Abstract
A composite material based on polyvinyl alcohol (PVA) and hydroxyapatite modified with magnesium (0.3; 0.5; 1.0 mol) was developed using the in situ mineralization method. A thorough analysis confirmed the formation of a two-phase system, with a uniform distribution of HA particles within the PVA matrix. In addition, the analysis confirmed the successful incorporation of magnesium into the crystal lattice without the formation of secondary phases. The material exhibited a developed macroporous structure, with porosities ranging from 50 to 200 μm. In order to ensure that the rheological properties of the composition were suitable for 3D printing, 4 wt.% gelatin was added, resulting in stable scaffolds. In vitro studies demonstrated high biocompatibility of the materials and a synergistic effect of the components: PVA has been demonstrated to neutralise the cytotoxic effects of HA, while magnesium has been shown to statistically significantly increase the viability of macrophages. The combination of a polymer matrix with an inorganic phase results in a material that exhibits both elasticity and bioactivity. The structural and functional characteristics of these systems render them promising materials for tissue engineering, particularly for bone regeneration and the creation of biocompatible 3D scaffolds.
1. Introduction
Bone tissue, which constitutes the basis of the human musculoskeletal system, plays a pivotal role in maintaining body structure, protecting internal organs, hematopoiesis, and calcium metabolism []. Bone diseases and injuries are a significant problem in modern medicine, as they result in the loss of structural integrity and dysfunction of the musculoskeletal system. In this category, fractures of the bone (including complex, non-union, and pathological fractures) are of particular significance. In addition, degenerative diseases (e.g., osteoarthritis and osteoporosis), infectious lesions (e.g., osteomyelitis), tumours, and congenital defects resulting in the formation of bone cavities and defects of varying severity are also of importance. The aetiology of such disorders can be attributed to a number of factors, including both external (e.g., mechanical impacts, falls, traffic accidents, industrial and sports injuries) and internal causes (e.g., decreased bone mineral density, age-related degenerative changes, endocrine disorders, inflammatory processes, and cancer). The combined effect of these factors leads to a deterioration in the ability of bone tissue to self-repair and increases the risk of developing chronic or extensive defects [,,]. These conditions are frequently accompanied by chronic pain, limited mobility, and a significant deterioration in the quality of life of patients, requiring comprehensive and effective treatment methods. Conventional methodologies employed in the field of bone tissue restoration encompass conservative therapeutic approaches, in addition to surgical interventions that are directed towards the stabilisation, restoration, or replacement of compromised bone regions [,]. In cases of extensive defects or irreversible damage, implants have been proven to be an indispensable solution, providing mechanical support and acting as scaffolds for new bone regeneration [,]. Three-dimensional (3D) printing is also one of the most promising approaches in modern tissue engineering, enabling the creation of customized and biocompatible structures that precisely replicate the anatomical shape of damaged bone. In contradistinction to conventional implant formation methodologies, 3D printing confers a high degree of control over the architecture, encompassing porosity, pore size, and interconnections, which are pivotal for cell migration, vascularization, and osseointegration [,,].
Modern orthopaedics and traumatology utilise a wide range of implants, which can be classified by their material and functional purpose.
- (i)
- Metal implants, for example, those made of titanium [] and cobalt-chromium alloys [], are valued for their high strength and are used for fracture fixation and total joint replacement. However, they have the potential to induce stress shielding, thereby weakening the surrounding bone and impeding active regeneration.
- (ii)
- Ceramic materials (e.g., hydroxyapatite, tricalcium phosphate) demonstrate high biocompatibility, osteoconductivity (i.e., the capacity to function as a matrix for bone growth) and chemical similarity to natural bone. These fillers are utilised in the treatment of bone defects, while their use as coatings for metal implants has also been demonstrated [,,]. The primary disadvantages associated with these materials pertain to their fragility and the challenge of constructing intricate structures.
- (iii)
- Polymeric materials (for instance, polymethyl methacrylate, polyetheretherketone, and biodegradable polymers such as polylactic acid or polyglycolic acid) possess the advantageous qualities of flexibility, controllable degradation rates, and the capacity to generate porous structures. These materials find application in a variety of contexts, including bone cements, scaffolds for tissue engineering, and specific types of prostheses. However, it has been observed that their mechanical strength frequently falls short of the requirements necessary for load-bearing applications [,].
In view of the limitations of monomaterial implants, contemporary research is increasingly oriented towards the development of composite biomaterials [,,]. The selection of biomaterial components is critical to achieving the desired biomechanical and biological properties required for effective tissue engineering. In the context of bone tissue regeneration, particular attention is being paid to polymer-ceramic composites, which combine the elasticity and controlled biodegradation of polymers with the bioactivity and mechanical strength of ceramic phases. Polyvinyl alcohol (PVA) and hydroxyapatite (HA) have been identified as the most promising materials for creating such composites, exhibiting complementary properties that make them ideal for use in this application.
PVA has been demonstrated to exhibit high biocompatibility and non-toxicity, as evidenced by numerous in vitro and in vivo studies, without causing significant immune reactions []. It has been demonstrated that the material exhibits controlled biodegradability within the body, a property which facilitates its utilisation as a temporary scaffold, which is then gradually replaced by new tissue []. The mechanical properties of the material, including its elasticity and strength, can be significantly enhanced through physical cross-linking processes, such as those induced by freeze–thaw cycles, or chemical cross-linking methods [,]. The primary constraint imposed by pure PVA, particularly in its uncross-linked state, pertains to its inadequate mechanical strength and rigidity. This deficiency renders it unsuitable for utilisation as load-bearing bone implants. Additionally, PVA itself does not possess osteoinductive properties, meaning that it does not actively stimulate the differentiation of stem cells into osteoblasts.
Hydroxyapatite (HAP) is a material that is frequently employed in tissue engineering applications due to its high degree of biocompatibility and pronounced osteoconductivity. These properties provide an effective matrix for the adhesion, growth, and migration of osteogenic cells, as well as facilitating the integration of the implant with the surrounding bone tissue []. In certain morphologies, such as nanoparticles or porous structures, it is also capable of exhibiting osteoinductive properties, thereby stimulating the formation of new bone []. Nevertheless, the inherent fragility of the material and its limited resistance to mechanical stress significantly restrict its independent utilisation, particularly in areas under strain. In this regard, an appropriate approach is the integration of HA into a polymer matrix, which provides elasticity, processability, and increased mechanical strength of the composite material. Furthermore, modification of HA with magnesium ions has been shown to bring its chemical composition closer to natural bone tissue, whilst also promoting increased biological activity, improved cell proliferation and stimulation of osteogenesis, thus expanding the possibilities of its use in bone regeneration [,,].
The combination of PVA and HA in a single composite material has been shown to create a synergistic effect, with the aim of overcoming the limitations of each component and significantly improving the functional properties for bone tissue engineering. PVA, functioning as a polymer matrix, endows the composite with elasticity and fracture toughness, while HA, operating as a reinforcing filler, substantially augments the material’s stiffness and elastic modulus. This enables an optimal balance to be achieved between strength and flexibility, thus bringing the composite’s properties closer to those of native bone. PVA’s water solubility and its capacity to form viscous solutions render it an optimal base for “bioink” in extrusion 3D printing. This process is instrumental in ensuring the requisite rheological properties for the effective printing of intricate, porous structures, characterised by a uniform distribution of HA particles within the matrix. The aim of this study was to create and characterize the composition of biocompatible composite materials based on polyvinyl alcohol and ion-modified hydroxyapatite suitable for obtaining materials by 3D printing.
2. Materials and Methods
Polyvinyl alcohol (PVA) with an average molecular weight of 50,000 g/mol was purchased from Sigma Aldrich (St. Louis, MO, USA), calcium hydroxide (GOST 9262-77) (Khimbaza, Moscow, Russia), ammonium hydrogen phosphate (GOST 3772-74) (Khimbaza, Moscow, Russia), and magnesium chloride (Khimbaza, Moscow, Russia), gelatin (HiMedia, Moscow, Russia).
2.1. Synthesis of Composite Material
Samples of composite material were prepared by means of the following procedure. A weighed portion of polyvinyl alcohol (4.5 g) was dissolved in 15 mL of distilled water. The solution was then subjected to vigorous stirring using an immersion stirrer, after which it was heated in a water bath at 80 °C. Following gelation, the calculated amount of magnesium chloride was added to the solution until complete dissolution. Subsequently, a suspension of calcium hydroxide was introduced and thoroughly amalgamated until a homogeneous structure was achieved. Thereafter, a solution of ammonium phosphate was incorporated. The reaction mixture was stirred for a period of six hours at a pH value ranging from 10 to 11. The reactions proceeded in accordance with the equations presented in Table 1. The final suspension contained 10 wt.% PVA and 10 wt.% HA.

Table 1.
Synthesis scheme of composite materials.
2.2. Three-Dimensional Printing Parameters
Three-dimensional printing was performed on a Regemat3D REG4LIFE bioprinter (Granada, Spain) using a 5 mL syringe and a 1.64 mm nozzle. The base height was 1.5 mm, and the layer height was maintained at 1.2 mm at a print speed of 2 mm/s. Printing was performed under ambient conditions (22–25 °C, relative humidity 40–50%).
2.3. Characterization Methods
Surface morphology was studied using scanning electron microscopy (SEM) on a Quanta 200 3D instrument (FEI Company, Hillsboro, OR, USA), and elemental analysis was performed using EDAX Genesis XM 2 60 (AMETEK, Berwyn, PA, USA). The phase composition of the samples was identified using X-ray diffraction analysis on an XRD-6000 diffractometer (Shimadzu, Kyoto, Japan) in the 2-theta range of 15–70°. The composition of functional groups was determined by IR spectroscopy on a Nicolet 670 instrument (Thermo Fisher Scientific, Madison, WI, USA) in the range of 4000–450 cm−1. The surface mass was calculated using the Owens-Wendt equation and the contact angle was measured using a DSA25 instrument (KRÜSS, Hamburg, Germany) using ADVANCE software 1.15-02.
2.4. In Vitro Experiments
Immune cell viability was assessed using monocytes after incubation on the surface of the test materials. Monocytes were isolated from the blood of three donors. Each sample was spiked with 2 mL of cell suspension at a concentration of 1 million/mL. The samples were incubated at 37 °C for 6 days. The supernatant was then removed from each well, leaving 500 μL of cell-containing medium in each well. Additionally, 50 μL of Alamar Blue reagent were added to the wells (Alamar Blue/cell medium ratio 1/10). Cells with Alamar Blue were incubated for 3 h at 37 °C in the dark. After incubation, cell medium containing Alamar Blue was added to a 96-well plate (three wells for each sample). Fluorescence signal intensity was measured using a Tecan Infinite 200 microreader (Tecan Group Ltd., Männedorf, Switzerland) at a wavelength of 540 nm.
3. Results and Discussion
IR spectroscopy of composite material samples obtained by in situ mineralization of a polyvinyl alcohol solution with ion-modified hydroxyapatite revealed bands characteristic of both polyvinyl alcohol and hydroxyapatite. The presence of broad bands corresponding to the stretching vibrations of –OH groups is observed in the range of 3400–3100 cm−1. The characteristic peaks of the CH2 groups of polyvinyl alcohol are recorded in the region of 2940 and 2900 cm−1. The peak with the higher wavenumber is associated with asymmetric stretching vibrations, while the peak with the lower wavenumber is associated with symmetric stretching vibrations. Absorptions around 1600 cm−1 are consistent with the stretching vibrations of C=O, which is presumed to be the residue of acetate fragments. The band at 1432 cm−1 is related to the scissor vibrations of CH groups. One of the hallmarks of hydroxyapatite formation is the presence of a broad band with a maximum at approximately 1000–1100 cm−1, which is attributed to the stretching vibrations of PO43−. In this region, a broadening of the bands is observable as the magnesium content in the sample increases, which may indicate structural changes in the hydroxyapatite crystal lattice due to the incorporation of magnesium ions (Figure 1) [].
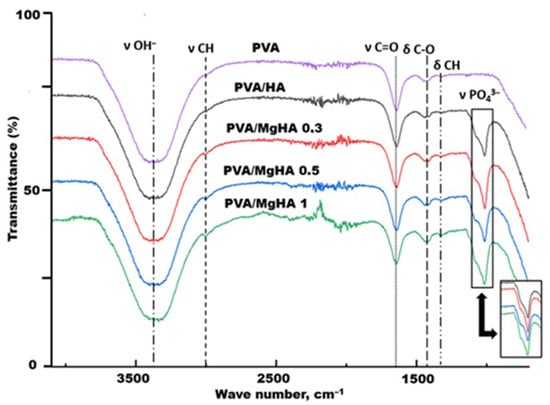
Figure 1.
IR spectra of composite material samples.
X-ray diffraction analysis of the composite material samples confirmed the presence of two main phases: polyvinyl alcohol and hydroxyapatite. The broad amorphous reflection at 2θ ≈ 20° corresponds to the polymer phase and gradually decreases in intensity with increasing HA content. The observed increase in the intensity of the diffraction peak near 2θ ≈ 20° with increasing magnesium content in the composite indicates partial ordering of the macromolecular chains of polyvinyl alcohol. This is due to the fact that Mg2+ ions, having a smaller ionic radius and greater polarizing capacity compared to Ca2+, are capable of forming stronger ion-dipole interactions with the hydroxyl groups of PVA. Such bonds serve as additional centers of orientation and crystallization of the polymer chains, promoting their closer packing. Taken together, these effects lead to an increase in the degree of crystallinity of the PVA matrix, which is reflected in the form of a narrower and more intense peak in the region of ~20° in the X-ray diffraction pattern. Hydroxyapatite is characterized by sharp diffraction peaks at 2θ ≈ 26°, 32–34°, 39°, 47°, 49°, and 53°, which completely coincide with the reflections of hexagonal HA (Figure 2). A comparison of the diffraction patterns revealed a systematic shift in reflections toward higher angles in the magnesium-modified samples, indicating the substitution of magnesium ions for calcium ions in the crystal lattice. This leads to a decrease in the unit cell parameters. No additional phases were detected, indicating the production of pure hydroxyapatite without byproducts. In previous studies, when obtaining pure hydroxyapatite in the presence of PVA, the formation of monophasic hydroxyapatite was also observed, which indicates that magnesium in the composition does not contribute to the formation of new phases, probably due to the smaller size of the atom in comparison with calcium, which can be located in the interstices of the crystal lattice [,].
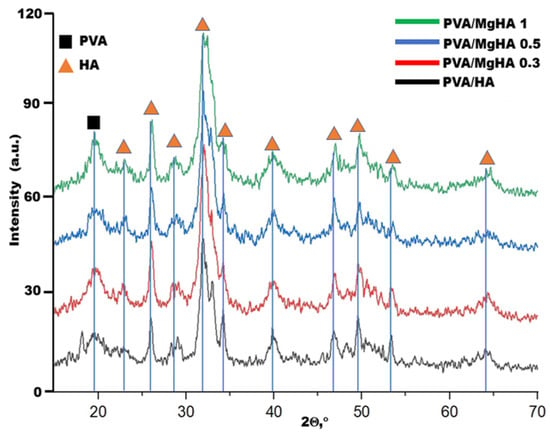
Figure 2.
Diffraction patterns of composite material samples.
The crystallite sizes for hydroxyapatite were calculated using the Selyakov-Scherrer formula, as were the interplanar spacings (Table 2).

Table 2.
Determination of crystallite sizes and interplanar spacing (D).
With the addition of magnesium, the average crystallite size decreases in comparison with pure HA. However, it can be concluded that crystallite size is independent of magnesium concentration in the sample. The interplanar spacing also changes slightly, suggesting that magnesium has been incorporated into the hydroxyapatite structure without significant structural changes to the crystal lattice.
Scanning electron microscopy (SEM) revealed surface heterogeneity of the composite materials and a porous structure with incorporated hydroxyapatite particles (Figure 3). Of particular interest is the uniform distribution of hydroxyapatite particles (1–5 μm in size) within the composite matrix. They form a rough surface capable of improving osteoblast adhesion. The observed material architecture demonstrates an optimal combination of macroporosity for cellular adhesion.
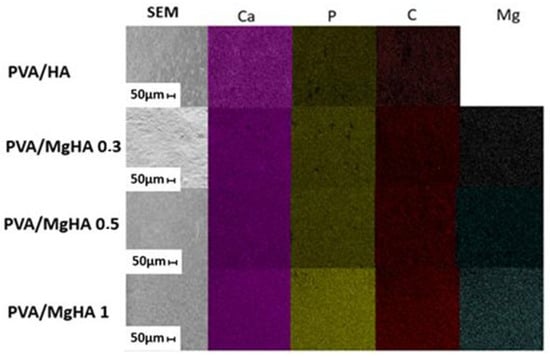
Figure 3.
SEM and distribution of elements on the surface of composites obtained by the Energy-dispersive X-ray spectroscopy.
The atomic content of surface elements obtained using the EDAX method demonstrated a consistent increase in magnesium content in the samples (see Table 3). It is evident that the elements in the sample are distributed uniformly. The obtained materials were found to be dominated by carbon, oxygen, phosphorus, and calcium; no traces of other elements typical of PVA/HA cryogels were detected. In the absence of foreign phases as indicated by X-ray diffraction analysis of the samples, it can be concluded that all magnesium introduced was incorporated into the apatite structure. An increase in magnesium content has been shown to result in a decrease in the amount of carbon on the surface. This phenomenon may be indicative of a change in the orientation of macromolecules on the surface of HA particles. The calcium-to-phosphorus ratio (Ca/P) deviates marginally from the stoichiometric value of 1.67, yet it remains within the acceptable range of 1.5 to 2.5. This phenomenon may be attributed to alterations in the crystallinity of the HA composition, given the variability of the calcium-to-phosphorus ratio that is characteristic of amorphous materials.

Table 3.
Atomic Content of Elements in the Composite Material.
The surface energy of the samples was measured and calculated using the contact angle measurement method (see Figure 4). The polar component of the surface energy (σP) is the primary contributor, a phenomenon that is characteristic of polyvinyl alcohol gel, as the presence of hydroxyl groups leads to the formation of a substantial number of hydrogen bonds. It is evident that the polar component is significantly influenced by the ionic structure of hydroxyapatite. As the magnesium content in the structure increases, the polar component of the surface energy concomitantly increases, which naturally indicates a change in the chemical composition of the particles, as magnesium is more electronegative than calcium. The value of the dispersion component of surface energy (σD) is negligible in comparison to the polar component. However, an increase in the dispersion component is observed with an increase in the amount of magnesium in the sample. An increase in the dispersion component of surface energy may indicate a change in the nature of the interaction of polymer chains with each other and with hydroxyapatite, since the interaction mechanism changes from polar to non-polar. It can be posited that the decline in carbon observed on the surface of magnesium-containing composites (see Table 3) is indicative of an intensified interaction between PVA molecules and the HA surface. This hypothesis suggests that the hydroxyl groups are spatially oriented towards the surface of the cations on the HA surface. This, in turn, leads to a change in the OH concentrations in the surface layer and an increase in the dispersion component with the growth of carbon chains facing the surface, contributing to the current ratio of surface energy components.
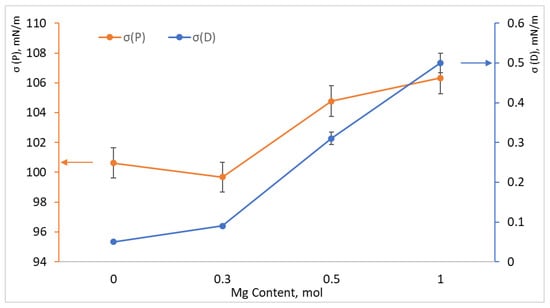
Figure 4.
Polar (orange graph and axis) and dispersion (blue graph and axis) components of surface energy depending on the amount of magnesium added to composite samples PVA/HA, PVA/MgHA 0.3, PVA/MgHA 0.5, PVA/MgHA 1.0.
The second step was to select the optimal solution consistency that would not degrade during layer-by-layer printing. To optimize printability, pure composite materials, composites with various gelling additives, and cryogenic treatment were tested (Table 4).

Table 4.
Printability test results for composite materials with various additives.
The experiment conducted using a 3D printing apparatus revealed that a pure polyvinyl alcohol solution is not compatible with the printing process, irrespective of concentration. As illustrated in Figure 5A, the sample undergoes structural degradation during the initial layer of printing, attributable to the inherently fragile intermolecular bonds inherent in the PVA molecule. Furthermore, the cryogenic treatment of the PVA solution was unsuccessful in achieving the desired outcomes. This is due to the fact that freezing occurs in an uneven manner, which subsequently affects the rheological properties of the solution. The solution under scrutiny comprises agglomerates, which impede the extrusion process (see Figure 5B). The incorporation of a sodium alginate solution into the polyvinyl alcohol solution resulted in an augmentation of the overall viscosity of the solution. During the extrusion process, a two-dimensional sample with clearly defined boundaries and stability was successfully printed. However, with subsequent layering, the sample lost shape, and the layers began to merge (Figure 5C). It was determined through rigorous experimentation that gelatin is the most optimal component for 3D printing. Following the incorporation of the gelatin solution, the mixture obtained the requisite rheological properties, thus enabling the extrusion process to proceed in an efficient manner. Subsequent layering enabled the fabrication of a three-dimensional porous scaffold that exhibited robust shape retention (Figure 5D).
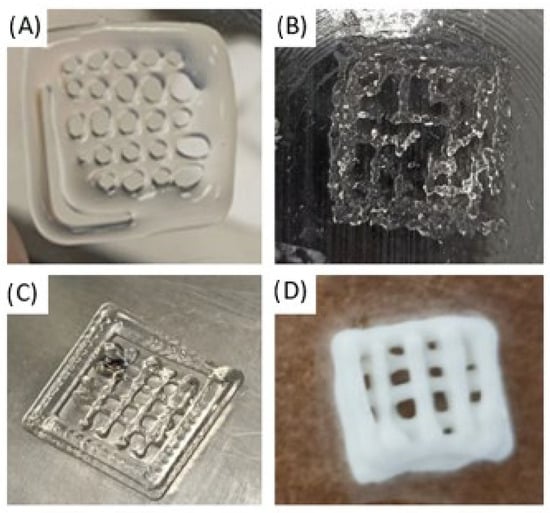
Figure 5.
Three-Dimensional printing with composite material solutions (A) pure polyvinyl alcohol solution, (B) cryogenic treatment of the PVA solution, (C) polyvinyl alcohol solution with sodium alginate, (D) polyvinyl alcohol solution with gelatin.
Gelatin at a concentration of 4 wt.% was added to all obtained suspensions of PVA/HA, PVA/MgHA 0.3, PVA/MgHA 0.5, PVA/MgHA 1.0, and 3D scaffolds with the following parameters were printed on their basis: width 10 mm, length 10 mm, height 5 mm.
The obtained 3D scaffolds were tested for the viability of macrophages in the presence of materials (Figure 6).
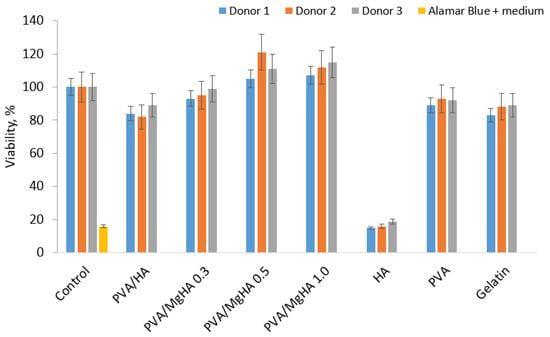
Figure 6.
Viability of human macrophages in the presence of 3D printed PVA/HA/gelatin composites and individual components.
A study of the viability of macrophages in the presence of the materials demonstrated that all composite materials exhibited a high level of cell survival after six days of incubation. A comparison of the viability level with pure components included in the system reveals a synergistic effect of properties, since the viability in the presence of composites is higher than in the presence of individual components. It is evident that an increase in magnesium content in the samples is accompanied by an enhancement in viability, suggesting a favourable impact of magnesium. In the case of the PVA/HA sample, it can be observed that PVA is able to neutralise the cytotoxic effect of HA by adsorbing on the surface of the particles, since the viability level of the PVA/HA and PVA samples is extremely close. The incorporation of gelatin did not exert a substantial influence on viability, as the viability level in the composites did not decline below the values of pure PVA.
Optical microscopy images demonstrate a contrast between flattened macrophages and apoptotic, rounded, wrinkled macrophages with dark nuclei in pure hydroxyapatite (Figure 7). These data correlate with the viability levels obtained using Alamar Blue.
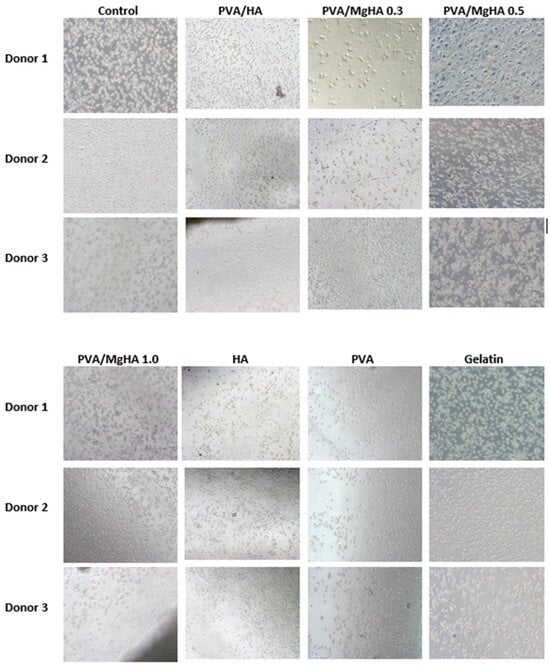
Figure 7.
Images of human macrophages after 6 days of incubation with 3D printed PVA/HA/gelatin composites and individual components.
4. Conclusions
A composite material based on polyvinyl alcohol (PVA) and magnesium-modified hydroxyapatite (MgHA) with magnesium contents of 0.3, 0.5, and 1.0 mol was successfully synthesised using the in situ mineralisation method. A thorough analysis confirmed the formation of a two-phase system, where HA particles are uniformly distributed in the polymer matrix. The introduction of magnesium ions into the HA structure has been shown to result in a decrease in the unit cell parameters and crystallite size, without the formation of secondary phases. The incorporation of magnesium has been demonstrated to enhance the polar component of surface energy, thereby signifying a modification in the chemical composition and the nature of interaction at the PVA-HA interface. In order to guarantee the rheological properties essential for layer-by-layer printing, 4 wt.% gelatin was incorporated into the suspensions. This modification enabled the successful fabrication of stable three-dimensional scaffolds that retained their shape following the printing process. All the resulting composites demonstrated high viability of macrophages during co-incubation. A synergistic effect of the components was observed: PVA has been demonstrated to neutralise the potential toxicity of GA, and the addition of magnesium has been shown to statistically significantly increase the viability of macrophages. The incorporation of gelatin did not exert a detrimental effect on cellular viability. The resulting composite materials, based on PVA and Mg-substituted hydroxyapatite, hold significant potential for further development in biomedicine. These findings have significant implications for future applications in tissue regeneration, where their use in the creation of biocompatible 3D scaffolds for guided bone tissue regeneration is particularly promising. Additionally, their potential extends to resorbable implants and coatings for orthopaedic devices, where their use can ensure improved osseointegration. It is evident that the adjustable bioactivity and mechanical properties of these materials enable their customisation to suit individual patient requirements, thus facilitating their application in the domain of personalised medicine. Moreover, they have the potential to function as carriers for the delivery of drugs and growth factors, thereby expanding the functionality of the composite in tissue engineering.
Author Contributions
Conceptualization, R.S.; Methodology, R.S., L.D., Z.R. and G.S.; Software, R.Z. and U.K.; Validation, R.Z., D.L. and I.K.; Formal analysis, R.Z., I.K. and A.T.; Investigation, G.M.; Resources, G.M., O.L., L.D., Z.R. and G.S.; Data curation, R.S., R.Z., U.K. and O.L.; Writing—original draft, R.S., D.L. and I.K.; Writing—review & editing, I.K. and A.T.; Supervision, G.M., O.L., L.D. and G.S.; Project administration, U.K. and O.L.; Funding acquisition, I.K. All authors have read and agreed to the published version of the manuscript.
Funding
This study was supported by the TSU Strategic academic leadership program Priority 2030.
Data Availability Statement
The original contributions presented in this study are included in the article. Further inquiries can be directed to the corresponding authors.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Florencio-Silva, R.; da Silva Sasso, G.R.; Sasso-Cerri, E.; Simões, M.J.; Cerri, P.S. Biology of bone tissue: Structure, function, and factors that influence bone cells. BioMed Res. Int. 2015, 1, 421746. [Google Scholar] [CrossRef]
- Saul, D.; Menger, M.M.; Ehnert, S.; Nüssler, A.K.; Histing, T.; Laschke, M.W. Bone healing gone wrong: Pathological fracture healing and non-unions—Overview of basic and clinical aspects and systematic review of risk factors. Bioengineering 2023, 10, 85. [Google Scholar] [CrossRef]
- Khan, A.A.; Slart, R.H.J.A.; Ali, D.S.; Bock, O.; Carey, J.J.; Camacho, P.; Engelke, K.; Erba, P.A.; Harvey, N.C.; Lems, W.F.; et al. Osteoporotic fractures: Diagnosis, evaluation, and significance from the International Working Group on DXA Best Practices. Mayo Clin. Proc. 2024, 99, 1127–1141. [Google Scholar] [CrossRef] [PubMed]
- De Pace, R.; Molinari, S.; Mazzoni, E.; Perale, G. Bone regeneration: A review of current treatment strategies. J. Clin. Med. 2025, 14, 1838. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.-Y.; Song, W.; Wang, J.-B.; Lv, R.-Q.; Zhao, F.-H.; Yang, D.-W. Surgical vs. conservative treatment for hip osteoporotic fracture in maintenance hemodialysis patients: A retrospective analysis. Front. Surg. 2024, 11, 1471101. [Google Scholar] [CrossRef]
- Ehlen, Q.T.; Costello, J.P.; Mirsky, N.A.; Slavin, B.V.; Parra, M.; Ptashnik, A.; Nayak, V.V.; Coelho, P.G.; Witek, L. Treatment of bone defects and nonunion via novel delivery mechanisms, growth factors, and stem cells: A review. ACS Biomater. Sci. Eng. 2024, 10, 7314–7336. [Google Scholar] [CrossRef]
- Todd, E.A.; Mirsky, N.A.; Silva, B.L.G.; Shinde, A.R.; Arakelians, A.R.L.; Nayak, V.V.; Marcantonio, R.A.C.; Gupta, N.; Witek, L.; Coelho, P.G. Functional scaffolds for bone tissue regeneration: A comprehensive review of materials, methods, and future directions. J. Funct. Biomater. 2024, 15, 280. [Google Scholar] [CrossRef]
- Yang, Y.; Yang, Y.; He, C.; Yang, W.; Qi, F.; Xie, D.; Shen, L.; Peng, S.; Shuai, C. Mg bone implant: Features, developments and perspectives. Mater. Des. 2020, 185, 108259. [Google Scholar] [CrossRef]
- Gungor-Ozkerim, P.S.; Inci, I.; Zhang, Y.S.; Khademhosseini, A.; Dokmeci, M.R. Bioinks for 3D bioprinting: An overview. Biomater. Sci. 2018, 5, 915–946. [Google Scholar] [CrossRef]
- Kačarević, Ž.P.; Rider, P.M.; Alkildani, S.; Retnasingh, S.; Smeets, R.; Jung, O.; Ivanišević, Z.; Barbeck, M. An introduction to 3D bioprinting: Possibilities, challenges and future aspects. Materials 2018, 11, 2199. [Google Scholar] [CrossRef]
- Fang, Y.; Guo, Y.; Liu, T.; Xu, R.; Mao, S.; Mo, X.; Zhang, T.; Ouyang, L.; Xiong, Z.; Sun, W. Advances in 3D bioprinting. Chin. J. Mech. Eng. 2022, 1, 100011. [Google Scholar] [CrossRef]
- Zhang, J.; Zhuang, Y.; Sheng, R.; Tomás, H.; Rodrigues, J.; Yuan, G.; Wang, X.; Lin, K. Smart stimuli-responsive strategies for titanium implant functionalization in bone regeneration and therapeutics. Mater. Horiz. 2024, 11, 12–36. [Google Scholar] [CrossRef] [PubMed]
- Mani, G.; Porter, D.; Collins, S.; Schatz, T.; Ornberg, A.; Shulfer, R. A review on manufacturing processes of cobalt-chromium alloy implants and its impact on corrosion resistance and biocompatibility. J. Biomed. Mater. Res. B Appl. Biomater. 2024, 6, e35431. [Google Scholar] [CrossRef]
- Baggio, A.M.P.; Sillmann, Y.M.; Eber, P.; Michallek, F.R.; Monteiro, J.L.; Bassi, A.P.; Guastaldi, F.P. How Does Ceramic-Based Scaffold Microarchitecture Impact Maxillofacial Bone Regeneration? A Systematic Review of Large Animal Models. Appl. Sci. 2025, 15, 6899. [Google Scholar] [CrossRef]
- Kucko, S.K.; Raeman, S.M.; Keenan, T.J. Current advances in hydroxyapatite-and β-tricalcium phosphate-based composites for biomedical applications: A review. Biomed. Mater. Devices 2023, 1, 49–65. [Google Scholar] [CrossRef]
- Somngam, C.; Samartkit, S.; Kanchanasurakit, S.; Strietzel, F.P.; Khongkhunthian, P. New bone formation of biphasic calcium phosphate bone substitute material: A systematic review and network meta-analysis of randomized controlled trials (RCTs). Int. J. Implant Dent. 2025, 11, 47. [Google Scholar] [CrossRef]
- Wachtman, J.B.; Cannon, W.R.; Matthewson, M.J. Mechanical Properties of Ceramics; John Wiley & Sons: Hoboken, NJ, USA, 2009. [Google Scholar]
- Elsayed, A.; Wille, S.; Al-Akhali, M.; Kern, M. Comparison of fracture strength and failure mode of different ceramic implant abutments. J. Prosthet. Dent. 2017, 4, 499–506. [Google Scholar] [CrossRef] [PubMed]
- Sadykov, R.; Lytkina, D.; Stepanova, K.; Kurzina, I. Synthesis of biocompatible composite material based on cryogels of polyvinyl alcohol and calcium phosphates. Polymers 2022, 16, 3420. [Google Scholar] [CrossRef]
- Shalygina, K.; Lytkina, D.; Sadykov, R.; Kurzina, I. Composite cryogels based on hydroxyapatite and polyvinyl alcohol and the study of physicochemical and mechanical properties. Materials 2024, 2, 403. [Google Scholar] [CrossRef]
- Stepanova, K.; Lytkina, D.; Sadykov, R.; Shalygina, K.; Khojazoda, T.; Mahmadbegov, R.; Kurzina, I. Composite cement materials based on β-tricalcium phosphate, calcium sulfate, and a mixture of polyvinyl alcohol and polyvinylpyrrolidone intended for osteanagenesis. Polymers 2022, 1, 210. [Google Scholar] [CrossRef]
- Lan, W.; Xu, M.; Qin, M.; Cheng, Y.; Zhao, Y.; Huang, D.; Wei, X.; Guo, Y.; Chen, W. Physicochemical properties and biocompatibility of the bi-layer polyvinyl alcohol-based hydrogel for osteochondral tissue engineering. Mater. Des. 2021, 204, 109652. [Google Scholar] [CrossRef]
- Yang, M.; Wang, Z.; Li, M.; Yin, Z.; Butt, H.A. The synthesis, mechanisms, and additives for bio-compatible polyvinyl alcohol hydrogels: A review on current advances, trends, and future outlook. J. Vinyl Addit. Technol. 2023, 6, 939–959. [Google Scholar] [CrossRef]
- Li, L.; Xu, X.; Liu, L.; Song, P.; Cao, Q.; Xu, Z.; Fang, Z.; Wang, H. Water governs the mechanical properties of poly (vinyl alcohol). Polymer 2021, 213, 123330. [Google Scholar] [CrossRef]
- Fumio, U.; Hiroshi, Y.; Kumiko, N.; Sachihiko, N.; Kenji, S.; Yasunori, M. Swelling and mechanical properties of poly (vinyl alcohol) hydrogels. Int. J. Pharm. 1990, 2, 135–142. [Google Scholar] [CrossRef]
- Shi, H.; Zhou, Z.; Li, W.; Fan, Y.; Li, Z.; Wei, J. Hydroxyapatite based materials for bone tissue engineering: A brief and comprehensive introduction. Crystals 2021, 2, 149. [Google Scholar] [CrossRef]
- DileepKumar, V.G.; Sridhar, M.S.; Aramwit, P.; Krut’ko, V.K.; Musskaya, O.N.; Glazov, I.E.; Reddy, N. A review on the synthesis and properties of hydroxyapatite for biomedical applications. J. Biomater. Sci. Polym. Ed. 2022, 2, 229–261. [Google Scholar] [CrossRef]
- Alanis-Gómez, R.P.; Hernández-Rosas, F.; Olivares-Hernández, J.D.; Rivera-Muñoz, E.M.; Zapatero-Gutiérrez, A.; Méndez-Lozano, N.; Alanis-Gómez, J.R.; Velázquez-Castillo, R. Magnesium-Doped Hydroxyapatite Nanofibers for Medicine Applications: Characterization, Antimicrobial Activity, and Cytotoxicity Study. Int. J. Mol. Sci. 2024, 25, 12418. [Google Scholar] [CrossRef]
- Santos, G.G.; Nunes, V.L.C.; Marinho, S.M.O.C.; Santos, S.R.A.; Rossi, A.M.; Miguel, F.B. Biological behavior of magnesium-substituted hydroxyapatite during bone repair. Braz. J. Biol. 2020, 1, 53–61. [Google Scholar] [CrossRef]
- Predoi, D.; Ciobanu, S.C.; Iconaru, S.L.; Predoi, M.V. Influence of the biological medium on the properties of magnesium doped hydroxyapatite composite coatings. Coatings 2023, 2, 409. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).