ Transmission Studies on the Kinetics of Paper Degradation via Hydrolytic and Oxidative Reaction Paths. Appl. Phys. A 2006, 83, 597–603. [Google Scholar] [CrossRef]
- Łojewska, J.; Miśkowiec, P.; Łojewski, T.; Proniewicz, L.M. Cellulose Oxidative and Hydrolytic Degradation: In Situ FTIR Approach. Polym. Degrad. Stab. 2005, 88, 512–520. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).