Abstract
Tissue regeneration remains a key challenge in modern dentistry, particularly in cases of bone loss that hinder implant placement. This study evaluates the regenerative potential of RegenerOss®, a porcine-derived bone substitute, through histological and histomorphometric analysis in patients with alveolar bone defects. Twelve patients underwent guided bone regeneration using RegenerOss®, followed by histological evaluation of newly formed bone (NFB) and residual biomaterial. The results indicate that RegenerOss® effectively promotes bone formation, with an average NFB percentage of 34% and a low residual biomaterial percentage (6%). Osteoclast-mediated resorption was observed, confirming its role in physiological remodeling. Biocompatibility was high, with minimal inflammatory infiltrates in most cases. A scoring system (SCORE2) integrating qualitative and quantitative variables demonstrated a strong positive correlation with NFB, providing a reliable metric for bone regeneration quality. Regression analysis confirmed a linear relationship between SCORE2 and bone formation. The study highlights RegenerOss® as a promising biomaterial with excellent osteoconductive properties, predictable integration, and progressive resorption. However, variability among patients suggests that regeneration is influenced by local and systemic factors. Further studies are required to explore its full potential in different bone defect scenarios.
1. Introduction
Tissue regeneration following pathological, traumatic, or surgical events represents one of modern medicine’s most significant and ambitious goals [1]. This field of research, which has seen increasing interest, spans numerous tissue types, from neurological and dermatological to bone tissues [2]. The primary aim is to develop innovative strategies to restore compromised tissues to their original physiological state, recovering structural and mechanical-functional characteristics and biochemical and biological properties [3].
In dentistry, bone tissue regeneration plays a central role. Bone loss occurs in various clinical scenarios, such as bone resorption caused by radicular infections, often resulting in the loss of affected teeth. This deficiency poses a significant obstacle to dental implant placement, a procedure essential for restoring masticatory function and the patient’s physiological conditions [4,5]. Bone regeneration in such contexts relies on biomaterials, which may consist of natural or synthetic substances designed to mimic the properties of natural bone [6].
An ideal bone substitute must meet specific criteria: osteoconduction, the ability to provide a scaffold for new tissue formation; osteoinduction, the ability to stimulate bone production; osteogenicity, the intrinsic capability to develop into bone tissue; resorbability; and, above all, biocompatibility [7]. Autologous bone, harvested from the patient, is considered the gold standard for bone regeneration as it possesses all these qualities [8]. However, using autologous bone is not without challenges, including the risk of excessive resorption, limited or inadequate availability, and additional surgical procedures to harvest the material, which increases operative risks and patient discomfort [9].
To address these challenges, numerous bone substitutes have been developed from diverse sources, including polymeric, metallic, siliceous materials, and animal-derived products such as bovine, porcine, equine, and coral-based substitutes [10]. Although these materials lack osteogenic properties, they can exhibit osteoinductive and, more commonly, osteoconductive capabilities, the minimum requirements for clinical efficacy [11]. Additionally, these materials, which generally have good biocompatibility, vary in their resorption rates: some degrade over few months, while others may persist in the body for longer periods [12].
The specific, allogeneic grafts from cadaveric or living human donors provide an off-the-shelf osteoconductive scaffold and can retain some osteoinductive capacity (e.g., demineralized bone matrix) without a second surgery, but they carry a small risk of immunogenic reaction and disease transmission and often remodel more slowly than autografts [13]. Xenogeneic substitutes, typically deproteinized bovine bone, are highly biocompatible, purely osteoconductive matrices that resorb very slowly, helping maintain volume stability of the augmented site [14]; indeed, xenografts have become reliable alternatives to autograft in procedures like sinus floor elevation and ridge preservation, though they lack intrinsic osteoinductive elements and serve mainly as a passive framework for new bone growth. Synthetic bone grafts (alloplasts)—such as hydroxyapatite and β-tricalcium phosphate ceramics or bioactive glass—are likewise osteoconductive and can be manufactured with controlled resorbability and excellent biocompatibility [15], with no risk of disease transmission; their limitation is the absence of living cells or bone-inductive proteins, so bone regeneration with synthetics relies entirely on colonization by host cells, sometimes augmented with exogenous growth factors or cells in advanced graft designs. Clinically, these graft materials are applied alone or in combination, often under barrier membranes in guided bone regeneration (GBR), for implant-site development, including maxillary sinus floor augmentation, post-extraction socket preservation, and horizontal or vertical alveolar ridge augmentation [16,17]. Recent systematic reviews and clinical studies (2019–2025) demonstrate high implant survival rates with all graft categories in such applications [18] but also underscore that none of these materials perfectly meet all ideal criteria (optimal osteogenic potential, integration, volume maintenance, and minimal morbidity), necessitating a case-by-case selection to balance the graft’s osteogenic capacity, structural support, resorbability, and clinical handling [19].
In dentistry, bone substitutes are widely used, with a vast array of options available on the market. Among xenogenic materials, anorganic bovine bone-derived substitutes are the most popular and documented. These materials undergo thermal and chemical treatments to remove organic components while preserving the mineralized matrix and original porosity. Once processed, they are ground into powder and made available for clinical use [20].
Recently, attention has shifted to other animal sources, like pigs. An example is RegenerOss® (ZimVie, Westminster, CO, USA), a porous carbonated apatite matrix derived from porcine bone. This material, with its distinct structural characteristics, including a porous and rough surface and a spongy structure with highly interconnected pores [21], holds great promise for bone regeneration. These properties enhance osteoconductivity, facilitating gas and nutrient exchange during the initial stages of regeneration and promoting angiogenesis in subsequent healing phases. The unique properties of RegenerOss® inspire hope for its potential in the field of bone regeneration [22].
This study aims to provide a histologic and histomorphometric assessment of bone regeneration achieved using RegenerOss® in alveolar bone defects.
2. Materials and Methods
The study was approved by the Institutional Review Board of Fondazione IRCCS Ca’ Granda Ospedale Maggiore Policlinico, Milan, Italy, under protocol number 420/01, RC2024, for retrospective studies on implant therapy and bone regeneration. All patients provided written informed consent for the treatment and for the use of the data deriving from analysis of their bone biopsies for scientific purposes.
2.1. Surgical Procedure
Twelve patients with horizontal, vertical, or combined alveolar bone defects were selected from a database of individuals who underwent alveolar bone regeneration procedures using RegenerOss® between June 2023 and April 2024. Considering the extent of the defects, a two-stage regenerative approach was adopted.
Inclusion criteria were as follows: patients aged between 18 and 70 years; presence of mandibular bone defects confirmed by panoramic radiographs and cone beam computed tomography (CBCT); general good health, classified as ASA-1 or ASA-2 according to the American Society of Anesthesiologists; non-smokers or moderate smokers (≤10 cigarettes/day); absence of active periodontal disease; ability to comply with the therapeutic protocol; and provision of written informed consent.
Exclusion criteria included pregnancy or breastfeeding, severe systemic conditions that could impair bone healing, and the use of medications known to affect bone metabolism. Patients with a history of radiotherapy or chemotherapy in the head and neck region, non-regenerative bone defects or active local infections were also excluded. Furthermore, individuals with alcohol or drug dependency, known allergies to the materials used, poor oral hygiene, or untreated periodontitis were not eligible for inclusion.
Additional exclusion criteria, in line with the absolute contraindications for implant therapy described by Wang and Hwang [23], comprised recent myocardial infarction or stroke, mitral valve surgery, immunosuppressive therapy, coagulation disorders, current treatment for malignancy, intravenous bisphosphonate therapy, psychiatric illness, and drug abuse. Patients presenting systemic diseases such as osteoporosis, osteopenia, uncontrolled diabetes, vitamin D deficiency, long-term glucocorticoid therapy, hypothyroidism, or uncontrolled cardiovascular conditions (e.g., hypertension, coronary heart disease, or congestive heart failure) were also excluded. Finally, patients who reported smoking more than 10 cigarettes per day or who presented with acute odontogenic infections or periapical lesions were not considered eligible.
All patients underwent thorough preoperative clinical and radiographic assessment, including panoramic radiographs (OPTs) and CBCT scans. No patient was subjected to additional procedures beyond the standard therapeutic protocol, and the material used for histological analysis was collected as biological waste material intended for disposal. All procedures were conducted in accordance with the Declaration of Helsinki.
No control group was included in this study, as the primary objective was to perform a qualitative histological evaluation of the biological response to the tested biomaterial rather than to conduct a comparative analysis. Accordingly, the study design focused on the descriptive assessment of tissue morphology in biopsies harvested after guided bone regeneration. The samples showed a stratified range of histological features, with variable amounts of new bone and residual biomaterial, which allowed for internal interpretation of the regenerative performance. Although this approach does not involve a conventional control group, it enables the characterization of the biomaterial’s behavior under clinical conditions.
During guided bone regeneration (GBR) procedures, the protocol involved the use of a custom-made titanium mesh (AccuraMesh, (AccuraMesh®, Zimmer Biomet Dental, Palm Beach Gardens, FL, USA)) covered with a porcine dermal collagen membrane (CopiOs® Bone Graft, ZimVie Inc., Palm Beach Gardens, FL, USA). The filling material consisted exclusively of a slow-resorbing xenograft based on porcine carbonate apatite (RegenerOss®, Zimmer Biomet Dental, Palm Beach Gardens, FL, USA).
Eight months after device placement, a second surgery was performed to remove the mesh and insert the implants, following the established therapeutic plan. During this phase, a trephine bur was used to prepare the site for dental implant insertion. The material collected was preserved in formalin for subsequent histological analysis. The steps of the procedure are illustrated in Figure 1.
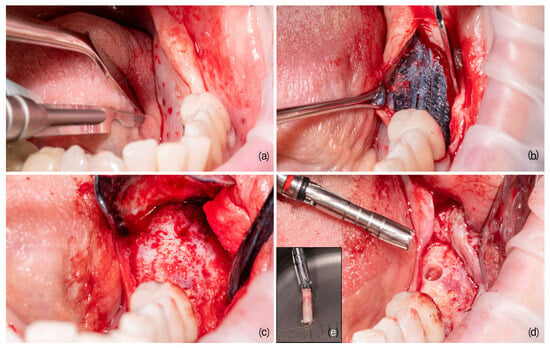
Figure 1.
The main steps of guided bone regeneration. (a) The pre-operative site, where the initial conditions are assessed; (b) the bone substitute placed under a customized titanium mesh to better define and maintain the spaces to be regenerated; (c) the site after the healing period, showing the progress of the regeneration; (d) the bone core harvesting site, indicating the area from which the sample is taken; and (e) the detailed view of the harvested bone core, providing insight into the newly formed bone structure.
2.2. Histological, Histomorphometric, and Statistical Analysis
The samples were fixed in buffered formalin (Carlo Erba Reagents S.r.l., Cornaredo, Italy) for 72 h and subsequently decalcified in disodium EDTA (Carlo Erba Reagents S.r.l., Cornaredo, Italy) using a microwave oven [24]. The endpoint of decalcification was assessed using the ammonium oxalate test [25]. After decalcification, the samples were dehydrated in ascending ethanol concentrations, starting at 70% and progressing to two final washes in 100% ethanol, clarified in xylene, and embedded in paraffin, with the final paraffin bath maintained under vacuum overnight [26]. Sections that were 4 µm thick were obtained from each sample using a Leica RM2245 rotary microtome (Leica Biosystems, Nussloch, Germany). These sections were mounted on polylysine-coated slides (VWR International, Radnor, PA, USA) and stained with hematoxylin and eosin (Carlo Erba Reagents S.r.l., Cornaredo, Italy) to visualize newly formed bone, residual bone substitute material, soft tissues, and inflammatory infiltrates.
Photographic acquisition was performed using an Olympus CX43 microscope equipped with an Olympus LC30 camera (Olympus Corporation, Tokyo, Japan), operated through CellSens software (Olympus Corporation, Tokyo, Japan). For each biopsy, three representative sections were selected. Each section was photographed through multiple high-resolution images and stitched together into a single composite image of the entire section using AutoStitch software v1.0 [27,28]. These composite images were used for subsequent histomorphometric analysis.
For each sample, the percentages of newly formed bone and residual bone substitute material were calculated by measuring the pixel areas corresponding to each tissue type and relating them to the total surface area of the sample [29]. Measurements were performed on three sections per sample and independently analyzed by two researchers to minimize human error. Image processing and analysis were conducted using ImageJ software (ImageJ software. National Institutes of Health, Bethesda, MD, USA. Available online: https://imagej.net/ij/, accessed on 15 January 2025).
To complement the quantitative analysis with a qualitative assessment of bone regeneration characteristics, a scoring table (Table 1) was developed.

Table 1.
Table explaining the semiquantitative values used to evaluate histological sections.
This table assigns specific values to the amount of newly formed bone, residual bone substitute material, bone architecture, and the presence of inflammatory foci. The scoring criteria are based on available literature data and inspired by similar tables previously validated and accepted in the literature [30,31,32]. The values were summed to obtain a total score that provides an overall evaluation of the analyzed samples. In addition, a correlation analysis was performed between qualitative and quantitative indices to identify the most relevant ones. Using this methodology, two scores were defined: SCORE and SCORE2. The first includes all indices, while the second excludes the inflammation value. The data collected underwent statistical analysis using the R software v 4.4.2 to summarize the main characteristics of the studied variables. In addition, correlation analyses among the measured parameters were performed using Pearson’s correlation coefficient, with a p-value < 0.05 considered statistically significant. The scoring table was validated by calculating Pearson’s correlation coefficient to assess the relationship between quantitative and semi-quantitative data. All statistical analyses were performed using IBM SPSS Statistics for Windows, Version 28.0 (IBM Corp., Armonk, NY, USA), with integration of the R programming language (R Core Team, 2022; R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria) via the “IBM SPSS Statistics—Essentials for R” package.
2.3. Physico-Chemical Characterization of the Biomaterial
RegenerOss® was previously characterized [33] using a range of physico-chemical analyses to assess its structure and composition. Scanning electron microscopy (SEM) revealed a highly porous and interconnected architecture, with a rough surface topography that supports cell adhesion and tissue integration. X-ray diffraction (XRD) analysis showed a low-crystallinity apatite phase, closely resembling the mineral composition of native bone. Fourier-transform infrared spectroscopy (FTIR) demonstrated characteristic phosphate absorption bands near 560 and 1030 cm−1, along with carbonate bands around 1420 and 1465 cm−1, confirming the presence of carbonate substitution within the apatite lattice. Morphometric data indicated that the pore size was predominantly in the 0.1–1.0 mm range, and the granule size distribution was weighted toward larger particles (0.85–2.0 mm). The volume fill capacity ranged from 2.86 to 4.35 cm3/g, with a total void volume of up to 95%, providing an ideal microenvironment for osteoconduction.
3. Results
3.1. Qualitative Evaluation of Newly Formed Bone
Optical microscopy analysis allowed for qualitative evaluation of the architecture of newly formed bone (NFB). It was observed that the bone structure was more defined with an increasing amount of newly formed bone and vice versa (Figure 2).
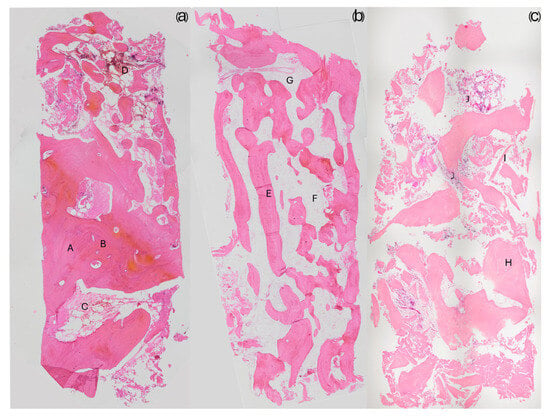
Figure 2.
The images depict three different bone qualities at 20X magnification. (a) A sample from a regeneration with more than 50% newly formed bone; compact bone portions are clearly visible (A) along with recognizable osteons (B), identifiable by their central Haversian canal and the concentric arrangement of osteocytes. This sample also contains a highly vascularized area of bone marrow tissue (C) and a concentration of inflammatory cells (D) in the upper part of the bone core. (b) A sample from a regeneration consisting of 30% newly formed bone; unlike the previous sample, its structure resembles trabecular bone, with well-defined and elongated trabeculae (E). Large areas of bone marrow tissue composed of loose connective tissue (F) are present, along with visible blood vessels (G). (c) A sample with less than 20% newly formed bone; some areas of dense bone (H) are present, but the majority of the structure appears poorly defined, with small trabeculae (I). Several inflammatory cell foci (J) are also observed. In histological sections, the biomaterial showed good integration with the newly formed bone (Figure 3a). Osteoclasts actively resorbing the biomaterial were also observed, demonstrating the biomaterial’s ability to participate in the physiological bone tissue remodeling process (Figure 3b). Additionally, inflammatory infiltrates were negligible or very small, with only one case showing significant levels of inflammation.
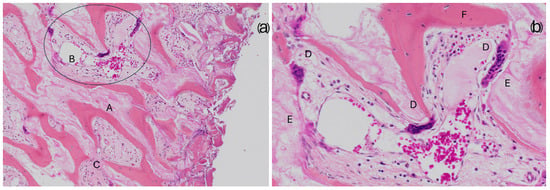
Figure 3.
The images illustrate the integration between Regeneross and newly formed bone. (a) 200× magnification shows the bone substitute (A) in continuity with the newly formed bone. Within the intratrabecular spaces, loose vascularized connective tissue (B) can be observed, along with compact mesenchymal tissue (C), which is likely to contribute to further bone formation. (b) 400× magnification, close-up view of the area highlighted by the circle in the previous image provides a detailed view of the circled area from the previous image, clearly displaying the structure of the bone substitute (E) and the newly formed bone (F). Notably, the presence of osteoclasts (D) actively resorbing the biomaterial is evident, demonstrating its potential to be gradually replaced by physiological bone through the natural remodeling processes of bone tissue. Quantification of newly formed bone and residual biomaterial.
All samples analyzed exhibited newly formed bone tissue, with an average NFB percentage of 34% (range from 11.8% to 56.5%). The average percentage of residual non-resorbed biomaterial was 6%, with two samples showing complete resorption (Table 2).

Table 2.
Descriptive statistics of histomorphometric analysis, showing the percentage of newly formed bone and residual material in the analyzed samples.
3.2. Correlation Analysis
Statistical analysis identified significant correlations among the variables studied (Table 3).

Table 3.
The table presents the Pearson correlation coefficients between the different variables considered in the study. The Pearson Correlation cell contains the correlation coefficient, which ranges from −1 to 1, where −1 indicates a strong inverse correlation, 1 indicates a strong direct correlation, and 0 indicates no correlation. Below each coefficient, the statistical significance of the correlation is reported, with p < 0.05 (*) and p < 0.01 (**). The N value represents the number of observations (i.e., the sample size) used for the analysis. Since the main focus of this study was on the NFB (newly formed bone) variable, its correlations with the other variables can be observed in the first column. All correlations with NFB are statistically significant, except for the INFL (Inflammation) variable.
The correlations of the observed variables with the two proposed scores, SCORE, and SCORE2 were also evaluated as potential indicators of newly formed bone (NFB).
- SCORE2 was proposed after it was observed that the INFL variable (inflammatory infiltrates) was not significantly correlated with NFB.
3.3. Linear Regression Models
Two linear regression models were built to assess the predictive performance of SCORE and SCORE2 concerning the amount of NFB:
- Model 1: SCORE as the independent variable.
- Model 2: SCORE2 as the independent variable.
The main characteristics of the models are summarized in Table 4.

Table 4.
Linear regression analysis results, showing the relationship between the predictor variables (SCORE and SCORE2) and the dependent variable. Unstandardized and standardized coefficients, significance values, and 95% confidence intervals are reported for each model.
Analysis of the β coefficient indicated that, with equivalent statistical significance, Model 2 performed better than Model 1. The scatter plots of the two models, shown in Figure 4 and Figure 5, include the regression line equation, the 95% confidence interval, and the R2 value. The relationship between SCORE2 and NFB was linear and positive: as SCORE2 increased, the amount of NFB also increased.
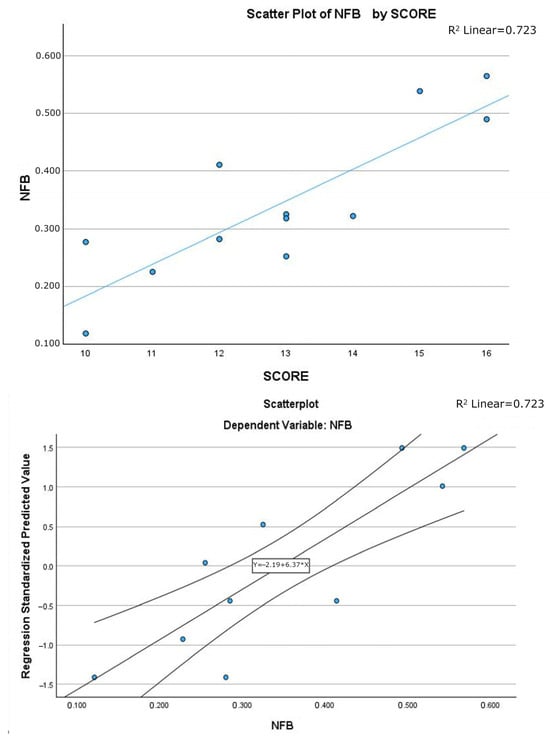
Figure 4.
Scatter plot of linear regression Model 1, with explicit line equation and 95% C.I. associated to the model and R2 value.
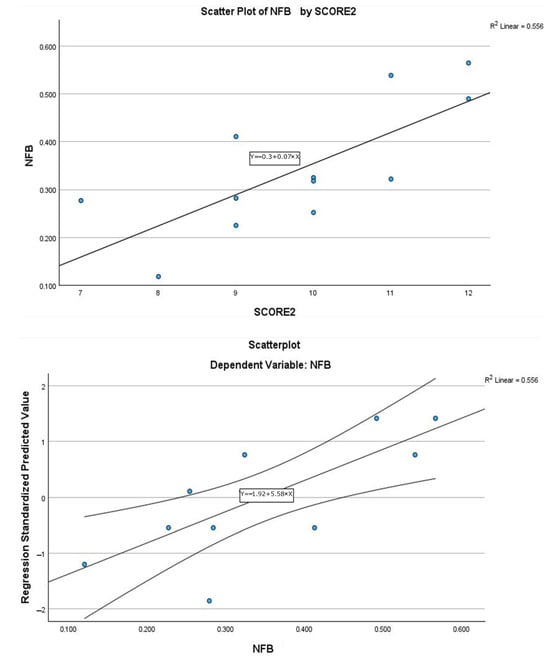
Figure 5.
Scatter plot of linear regression Model 2, with explicit line equation and 95% C.I. associated to the model and R2 value.
Qualitative classification using SCORE2:
Using SCORE2 to classify the quality of bone regeneration, an average score of 9.83/13 and a median of 10 were obtained. No sample achieved the maximum score.
- Insufficient samples: 5 samples with scores < 10, including 3 with 9.
- Sufficient samples: 3 samples with a score of 10.
- Good/excellent samples: 4 samples with a score ≥ 11 (75th percentile) are considered high-quality samples [34].
4. Discussion
Bone regeneration remains one of the most complex challenges in dentistry, as bone deficiency compromises the patient’s quality of life and impacts the success of many treatments, like dental implant placement. This study evaluated the potential of the porcine-derived bone substitute RegenerOss® through histological and histomorphometric analysis of bone regeneration in patients with horizontal, vertical, and combined bone defects. The results indicate that RegenerOss® effectively promotes the formation of newly formed bone (NFB). However, a wide variability was observed among samples, which might depend on patient-specific factors such as local vascularization which influence nutrient availability and therefore the rate of bone regeneration [35]. Regeneration does not appear to co-occur across the entire volume occupied by the biomaterial, suggesting a dynamic process reflecting the progression of angiogenesis and the maturation of bone tissue. Integration of the biomaterial with newly formed bone tissue was optimal, highlighting good osteoconduction, a fundamental requirement for promoting new bone formation.
Another relevant finding of this study is that RegenerOss® can be effectively resorbed and progressively replaced by natural bone. Osteoclasts actively resorbing the biomaterial confirms its involvement in physiological remodeling processes [36]. The average percentage of residual biomaterial was as low as 6%, with two samples showing no residual biomaterial. This suggests that resorption may be influenced by individual patient factors such as age, bone metabolism, and overall health conditions. From a biocompatibility perspective, the results were highly positive. Most samples showed negligible or very low levels of inflammatory infiltrates, with only one case displaying significant inflammation. This indicates that RegenerOss® elicits a minimal immune response, consistent with normal healing processes, confirming its high biocompatibility. Introducing a scoring system that integrates qualitative and quantitative variables added value to the analysis of bone regeneration. SCORE2, developed by excluding variables not directly correlated with new bone formation (such as inflammatory infiltrates), proved to be a reliable indicator of the quality of bone regeneration. Statistical analysis revealed a significant positive correlation between SCORE2 and the amount of NFB, with a linear positive relationship confirmed by regression analysis. Using SCORE2, the quality of bone regeneration resulted generally good, with an average score of 9.5/13 and a median of 10. Three samples scored below 9, indicating lower regeneration quality, while four samples reached or exceeded the 75th percentile (≥11), classifying them as high-quality regeneration. To contextualize these results, a comparison with the gold standard xenograft material, Bio-Oss®, is warranted. The histomorphometric outcomes obtained in this study with RegenerOss® appear consistent with those reported for the most widely used xenograft material, Bio-Oss®. In clinical studies involving human biopsies, Bio-Oss has shown newly formed bone (NFB) values typically ranging from 20% to 40% after 4 to 6 months, depending on the defect size and anatomical site [37]. For example, in sinus floor elevation procedures, average NFB values of ~30% and residual biomaterial values of ~40% have been reported after 6 months [38]. In smaller defects, such as post-extraction sockets, NFB percentages around 33–35% and lower residual biomaterial (~20–30%) are frequently observed [39]. In our sample, the average NFB was 34.4%, with a notably lower residual biomaterial of 6.1%, suggesting a favorable resorption profile for RegenerOss®. The presence of active osteoclasts supports this hypothesis, indicating the participation of the material in physiological bone remodeling. While Bio-Oss is known for its excellent biocompatibility and long-term volume stability, its slow resorption may limit the rate of native bone substitution in the early healing phases [40]. In contrast, the more rapid degradation observed for RegenerOss® may promote a more dynamic bone turnover without eliciting inflammatory responses, as confirmed by the minimal inflammation detected in our samples. Taken together, these findings support the clinical validity of RegenerOss® as a bone substitute with comparable osteoconductive capacity to Bio-Oss, and potentially advantageous remodeling characteristics.
5. Conclusions
RegenerOss® is a promising biomaterial, capable of predictable integration with bone tissue and promoting high-quality regeneration in most cases. However, this exploratory study is not intended to provide a comprehensive assessment of all the characteristics of this material. Further research involving a larger number of cases and a broader range of clinical scenarios is necessary to fully evaluate its performance. Nonetheless, the results obtained suggest that RegenerOss® has strong potential and integrates well into the biological processes of bone regeneration.
Author Contributions
Conceptualization, P.S. and M.D.F.; methodology, P.S. and V.M.S.; software, V.M.S.; validation, P.S., M.D.F., and F.S.; formal analysis, P.S.; investigation, P.S. and D.K.; resources, M.D.F. and L.G. (Luciano Giardino); data curation, D.K.; writing—original draft preparation, L.G. (Luciano Giardino); writing—review and editing, M.D.F., L.G. (Luciano Giardino), and F.S.; visualization, L.G. (Luigi Generali) supervision, M.D.F. and F.S.; project administration, P.S. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
The study was approved by the Institutional Review Board of Fondazione IRCCS Ca’ Granda Ospedale Maggiore Policlinico, Milan, Italy, under protocol number 420/01, RC2024, for retrospective studies on implant therapy and bone regeneration.
Informed Consent Statement
All patients provided written informed consent for the treatment and for the use of the data deriving from analysis of their bone biopsies for scientific purposes.
Data Availability Statement
The data will be made available upon request by contacting the corresponding authors.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Tatullo, M.; Zavan, B.; Piattelli, A. Critical overview on regenerative medicine: New insights into the role of stem cells and innovative biomaterials. Int. J. Mol. Sci. 2023, 24, 7936. [Google Scholar] [CrossRef] [PubMed]
- Ntege, E.H.; Sunami, H.; Shimizu, Y. Advances in regenerative therapy: A review of the literature and future directions. Regen. Ther. 2020, 14, 136–153. [Google Scholar] [CrossRef] [PubMed]
- Petrosyan, A.; Martins, P.N.; Solez, K.; Uygun, B.E.; Gorantla, V.S.; Orlando, G. Regenerative medicine applications: An overview of clinical trials. Front. Bioeng. Biotechnol. 2022, 10, 942750. [Google Scholar] [CrossRef] [PubMed]
- Smeets, R.; Matthies, L.; Windisch, P.; Gosau, M.; Jung, R.; Brodala, N.; Stefanini, M.; Kleinheinz, J.; Payer, M.; Henningsen, A.; et al. Horizontal augmentation techniques in the mandible: A systematic review. Int. J. Implant Dent. 2022, 8, 23. [Google Scholar] [CrossRef]
- Al-Nawas, B.; Schiegnitz, E. Augmentation procedures using bone substitute materials or autogenous bone—A systematic review and meta-analysis. Eur. J. Oral Implantol. 2014, 7 (Suppl. 2), S219–S234. [Google Scholar]
- Tumedei, M.; Savadori, P.; Del Fabbro, M. Synthetic blocks for bone regeneration: A systematic review and meta-analysis. Int. J. Mol. Sci. 2019, 20, 4221. [Google Scholar] [CrossRef]
- O’Brien, F.J. Biomaterials & scaffolds for tissue engineering. Mater. Today 2011, 14, 88–95. [Google Scholar] [CrossRef]
- Ciszyński, M.; Dominiak, S.; Dominiak, M.; Gedrange, T.; Hadzik, J. Allogenic Bone Graft in Dentistry: A Review of Current Trends and Developments. Int. J. Mol. Sci. 2023, 24, 16598. [Google Scholar] [CrossRef]
- Schmidt, A.H. Autologous bone graft: Is it still the gold standard? Injury 2021, 52 (Suppl. 2), S18–S22. [Google Scholar] [CrossRef]
- Georgeanu, V.A.; Gingu, O.; Antoniac, I.V.; Manolea, H.O. Current options and future perspectives on bone graft and biomaterials substitutes for bone repair, from clinical needs to advanced biomaterials research. Appl. Sci. 2023, 13, 8471. [Google Scholar] [CrossRef]
- De Long WGJr Einhorn, T.A.; Koval, K.; McKee, M.; Smith, W.; Sanders, R.; Watson, T. Bone grafts and bone graft substitutes in orthopaedic trauma surgery: A critical analysis. J. Bone Jt. Surg. Am. Vol. 2007, 89, 649–658. [Google Scholar] [CrossRef]
- Jing, L.; Su, B. Resorption rates of bone graft materials after crestal maxillary sinus floor elevation and its influencing factors. J. Funct. Biomater. 2024, 15, 133. [Google Scholar] [CrossRef] [PubMed]
- Starch-Jensen, T.; Deluiz, D.; Tinoco, E.M.B. Horizontal Alveolar Ridge Augmentation with Allogeneic Bone Block Graft Compared with Autogenous Bone Block Graft: A Systematic Review. J. Oral Maxillofac. Res. 2020, 11, e1. [Google Scholar] [CrossRef] [PubMed]
- De Azambuja Carvalho, P.; De Oliveria Ciaramicolo, N.; Ferreira, J.O.; Pereira-Filho, V. Clinical and laboratorial outcomes of xenogenic biomaterials: Literature review. Front. Oral Maxillofac. Med. 2021, 5, 8. Available online: https://fomm.amegroups.org/article/view/56185 (accessed on 28 January 2025).
- Santoro, A.; Voto, A.; Fortino, L.; Guida, R.; Laudisio, C.; Cillo, M.; D’Ursi, A.M. Bone Defect Treatment in Regenerative Medicine: Exploring Natural and Synthetic Bone Substitutes. Int. J. Mol. Sci. 2025, 26, 3085. [Google Scholar] [CrossRef]
- Starch-Jensen, T.; Deluiz, D.; Vitenson, J.; Bruun, N.H.; Tinoco, E.M.B. Maxillary Sinus Floor Augmentation with Autogenous Bone Graft Compared with a Composite Grafting Material or Bone Substitute Alone: A Systematic Review and Meta-Analysis Assessing Volumetric Stability of the Grafting Material. J. Oral Maxillofac. Res. 2021, 12, e1. [Google Scholar] [CrossRef]
- Vanka, S.; Kasem, F.A.; Kailani, T.; Wali, O.; Vanka, A. Bone graft substitutes and dental implant stability in immediate implant surgery: A systematic review and meta-analysis. Evid.-Based Dent. 2024, 26, 70. [Google Scholar] [CrossRef]
- Azadi, A.; Hazrati, P.; Tizno, A.; Rezaei, F.; Akbarzadeh Baghban, A.; Tabrizi, R. Bone expansion as a horizontal alveolar ridge augmentation technique: A systematic review and meta-analysis. Oral Maxillofac. Surg. 2025, 29, 32. [Google Scholar] [CrossRef]
- Zhao, R.; Yang, R.; Cooper, P.R.; Khurshid, Z.; Shavandi, A.; Ratnayake, J. Bone Grafts and Substitutes in Dentistry: A Review of Current Trends and Developments. Molecules 2021, 26, 3007. [Google Scholar] [CrossRef]
- Perić Kačarević, Z.; Kavehei, F.; Houshmand, A.; Franke, J.; Smeets, R.; Rimashevskiy, D.; Wenisch, S.; Schnettler, R.; Jung, O.; Barbeck, M. Purification processes of xenogeneic bone substitutes and their impact on tissue reactions and regeneration. Int. J. Artif. Organs 2018, 41, 789–800. [Google Scholar] [CrossRef]
- Akita, K.; Fukuda, N.; Kamada, K.; Kudoh, K.; Kurio, N.; Tsuru, K.; Ishikawa, K.; Miyamoto, Y. Fabrication of porous carbonate apatite granules using microfiber and its histological evaluations in rabbit calvarial bone defects. J. Biomed. Mater. Res. Part A 2020, 108, 709–721. [Google Scholar] [CrossRef] [PubMed]
- Battafarano, G.; Rossi, M.; De Martino, V.; Marampon, F.; Borro, L.; Secinaro, A.; Del Fattore, A. Strategies for bone regeneration: From graft to tissue engineering. Int. J. Mol. Sci. 2021, 22, 1128. [Google Scholar] [CrossRef] [PubMed]
- Hwang, D.; Wang, H.L. Medical contraindications to implant therapy: Part I: Absolute contraindications. Implant Dent. 2006, 15, 353–360. [Google Scholar] [CrossRef] [PubMed]
- Savadori, P.; Dalfino, S.; Piazzoni, M.; Inchingolo, F.; Del Fabbro, M.; Tartaglia, G.M.; Giardino, L. Arduino Automated Microwave Oven for Tissue Decalcification. Bioengineering 2023, 10, 79. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Giardino, L.; Grande, N.M.; Savadori, P.; Fabbro, M.D.; Plotino, G. Clinical and Histological Findings of Post-Treatment Infection in the Presence of Vertical Root Fracture and Apical Periodontitis: Case Reports. Eur. Endod. J. 2019, 4, 45–48. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Giardino, L.; Generali, L.; Del Fabbro, M.; Tartaglia, G.M.; Bidossi, A.; Savadori, P. Detection of bacteria in dental samples using the Periodic acid-Schiff (PAS) histological stain. Micron 2023, 172, 103498. [Google Scholar] [CrossRef] [PubMed]
- Brown, M.; Lowe, D.G. Recognising Panoramas. In Proceedings of the 9th IEEE International Conference on Computer Vision, Nice, France, 13–16 October 2003. [Google Scholar]
- Canullo, L.; Donato, A.; Savadori, P.; Radovanovic, S.; Iacono, R.; Rakic, M. Effect of argon plasma abutment activation on soft tissue healing: RCT with histological assessment. Clin. Implant Dent. Relat. Res. 2024, 26, 226–236. [Google Scholar] [CrossRef] [PubMed]
- Minetti, E.; Palermo, A.; Savadori, P.; Patano, A.; Inchingolo, A.D.; Rapone, B.; Malcangi, G.; Inchingolo, F.; Dipalma, G.; Tartaglia, F.C.; et al. Socket Preservation Using Dentin Mixed with Xenograft Materials: A Pilot Study. Materials 2023, 16, 4945. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Söhling, N.; Von Jan, O.; Janko, M.; Nau, C.; Ritz, U.; Marzi, I.; Henrich, D.; Verboket, R.D. Measuring Bone Healing: Parameters and Scores in Comparison. Bioengineering 2023, 10, 1011. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Lucaciu, O.; Gheban, D.; Soriţau, O.; Băciuţ, M.; Câmpian, R.S.; Băciuţ, G. Comparative assessment of bone regeneration by histometry and a histological scoring system/Evaluarea comparativă a regenerării osoase utilizând histometria și un scor de vindecare histologică. Rev. Romana Med. Lab. 2015, 23, 31–45. [Google Scholar] [CrossRef]
- Gerstenfeld, L.C.; Wronski, T.J.; Hollinger, J.O.; Einhorn, T.A. Application of histomorphometric methods to the study of bone repair. J. Bone Miner. Res. 2005, 20, 1715–1722. [Google Scholar] [CrossRef] [PubMed]
- Li, S.-T.; Chen, H.-C.; Yuen, D. Isolation and Characterization of a Porous Carbonate Apatite from Porcine Cancellous Bone. Sci. Technol. Innov. 2014, 1, 13. [Google Scholar]
- Nease, R.F., Jr.; Owens, D.K.; Sox, H.C., Jr. Threshold analysis using diagnostic tests with multiple results. Med. Decis. Making 1989, 9, 91–103. [Google Scholar] [CrossRef] [PubMed]
- Dalfino, S.; Savadori, P.; Piazzoni, M.; Connelly, S.T.; Giannì, A.B.; Del Fabbro, M.; Tartaglia, G.M.; Moroni, L. Regeneration of Critical-Sized Mandibular Defects Using 3D-Printed Composite Scaffolds: A Quantitative Evaluation of New Bone Formation in In Vivo Studies. Adv. Healthc. Mater. 2023, 12, e2300128. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Buccino, F.; Zagra, L.; Savadori, P.; Galluzzo, A.; Colombo, C.; Grossi, G.; Banfi, G.; Vergani, L.M. Mapping local mechanical properties of human healthy and osteoporotic femoral heads. Materialia 2021, 20, 101229. [Google Scholar] [CrossRef]
- Figueiredo, A.; Coimbra, P.; Cabrita, A.; Guerra, F.; Figueiredo, M. Comparison of a xenogeneic and an alloplastic material used in dental implants in terms of physico-chemical characteristics and in vivo inflammatory response. Mater. Sci. Eng. C Mater. Biol. Appl. 2013, 33, 3506–3513. [Google Scholar] [CrossRef]
- Giardino, L.; Bidossi, A.; Del Fabbro, M.; Savadori, P.; Maddalone, M.; Ferrari, L.; Ballal, N.V.; Das, S.; Rao, B.S.S. Antimicrobial activity, toxicity and accumulated hard-tissue debris (AHTD) removal efficacy of several chelating agents. Int. Endod. J. 2020, 53, 1093–1110. [Google Scholar] [CrossRef]
- Ferraz, M.P. Bone Grafts in Dental Medicine: An Overview of Autografts, Allografts and Synthetic Materials. Materials 2023, 16, 4117. [Google Scholar] [CrossRef]
- Krennmair, S.; Postl, L.; Schwarze, U.Y.; Malek, M.; Stimmelmayr, M.; Krennmair, G. Clinical, radiographic, and histological/histomorphometric analysis of maxillary sinus grafting with deproteinized porcine or bovine bone mineral: A randomized clinical trial. Clin. Oral Implants Res. 2023, 34, 1230–1247. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).