Effect of the Addition of Banana Stem Lignin (Musa acuminata ssp. balbisiana var. Dominico-Harton) on the Physicochemical Properties of Biodegradable Composites Based on Methylhydroxyethylcellulose
Abstract
1. Introduction
2. Materials and Methods
2.1. Obtaining Lignin from the Pseudostem of the Dominico-Hartón Banana
2.2. Characterisation of the Pseudostem Lignin of the Dominico-Hartón Banana
2.3. Obtaining Biodegradable Films from MHEC with Incorporation of the Obtained Lignin
Lignin Yield
2.4. Characterisation of Biodegradable Films from MHEC with Incorporation of the Obtained Lignin
2.4.1. Mechanical and Thickness Properties
2.4.2. Colour
2.4.3. Gloss
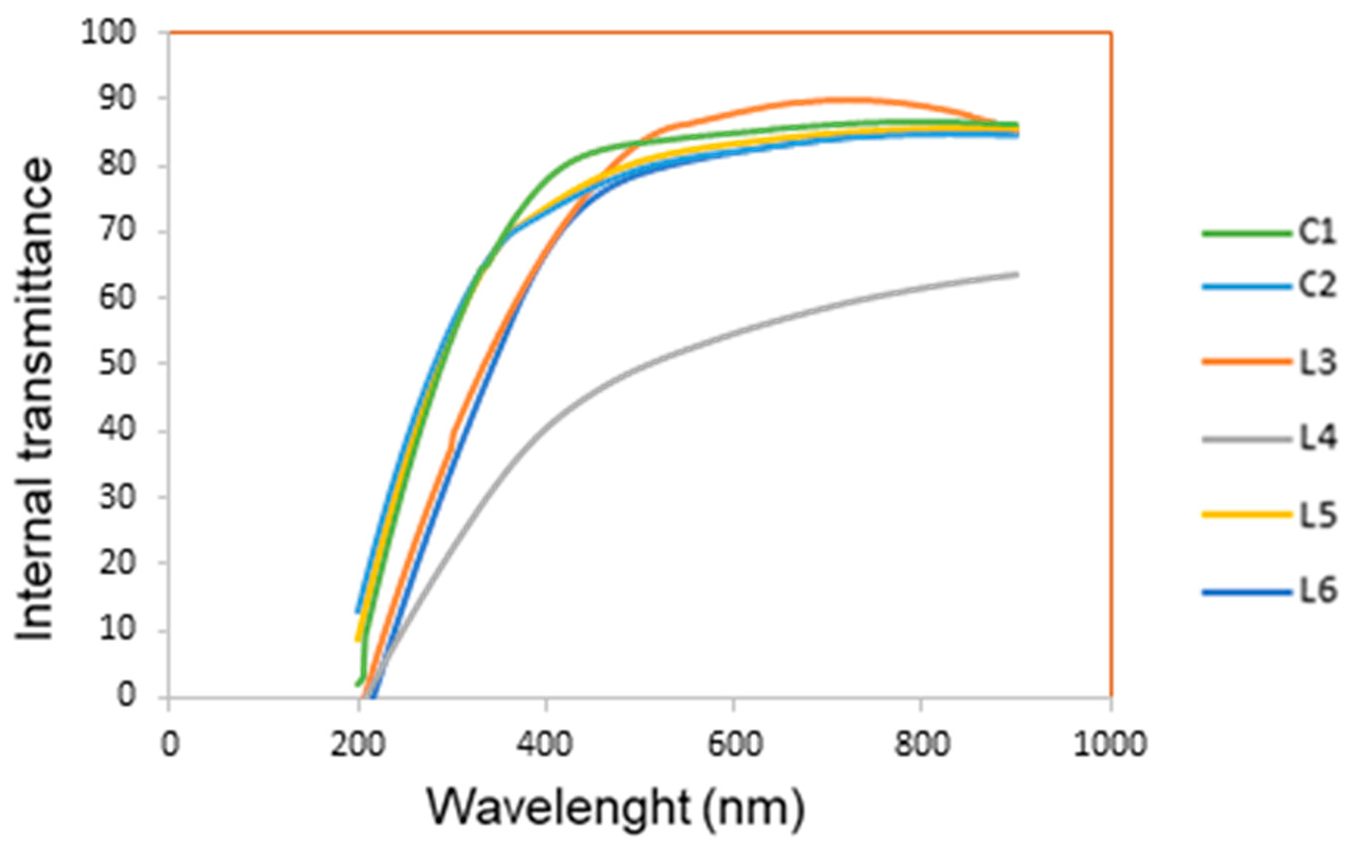
2.4.4. UV-Vis Internal Transmittance
2.4.5. Opacity
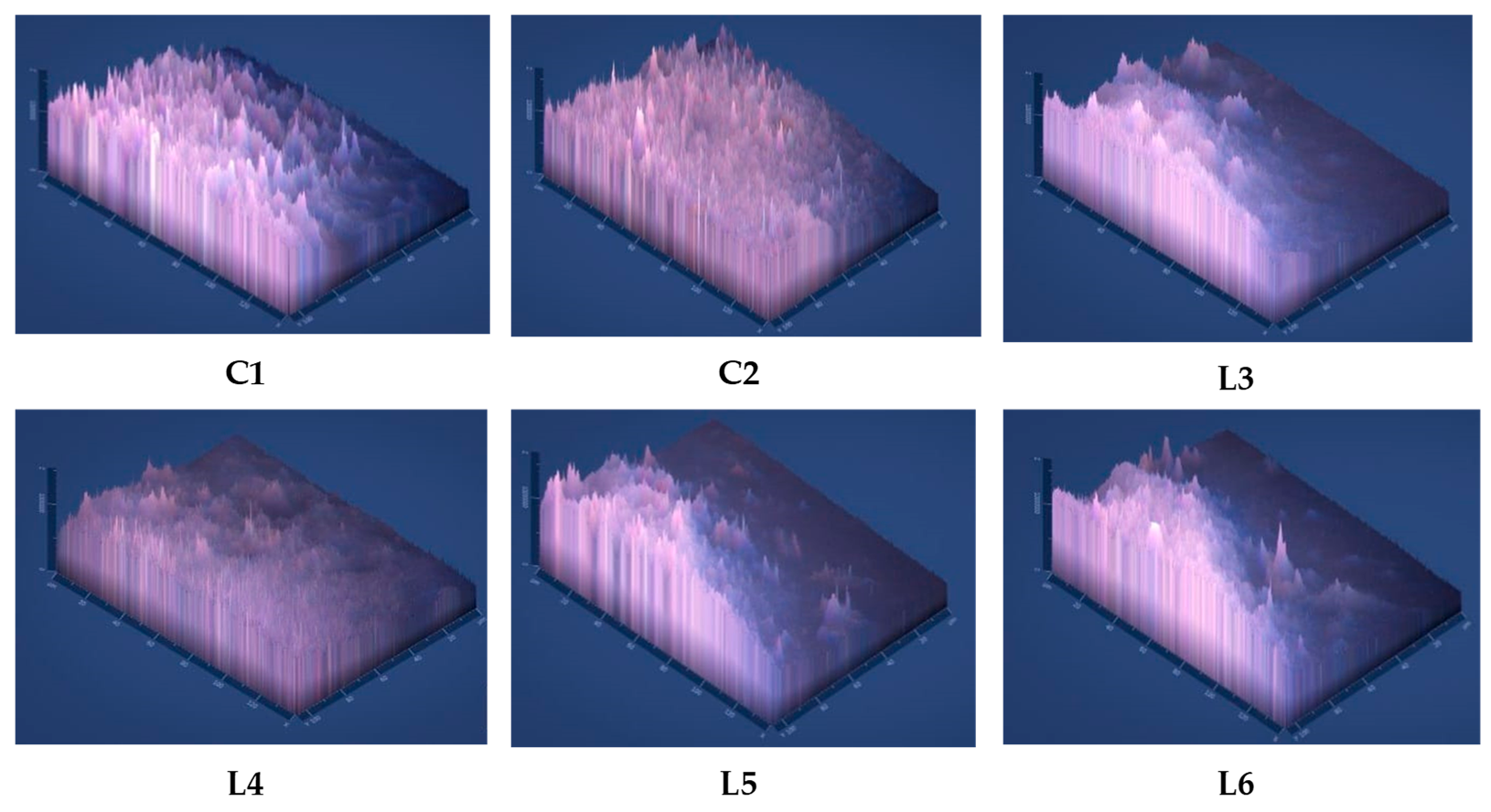
2.4.6. Optical Micrography
2.4.7. Moisture Content
2.4.8. Contact Angle
2.4.9. Water Vapour Permeability
2.4.10. Antioxidant Capacity (DPPH) and (ABTS)
2.4.11. Statistical Analysis
3. Results
3.1. Characterisation of Lignin
3.1.1. Lignin Yield from the Banana Pseudostem (Dominico-Hartón)
3.1.2. Antioxidant Capacity
3.2. Characterisation of Biodegradable MHEC Films with Lignin Incorporation
3.2.1. Physical Properties
3.2.2. Mechanical Properties
3.2.3. Colour
3.2.4. UV-Vis Internal Transmittance
3.2.5. Opacity
3.2.6. Contact Angle
3.2.7. Optical Micrography
3.2.8. Antioxidant Capacity (DPPH) and (ABTS)
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Rivera, C.; Contreras, F.; Ariza, W.; Bonilla, S.; Cruz, A. Los empaques biodegradables, una respuesta a la consciencia ambiental de los consumidores. Real. Empres. 2019, 7, 2–8. [Google Scholar] [CrossRef]
- Flury, M.; Narayan, R. Biodegradable plastic as an integral part of the solution to plastic waste pollution of the environment. Curr. Opin. Green Sustain. Chem. 2021, 30, 100490. [Google Scholar] [CrossRef]
- Martha-Aguilar, N.G. Biopolímeros: La explosión de la era eco-amigable. RD-ICUAP 2022, 8, 60–67. [Google Scholar] [CrossRef]
- Maceda, A.; Soto-Hernández, M.; Peña-Valdivia, C.B.; Trejo, C.; Terrazas, T. Lignina: Composición, síntesis y evolución. Madera Y bosques 2021, 27, 2. [Google Scholar] [CrossRef]
- López, A.; Abigail, S. Obtención Y Caracterización de Una Biopelícula a Partir de Plátano Verde Dominico-Hartón (Musa AAB Simmonds) Para el Uso en Alimentos; Escuela Superior Politécnica de Chimborazo: Riobamba, Ecuador, 2021. [Google Scholar]
- Abd-El Kader, F.H.; Said, G.; El-Naggar, M.M.; Anees, B.A. Characterization and electrical properties of methyl-2-hydroxyethyl cellulose doped with erbium nitrate salt. J. Appl. Polym. Sci. 2006, 102, 2352–2361. [Google Scholar] [CrossRef]
- Castañeda-Niño, J.P.; Mina-Hernández, J.H.; Solanilla-Duque, J.F. Extraction and Characterization of Starches from the Pulp and Peel of Native Plantain (Musa AAB Simmonds) from Two Colombian Departments. Polysaccharides 2025, 6, 34. [Google Scholar] [CrossRef]
- Zhang, W.; Li, X.; Jiang, W. Development of antioxidant chitosan film with banana peels extract and its application as coating in maintaining the storage quality of apple. Int. J. Biol. Macromol. 2020, 154, 1205–1214. [Google Scholar] [CrossRef]
- Alcivar-Gavilanes, M.G.; Carrillo-Anchundia, K.L.; Rieral, M.A. Development of a bioplastic from banana peel. Ing. Investig. 2022, 42, e92768. [Google Scholar] [CrossRef]
- Porta, R. The plastics sunset and the bioplastics sunrise. Coatings 2019, 9, 526. [Google Scholar] [CrossRef]
- Poveda-Giraldo, J.A.; Solarte-Toro, J.C.; Alzate, C.A.C. The potential use of lignin as a platform product in biorefineries: A review. Renew. Sustain. Energy Rev. 2021, 138, 110688. [Google Scholar] [CrossRef]
- González, A.; Sánchez, A.; Rodríguez, A. Extracción de lignina para la producción de biopolímeros biodegradables. Rev. Investig. Académica 2020, 32, 1–10. [Google Scholar]
- HPS, A.K.; Saurabh, C.K.; AS, A.; Nurul Fazita, M.R.; Syakir, M.I.; Davoudpour, Y.; Rafatullah, M.; Abdullah, C.K.; Haafiz, M.K.; Dungani, R. A review on chitosan-cellulose blends and nanocellulose reinforced chitosan biocomposites: Properties and their applications. Carbohydr. Polym. 2016, 150, 216–226. [Google Scholar] [CrossRef]
- Platzer, M.; Kiese, S.; Herfellner, T.; Schweiggert-Weisz, U.; Miesbauer, O.; Eisner, P. Common trends and differences in antioxidant activity analysis of phenolic substances using single electron transfer based assays. Molecules 2021, 26, 1244. [Google Scholar] [CrossRef] [PubMed]
- Moreno-Ricardo, M.A.; Gómez-Contreras, P.; González-Delgado, Á.D.; Hernández-Fernández, J.; Ortega-Toro, R. Development of films based on chitosan, gelatin and collagen extracted from bocachico scales (Prochilodus magdalenae). Heliyon 2024, 10, e25194. [Google Scholar] [CrossRef]
- ASTM D882; Standard Test Method for Tensile Properties of Thin Plastic Sheeting. ASTM: West Conshohocken, PA, USA, 2002.
- Ortega-Toro, R.; Collazo-Bigliardi, S.; Roselló, J.; Santamarina, P.; Chiralt, A. Antifungal starch-based edible films containing Aloe vera. Food Hydrocoll. 2017, 72, 1–10. [Google Scholar] [CrossRef]
- ASTM E96-95; Standard Test Method for Water Vapor Transmission of Materials. American Society for Testing and Materials: Philadelphia, PA, USA, 1999.
- Tavares, D.; Cavali, M.; Tanobe, V.D.O.A.; Torres, L.A.Z.; Rozendo, A.S.; Zandoná Filho, A.; Soccol, C.R.; Woiciechowski, A.L. Lignin from residual sawdust of Eucalyptus spp.—Isolation, characterization, and evaluation of the antioxidant properties. Biomass 2022, 2, 195–208. [Google Scholar] [CrossRef]
- Mahmood, Z.; Yameen, M.; Jahangeer, M.; Riaz, M.; Ghaffar, A.; Javid, I. Lignin as natural antioxidant capacity. Lignin-Trends Appl. 2018, 10, 181–205. [Google Scholar] [CrossRef]
- Bülichen, D.; Kainz, J.; Plank, J. Working mechanism of methyl hydroxyethyl cellulose (MHEC) as water retention agent. Cem. Concr. Res. 2012, 42, 953–959. [Google Scholar] [CrossRef]
- Gaikwad, K.K.; Singh, S.; Ajji, A. Moisture absorbers for food packaging applications. Environ. Chem. Lett. 2019, 17, 609–628. [Google Scholar] [CrossRef]
- Jayakumar, A.; Radoor, S.; Kim, J.T.; Rhim, J.W.; Nandi, D.; Parameswaranpillai, J.; Siengchin, S. Recent innovations in bionanocomposites-based food packaging films–A comprehensive review. Food Packag. Shelf Life 2022, 33, 100877. [Google Scholar] [CrossRef]
- Ruwoldt, J.; Blindheim, F.H.; Chinga-Carrasco, G. Functional surfaces, films, and coatings with lignin–a critical review. RSC Adv. 2023, 13, 12529–12553. [Google Scholar] [CrossRef]
- Jiang, Y.; Liu, X.; Yang, Q.; Song, X.; Qin, C.; Wang, S.; Li, K. Effects of residual lignin on composition, structure and properties of mechanically defibrillated cellulose fibrils and films. Cellulose 2019, 26, 1577–1593. [Google Scholar] [CrossRef]
- Câmara Mileo, P.; De Oliveira, M.F.; Luz, S.M.; Rocha, G.J.; Gonçalves, A.R. Evaluation of castor oil polyurethane reinforced with lignin and cellulose from sugarcane straw. Adv. Mater. Res. 2010, 123, 1143–1146. [Google Scholar] [CrossRef]
- Ye, H.; He, Y.; Li, H.; You, T.; Xu, F. 3D-Printed polylactic acid/Lignin films with great mechanical properties and Tunable functionalities towards superior UV-shielding, haze, and antioxidant properties. Polymers 2023, 15, 2806. [Google Scholar] [CrossRef]
- Mora Antivar, J.D.; Acosta León, G.A. Caracterización de Compuestos Bioactivos en el Rambután (Nephelium lappaceum) e Implementación de Lignina Extraída del Pericarpio como Materia Prima en Biopelículas. Bachelor’s Thesis, Universidad de Bogotá Jorge Tadeo Lozano, Bogotá, Colombia, 2019. Available online: https://expeditiorepositorio.utadeo.edu.co/handle/20.500.12010/7766 (accessed on 10 February 2025).
- Paudel, S.; Regmi, S.; Janaswamy, S. Effect of glycerol and sorbitol on cellulose-based biodegradable films. Food Packag. Shelf Life 2023, 37, 101090. [Google Scholar] [CrossRef]
- Salgado González, I.D. Efecto de la Adición de Quitosano en Las Propiedades Mecánicas, Permeabilidad al Vapor de Agua Y Oxígeno, Brillo Y Microestructura en Films Comestibles a Base de Almidón de Trigo. Master’s Thesis, Universitat Politècnica de València, Valencia, Spain, 2013. [Google Scholar]
- Ban, W.; Song, J.; Lucia, L.A. Influence of natural biomaterials on the absorbency and transparency of starch-derived films: An optimization study. Ind. Eng. Chem. Res. 2007, 46, 6480–6485. [Google Scholar] [CrossRef]
- Parit, M.; Saha, P.; Davis, V.A.; Jiang, Z. Transparent and homogenous cellulose nanocrystal/lignin UV-protection films. ACS Omega 2018, 3, 10679–10691. [Google Scholar] [CrossRef]
- Silva, L.; Colussi, F.; Martins, J.T.; Vieira, J.M.; Pastrana, L.M.; Teixeira, J.A.; Cerqueira, M.A.; Michelin, M. Strategies for the incorporation of organosolv lignin in hydroxypropyl methylcellulose-based films: A comparative study. Int. J. Biol. Macromol. 2024, 280, 135498. [Google Scholar] [CrossRef]
- Boarino, A.; Schreier, A.; Leterrier, Y.; Klok, H.A. Uniformly dispersed poly (lactic acid)-grafted lignin nanoparticles enhance antioxidant activity and UV-barrier properties of poly (lactic acid) packaging films. ACS Appl. Polym. Mater. 2022, 4, 4808–4817. [Google Scholar] [CrossRef]
- Souza de Miranda, C.; Ferreira, M.S.; Magalhães, M.T.; Gonçalves, A.P.B.; Carneiro de Oliveira, J.; Guimarães, D.H.; José, N.M. Effect of the glycerol and lignin extracted from Piassava fiber in cassava and corn starch films. Mater. Res. 2015, 18, 260–264. [Google Scholar] [CrossRef][Green Version]
- Pham, C.D.; Truong, T.M.; Ly, T.B.; Le, P.K. Application of lignin from cellulose isolation process in the fabrication of chitosan/lignin film for UV-light blocking and anti-oxidation. Waste Biomass Valori. 2024, 15, 1881–1894. [Google Scholar] [CrossRef]

| Formulations | MHEC (p/p)% | Glycerol (p/p)% | Lignin (p/p)% | H2O (mL) |
|---|---|---|---|---|
| Control 1 (C1) | 1.5 | 15 | 0 | 200 |
| Control 2 (C2) | 1.5 | 20 | 0 | 200 |
| Lignin 3 (L3) | 1.5 | 15 | 0.5 | 200 |
| Lignin 4 (L4) | 1.5 | 20 | 1 | 200 |
| Lignin 5 (L5) | 1.5 | 15 | 1 | 200 |
| Lignin 6 (L6) | 1.5 | 20 | 0.5 | 200 |
| Formulations | Thickness (μm) | WVP (g mm/kPa-h-m2) | Xw (g Water/g Dry Film) | Wca (g Dry Film/g Wet Film) |
|---|---|---|---|---|
| C1 | 177.8 ± 0.02 c | 4.14 ± 0.02 d | 0.24 ± 0.04 c | 0.147 ± 0.22 a |
| C2 | 183.5 ± 0.02 c | 4.20 ± 0.06 d | 0.11 ± 0.06 d | 0.130 ± 0.42 bc |
| L3 | 232.8 ± 0.02 b | 5.39 ± 0.04 b | 0.29 ± 0.03 c | 0.131 ± 0.44 d |
| L4 | 225.5 ± 0.01 b | 5.15 ± 0.08 c | 0.65 ± 0.09 a | 0.122 ± 0.37 bcd |
| L5 | 269.0 ± 0.01 a | 6.20 ± 0.05 a | 0.23 ± 0.03 c | 0.131 ± 0.47 b |
| L6 | 224.4 ± 0.02 b | 5.85 ± 0.01 e | 0.56 ± 0.05 b | 0.116 ± 0.43 cd |
| Formulations | TS (MPa) | ME (MPa) | E (%) |
|---|---|---|---|
| C1 | 11.5 ± 0.3 b | 295 ± 3 ab | 38.2 ± 0.5 c |
| C2 | 10.9 ± 0.2 a | 287 ± 5 a | 45.3 ± 0.4 a |
| L3 | 12.1 ± 0.5 b | 312 ± 3 c | 36.7 ± 0.4 d |
| L4 | 15.4 ± 0.2 c | 318 ± 2 d | 37.2 ± 0.2 cd |
| L5 | 17.1 ± 0.4 d | 325 ± 5 e | 34.4 ± 0.8 e |
| L6 | 11.8 ± 0.5 b | 301 ± 4 b | 40.5 ± 1.5 b |
| Formulations | Gloss at 60° | Colour Parameters | ΔE | ||||
|---|---|---|---|---|---|---|---|
| L* | A* | B* | C | h | |||
| C1 | 8.2 ± 0.9 c | 82.7 ± 3.2 a | −1.8 ± 0.6 ab | 3.4 ± 1.5 a | 3.8 ± 1.6 abc | 117.9 ± 2.3 a | - |
| C2 | 8.6 ± 0.9 c | 82.5 ± 1.4 a | −2.3 ± 0.4 b | 3.6 ± 0.4 a | 4.2 ± 0.5 a | 122.2 ± 4.6 a | - |
| L3 | 6.0 ± 0.8 d | 85.2 ± 1.2 a | −1.5 ± 0.2 ab | 2.0 ± 0.4 b | 2.5 ± 0.4 c | 126.5 ± 1.9 a | 4.1 ± 0.8 a |
| L4 | 13.3 ± 1.7 a | 83.1 ± 0.2 a | −1.9 ± 0.1 ab | 3.4 ± 0.6 a | 3.9 ± 0.5 abc | 119.2 ± 4.2 a | 2.9 ± 0.8 a |
| L5 | 13.8 ± 0.3 a | 83.4 ± 1.3 a | −2.0 ± 0.6 ab | 3.4 ± 0.1 a | 4.0 ± 0.3 ab | 119.9 ± 6.5 a | 2.6 ± 0.2 ab |
| L6 | 11.3 ± 1.8 b | 85.5 ± 1.8 a | −0.9 ± 1.5 a | 2.4 ± 0.5 ab | 2.8 ± 0.6 bc | 121.7 ± 7.6 a | 4.8 ± 0.5 a |
| Formulations | CAw |
|---|---|
| C1 | 58.333 ± 0.8 c |
| C2 | 55.000 ± 0.5 d |
| L3 | 52.000 ± 0.5 e |
| L4 | 80.333 ± 2.5 a |
| L5 | 70.000 ± 2.2 b |
| L6 | 69.333 ± 2.1 b |
| Formulations | DPPH IC50 (μg/g Dry Film) | ABTS IC50 (μg/g Dry Film) |
|---|---|---|
| C1 | --- | --- |
| C2 | --- | --- |
| L3 | 720 ± 2 a | 501 ± 2 a |
| L4 | 495 ± 2 b | 342 ± 5 b |
| L5 | 504 ± 5 b | 347 ± 2 b |
| L6 | 715 ± 6 a | 498 ± 3 a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ocampo-Gómez, Y.A.; Rico-Rodríguez, F.; González-Cuello, R.; Hernández-Fernández, J.; Ortega-Toro, R. Effect of the Addition of Banana Stem Lignin (Musa acuminata ssp. balbisiana var. Dominico-Harton) on the Physicochemical Properties of Biodegradable Composites Based on Methylhydroxyethylcellulose. J. Compos. Sci. 2025, 9, 244. https://doi.org/10.3390/jcs9050244
Ocampo-Gómez YA, Rico-Rodríguez F, González-Cuello R, Hernández-Fernández J, Ortega-Toro R. Effect of the Addition of Banana Stem Lignin (Musa acuminata ssp. balbisiana var. Dominico-Harton) on the Physicochemical Properties of Biodegradable Composites Based on Methylhydroxyethylcellulose. Journal of Composites Science. 2025; 9(5):244. https://doi.org/10.3390/jcs9050244
Chicago/Turabian StyleOcampo-Gómez, Yonier Alejandro, Fabian Rico-Rodríguez, Rafael González-Cuello, Joaquín Hernández-Fernández, and Rodrigo Ortega-Toro. 2025. "Effect of the Addition of Banana Stem Lignin (Musa acuminata ssp. balbisiana var. Dominico-Harton) on the Physicochemical Properties of Biodegradable Composites Based on Methylhydroxyethylcellulose" Journal of Composites Science 9, no. 5: 244. https://doi.org/10.3390/jcs9050244
APA StyleOcampo-Gómez, Y. A., Rico-Rodríguez, F., González-Cuello, R., Hernández-Fernández, J., & Ortega-Toro, R. (2025). Effect of the Addition of Banana Stem Lignin (Musa acuminata ssp. balbisiana var. Dominico-Harton) on the Physicochemical Properties of Biodegradable Composites Based on Methylhydroxyethylcellulose. Journal of Composites Science, 9(5), 244. https://doi.org/10.3390/jcs9050244








