Abstract
Staining removal is an issue of interest in dentistry. Current treatments deal with staining removal on enamel, while few studies concentrate on resin composites. The aim of the current study is to evaluate the efficacy in staining removal of an ozonated gel on dental composites. The study sample consisted of 40 specimens of restorative composites: 20 specimens were stained for 1 day in tea solution (tea group) and 20 specimens were stained for 1 day in physiological solution (NaCl group). Both the tea and NaCl groups underwent the experimental treatment as follows: five specimens underwent ozonized gel application, five specimens underwent an ozonized spray, five specimens underwent an application of olive oil, and five specimens were not treated. A colorimetric evaluation was performed with a spectrophotometer, using CIEDE2000 data elaboration at the baseline (T0), after staining (T1), and after staining removal (T2). In the T0–T1 time frame, significantly different color changes (ΔE00) were found between tea groups and NaCl groups (p < 0.05), except for control groups (p > 0.05). After staining removal in the T1–T2 period, no significant differences in ΔE00 were found (p > 0.05). Higher values were found for groups treated with ozonized gel, denoting a stain removal effect. The groups treated with olive oil, instead, exhibited higher ΔE00 values, showing a greater staining effect. In conclusion, the ozonized gel tested showed staining removal activity on restorative resin composites. Future clinical applications are required to validate the in vitro results obtained.
1. Introduction
Resin composites are extensively used dental restorative materials with biocompatibility, high aesthetic properties, and greater antimicrobial power compared to traditional restorative materials [1]. Bisphenol A-glycidyl methacrylate (Bis-GMA)-based dental polymer composites are now the dentist’s first choice for both direct and indirect restorations in both the posterior and anterior sectors; Bis-GMA has low polymerization shrinkage, low volatility, and high viscosity [2]. Accordingly, composite resins can be used to improve aesthetics at a low cost, while offering a good clinical performance if correct bonding procedures are strictly followed [1].
Although dental resins are widely used in everyday dental practice, they still have several limitations. Apart from conformational changes after polymerization and the risk of secondary caries, plaque accumulation and discoloration are the main concerns [3]. The pigmentation of composite resins can be the result of the action of internal or external agents [4,5,6,7]. In addition, pigmentation can result from a modification of the surface layer of the resin that causes the surface morphology to retain a lot of external pigment [8]. In particular, the affinity of resins for extrinsic coloring agents is dependent on the conversion rate and the physico-chemical properties of the resins [9,10]. Specific factors that modify color properties are surface roughness, integrity and the polishing technique; low homogeneity of the surface layer with a high number of irregularities tends to cause the retention of exogenous pigments. Studies on color stability have shown that, in addition to smoking, mouthwashes and common beverages, especially soft drinks, have varying degrees of staining effect on self-curing and light-curing dental composites; the staining effect of such substances varies according to their composition and properties [11,12,13]. In this area, the current products available for dental bleaching, such as carbamide peroxide and hydrogen peroxide, have been extensively used for resin-based composite bleaching, with demonstrated efficacy in in vitro settings [14].
In recent years, ozone has been employed in the dental field to treat various conditions of both soft and hard tissues [15]. Several laboratory studies have shown a high antiseptic efficacy of ozonized products against various pathogens [16,17,18,19,20,21,22,23]. In addition, ozone has been reported to have immunomodulatory and anti-inflammatory effects, as well as anti-hypoxic and biosynthetic effects [24]. A relevant application of ozone in dentistry is the treatment of periodontal and peri-implant conditions. Broad-spectrum antiseptics such as chlorhexidine have generally been considered, but these substances have some limitations when used for long periods; in particular, they are associated with tooth discoloration and the occurrence of dysgeusia. On the contrary, a broad spectrum of antimicrobial action is reported for ozone, with reduced toxicity and side effects [25]. According to a recent review of the literature evaluating the evidence for ozone therapy in medicine and dentistry, ozone has shown several beneficial effects in the medical field. However, despite the encouraging in vitro evidence, the clinical use of ozone has not yet been shown to be highly effective, which may be due to the risk of bias in the research conducted to date [26]. Furthermore, only two previous studies assess the effects of ozone as a bleaching agent for resin composites [27,28]. In this context, ozone, especially via the hydroxyl radical (OH−) that arises from its decomposition, can play a key role in the oxidation process. When ozone decomposes in an aqueous solution, peroxide radicals (HO2) initially form, followed by hydroxyl radicals (OH·). These reactions occur simultaneously, leading to the breakdown of chromophore groups by ozone and the formation of smaller molecules. This bleaching process occurs through several fundamental mechanisms: (a) an addition mechanism, in which ozone binds via a double bond to form ozonides; however, in the presence of water, ozone hydrolyzes, leading to the cleavage of the double bond; (b) a substitution mechanism, where an atom or functional group is replaced; and (c) a cleavage mechanism, in which a carbon–carbon bond is broken, producing smaller organic fragments [27]. Supposing that chromophores can be deposited on resin composite surfaces, ozone must be tested on composites to understand its ability to exert a bleaching effect. Considering that in vitro studies have certain limitations in investigating phenomena involving clinical areas, and that clinical studies should follow to validate their results, the aim of this study is therefore to determine whether ozonated substances could be effective in causing a color change in composite resins after pigmentation with some of the most commonly used coloring agents. The current study was conducted following the methodology of a previous study [28].
The statistical null hypothesis was that there would be no significant color change among groups over time.
2. Materials and Methods
2.1. Specimens’ Preparation
Sample size calculation for two independent study groups considering a continuous primary outcome was performed using an online sample size calculator (Clincalc.com; https://clincalc.com/stats/samplesize.aspx, accessed on 15 September 2022). Alpha was set at 0.05 with 80% power. ΔE00 was the primary outcome, an expected mean of 6.08 with an SD of 2.79 was considered, and the expected difference between means (bleaching effect vs. control) was set at 4.8 based on the previous literature [29]; therefore, 5 specimens were required for each group.
By light-curing an A3 shade restorative composite (G-aenial, GC Italia, Vimodrone, Milan, Italy), 40 discs with dimensions of 6 mm diameter and 2 mm height were obtained (the characteristics of the composite are shown in Table 1).

Table 1.
Characteristics of the tested restorative composite resin.
The specimens were prepared by placing the composite in a stainless-steel mold with an external diameter of 8 mm, an internal diameter of 6 mm, and a height of 4 mm. The molds were placed on dark paper with a polyester matrix strip (Mylar strip, Henry Schein, Melville, NY, USA) in between [30]. Each mold was slightly overfilled, and an analog polyester matrix strip was placed on the upper surface to avoid oxygen interference with the polymerization process [31]; the excess composite was removed to obtain flat surfaces. Light curing (output irradiance of 1000 mW/cm2 for 40 s) was performed on each specimen with the Celalux 2 LED unit (Voco, Cuxhaven, Germany) at full charge and after checking the irradiance with a radiometer before each use [32].
Finally, fine and superfine discs (Sof-Lex Pop On; 3M ESPE, St. Paul, MN, USA) were used to finish the composite surfaces and obtain regular surfaces of the specimens. The specimens were then remeasured to confirm their original size; if the original height of the specimens (2 mm) was reduced, they were excluded.
Specimens were kept in complete darkness in physiological solution for 48 h to allow for the completion of hardening [33]. An example of the specimens used is shown in Figure 1.
Figure 1.
Specimens before staining procedures.
2.2. Experimental Procedure
We subdivided the 40 specimens as follows:
- -
- Group Tea: Twenty specimens underwent a pigmentation treatment consisting of a 1 h immersion in a 37 °C tea solution assessed with a contact thermometer (PeakTech® Digital Thermometer 5135/5140 Prilf—und Messtechnik, GmbH, Ahrensburg, Germany); the solution was obtained with 10 applications of 10′ each of the teabag (Lipton, Glasgow, Scotland). The time of application was chosen to simulate a weekly consumption of tea.
- -
- Group NaCl: Twenty specimens were immersed in physiological solution (B. Braun, Milan, Italy) for 1 h.
In Figure 2, specimens are shown after the staining procedures.
Figure 2.
Specimens after staining procedures.
Then, specimens were sub-aggregated according to the treatment performed:
- -
- O3 gel: Two applications of 1 h each of ozonized gel (Gelio3, Bioemmei Srl, 36100 Vicenza, Italy) containing a bio-ozonized olive oil, hydrated silica, and arnica;
- -
- O3 spray: Two applications of 1 h each of ozonized spray (Gelio3 without hydrated silica);
- -
- Olive oil: Two applications of 1 h each of ozonized oil (Gelio3 without hydrated silica and ozone);
- -
- Control: Maintenance in physiological solution for 1 h.
Each treatment was performed on 5 specimens from Group Tea and 5 specimens from Group NaCl. This study was designed to assess the effect of the complete gel (Gelio3) compared to the spray form (without hydrated silica) and to oil (without ozone and hydrated silica). The application was conducted considering a standard quantity of application of periodontal gels in the oral environment. In Figure 3, specimens after the bleaching procedures are shown.
Figure 3.
Specimens after bleaching procedures.
In Table 2, the characteristics of the staining products are shown.

Table 2.
Characteristics of staining materials.
At the end of the procedure, the samples were rinsed with physiological solution, dried gently in air, and stored in distilled water at 37 °C.
2.3. Colorimetric Evaluation
The samples were subjected to a colorimetric analysis at three consecutive times: after 24 h of maintenance in physiological medium before the start of the experimental procedures (T0), after the assignment to groups Tea and NaCl, which represents the pigmentation for the Group Tea samples and maintenance in physiological medium for the Group NaCl samples (T1), and after the division into the four subgroups, consisting of the application of the products versus the control (T2).
The CIEDE2000 method was used for the colorimetric evaluation of color difference ΔE00, based on the following formula [34]:
where ΔL′ is lightness difference, ΔC′ is chroma difference, ΔH′ is hue angle difference, SL, SC, and SH are weighting functions of lightness, chroma, and hue components in order to better match human visual perception, kL, kC, and kH are parametric factors (usually set at 1), and RT is a rotation factor that accounts for the interaction between chroma and hue differences. The parameters needed for the calculation mentioned above were digitally provided by a spectrophotometer for dental use (Vita Easyshade® V, Vita Zahnfabrik, Bad Säckingen Germany), which was used with its tip perpendicular and in direct contact with the specimens. The experimentation was conducted in a dark room [8].
2.4. Statistical Analysis
The mean, standard deviation, minimum, median, and maximum (descriptive statistics) were calculated for each variable. The Shapiro–Wilk test was conducted to verify data normality. Inferential statistics were calculated through the ANOVA test followed by Tukey’s multiple comparison test, setting a significance threshold of p < 0.05. The statistical analysis was performed through R software (version 3.1.3, Core Team, R Foundation for Statistical Computing, Vien, Austria).
3. Results
During the T0–T1 period (Table 3), significant differences in terms of color change (ΔE00) between the Tea and NaCl groups were found (p < 0.05), except for Tea Control vs. NaCl groups (p > 0.05). The highest ΔE00 values were found in the Tea Olive Oil and Tea O3 groups, denoting a staining effect of the tea solution. On the other hand, NaCl groups exhibited lower ΔE00 values, demonstrating that the physiological solution did not contribute significantly to staining. No significant intragroup differences occurred (p > 0.05).

Table 3.
Color variation (ΔE00) for the two groups between T0 and T1. * Means with the same letters do not present significant intergroup and intragroup differences (p > 0.05).
After the T1–T2 period (Table 4), in which bleaching agents were used, no statistically significant differences in ΔE00 values were found among the study groups (p > 0.05). From a descriptive point of view, higher ΔE00 values were found in Tea O3 gel and NaCl O3 gel, suggesting a potential bleaching effect of the tested ozonized gel. The Olive oil groups presented high ΔE00, demonstrating a staining effect of the product. The Control groups showed the lowest ΔE00, confirming the limited recovery from staining.

Table 4.
Color variation (ΔE00) for the two groups between T1 and T2. * Means with the same letters do not present significant intergroup and intragroup differences (p > 0.05).
In Figure 4, the results of the four bleaching treatments after the effect of the two staining solutions are shown. Significant T0–T1 vs. T1–T2 comparisons were found between the O3 spray Tea and Control Tea groups (p < 0.05).
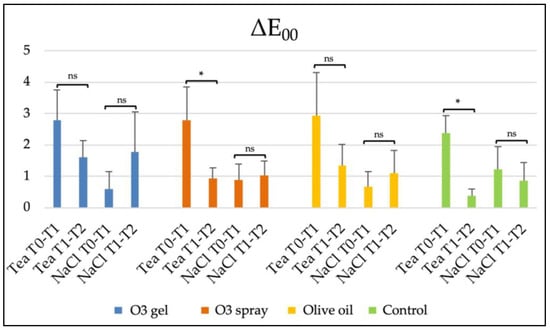
Figure 4.
Color variation (ΔE00) for the study groups. Tea and NaCl correspond to the two staining solutions in which specimens were immersed in the T0–T1 period. Comparisons between T0–T1 staining and T1–T2 bleaching procedures are shown together with the tested products: ozonized gel (O3 gel), ozonized spray (O3 spray), olive oil, and control. Legend: *, p < 0.05; ns, not significant.
4. Discussion
Increasing research has been conducted in recent years to evaluate the potential use of ozone in dental care, as demonstrated by recent systematic reviews [35,36] and a meta-analysis [37] published in the last two years. However, no comprehensive studies have been conducted to evaluate the efficacy of ozone as a bleaching agent on pigmented dental composites. In a previous study [28], forty discs of composite resin were divided into two groups and subjected to a pigmentation treatment corresponding to the application of 1% chlorhexidine-based gel (experimental group), known for its pigmentation effect, and to storage in physiological solution (control group). Subsequently, the samples from both groups were divided into four subgroups and subjected to four different protocols, three of which consisted of exposure to different ozonized products. The same methodology was used in the current study. However, the novelty of this study with respect to the previous one is the use of a staining agent other than chlorhexidine, i.e., tea. This latter substance is also known for its staining effect on teeth, and this effect is much more common due to the greater use of this substance compared to chlorhexidine [38].
Colorimetric analysis is an important aspect of dentistry, given the need to achieve the highest degree of homogeneity between natural teeth and restorative or prosthetic rehabilitation. This field of dentistry has various applications for colorimetry, such as the evaluation of accuracy and precision of the available measuring systems and, from the patient’s side, the acceptability and perceptibility of a color difference. This has led to different methods of assessing color change in dental research [39]. Today, patients are increasingly demanding aesthetic results, especially in the anterior region of the dental arch [40,41], sometimes leading to overtreatment in aesthetic dental procedures driven by patients’ requests and expectations [42]. To facilitate the clinician’s assessment, color evaluation can be performed using dental spectrophotometers [40,43]. This method avoids potential operator error and is much more accurate than the human eye in assessing small differences [44,45]. Spectrophotometers quantify the amount of light reflected by the red, green, and blue colors. The colorimetric system used is CIEDE2000, according to the literature [46], based on the quantification of color variations between repeated color measurements (ΔE00) [47].
The chemical composition of composite resins and the characteristics of their particles are directly related to the surface profile and thus to the risk of extrinsic pigmentation. In addition, the finishing and polishing methods also influence the characteristics of the outer surface of the composite and are therefore related to early discoloration [8,48,49].
Recently, ozonated products have been extensively studied and used to treat various clinical conditions in dentistry, such as gingivitis and periodontitis [38].
The aim of the present study was to evaluate whether ozonized products could be used as bleaching agents on pigmented dental composite resins. Although several studies have evaluated the bleaching power of ozone, no comprehensive research has yet been conducted to clarify its potential bleaching effect on dental composite resins. However, this is an important issue in dentistry as such materials can be subjected to pigmentation [50].
The statistical null hypothesis of this study was partially accepted; in fact, no significant color changes were found between the Tea and NaCl groups after bleaching in the T1–T2 evaluation. However, it should be noted that in the comparison of T0–T1 vs. T1–T2, only the Tea O3 spray and Tea Control groups resulted in a significantly lower ΔE00, highlighting that O3 gel was effective in the bleaching procedure, while olive oil contributed to additional staining. In the case of the ozonized spray, the absence of a color change effect may seem inconsistent with the bleaching effect assessed for the ozonized gel; probably, the difference in density between the spray and the gel, with the gel remaining in contact with the substrate for longer than the spray, could justify the difference between the two products.
To date, the effect of ozonated products on the bleaching of resin composites has not been extensively studied. Only two studies have been carried out [27,28], one of which was conducted by the present research group to evaluate the effect of the same products tested in the current study on the bleaching of chlorhexidine pigmented resin composites, which showed a positive bleaching effect of the ozonized products [28]. The results are in line with the present report, as almost no significant differences among the groups were found. The data suggest a bleaching effect of the ozonized gel, but it should be considered that in the present study a different staining agent and a different formula for color change assessment (CIEDE2000) were adopted; therefore, a reliable comparison is not possible. As mentioned above, the methodology of the current study is the same as that of the previous study mentioned above. The novelty of the present study is the use of a different staining agent than chlorhexidine, namely tea. This latter substance is also known for its staining effect on teeth, and this effect is much more common due to the greater use of this substance compared to chlorhexidine [51].
The present study is in line with the results obtained previously [27,33]. In fact, ozone seems to be an effective bleaching agent for pigmented composites, independent of the pigment.
Ozone may serve as an effective bleaching agent not only on resin composites but also on teeth, without the reported side effects associated with traditional bleaching agents such as hydrogen peroxide and carbamide peroxide [44,52]. These conventional tooth whiteners could be responsible for changes in the enamel, with a decrease in its microhardness. In particular, the effect of whitening agents on microhardness reduction depends on their pH [53]. In general, it is important to consider the surface properties of enamel after bleaching to ensure that bleaching provides adequate conditions for a healthy dental substrate [54]. Carvalho et al. [55] conducted an in vitro study to evaluate the effects of bleaching with 10% carbamide peroxide (CP), with or without ozone, on the microhardness (Knoop), roughness (Ra), and micromorphology (SEM) of the enamel surface. The results showed that enamel microhardness decreased after treatment with carbamide peroxide and ozone but remained unchanged when treated with the combination of ozone and carbamide peroxide. Furthermore, the latter treatment resulted in a much lower enamel microhardness than the former. Finally, SEM evaluation showed that carbamide peroxide produced slight irregularities in the micromorphology of the enamel after the whitening treatment. Conversely, the combination of this substance with ozone preserved the mechanical and physical properties of the microhardness and micromorphology of the enamel surface and either maintained or reduced the surface roughness.
Considering hydrogen peroxide and ozone, a systematic review with meta-analysis [52] found that bleaching with ozone produced statistically similar results to the groups using hydrogen peroxide; however, studies show that hydrogen peroxide gel has a more powerful whitening effect than ozone and that ozone does not have a synergistic effect when used simultaneously with hydrogen peroxide. It should be considered that bleaching can lead to dental sensitivity—in particular, when using hydrogen peroxide—but it seems that ozone, alone or after hydrogen peroxide use, can reduce tooth sensitivity. In this regard, bleaching with HP has been reported to induce tooth sensitivity [52]; therefore, this aspect should be considered when testing bleaching materials. These findings are confirmed by more recent in vitro and clinical studies [56,57], indicating that ozone could offer a viable alternative to hydrogen peroxide, both in terms of outcomes and in terms of dental sensitivity.
A limitation of the present study is related to its in vitro design, which does not take into account other factors that may occur in the oral cavity and influence the clinical discoloration of the resin (e.g., pH of the oral cavity, composition and amount of saliva, etc.). A single composite was used, corresponding to a single shade. It should also be considered that Easyshade (Vita Zahnfabrik, Bad Säckingen, Germany) is a clinical device and is generally not recommended for in vitro testing [58]. Moreover, other external agents may affect the colorimetric stability of resin composites, and the bleaching effect of ozone may differ between them. Further research addressing these issues is needed to clarify the potential for ozone to bleach both enamel and dental composite resins, as well as other materials commonly used in dental clinical practice. In addition, it could be valuable to understand if ozone might show a different outcome based on specific composites used for different clinical purposes, such as flow composites, bulk composites, etc. The same can be said considering other staining materials and different time intervals and durations of application. Testing a greater number of materials with similar compositions would be suitable to determine which components of composite materials may influence discoloration. Finally, to overcome in vitro limitations, clinical studies evaluating the bleaching efficacy of ozonated materials are certainly needed, as is the evaluation of other delivery methods, such as ozone gas; the lack of long-term durability assessment of ozone’s bleaching effect should be solved using well-designed studies with appropriate follow-ups. Another aspect that should be taken into consideration is the possibility of the alteration of the bond strength of dentin after ozone application, which could compromise the survival rate of composite restorations—in particular, Black’s Class V restorations [59]. At last, consideration of additional parameters for measuring color in dentistry, such as the Whiteness Index for Dentistry (WID), would be desirable [60], together with SEM/EDS and roughness profile analysis.
5. Conclusions
The results of the present study highlighted that the tested ozonized gel exerted a bleaching activity on composite samples, as higher ΔE00 values were found in O3 gel groups, even though with no significant differences. Further evaluations will consider additional staining substances, different composite resins and staining protocols.
Author Contributions
Conceptualization, A.S. and C.P.; methodology, A.S. and C.P.; software, A.S.; validation, P.Z.; formal analysis, M.C. and A.S.; investigation, J.T.; data curation, A.S. and J.T.; writing—original draft preparation, S.G.; writing—review and editing, A.S. and M.P.; visualization, C.P.; supervision, P.Z.; project administration, C.P. and M.C.; funding acquisition, C.P. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
All the data are available within the manuscript.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Owens, B.M. Replacement and initial placement of tooth colored restorations: A review and discussion. J. Tenn. Dent. Assoc. 1998, 78, 26–29. [Google Scholar]
- Hickel, R.; Heidemann, D.; Staehle, H.J.; Minnig, P.; Wilson, N.H. Direct composite restorations: Extended use in anterior and posterior situations. Clin. Oral. Investig. 2004, 8, 43–44. [Google Scholar] [PubMed]
- Janda, R.; Roulet, J.F.; Kaminsky, M.; Steffin, G.; Latta, M. Color stability of resin matrix restorative materials as a function of the method of light activation. Eur. J. Oral Sci. 2004, 112, 280–285. [Google Scholar] [CrossRef] [PubMed]
- Stober, T.; Gilde, H.; Lenz, P. Color stability of highly filled composite resin materials for facings. Dent. Mater. 2001, 17, 87–94. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.K.; Lim, B.S.; Rhee, S.H.; Yang, H.C.; Powers, J.M. Color and translucency of A2 shade composite resins after curing, polishing and thermocycling. Oper. Dent. 2005, 30, 436–442. [Google Scholar]
- HashemiKamangar, S.S.; Jafari, S.; Rouhaninasab, M. Effects of curing time and intensity and polishing technique on color stability of bleach-shade composite resins. Dent. Res. J. (Isfahan) 2023, 20, 67. [Google Scholar] [CrossRef]
- Schulze, K.A.; Marshall, S.J.; Gansky, S.A.; Marshall, G.W. Color stability and hardness in dental composites after accelerated aging. Dent. Mater. 2003, 19, 612–619. [Google Scholar] [CrossRef]
- Farah, R.I.; Elwi, H. Spectrophotometric evaluation of color changes of bleach-shade resin-based composites after staining and bleaching. J. Contemp. Dent. Pract. 2014, 15, 587–594. [Google Scholar] [CrossRef]
- de Gee, A.J.; ten Harkel-Hagenaar, E.; Davidson, C.L. Color dye for identification of incompletely cured composite resins. J. Prosthet. Dent. 1984, 52, 626–631. [Google Scholar] [CrossRef]
- Gül, P.; Harorli, O.T.; Akgül, N.; Gündoğdu, M. Effect of different bleaching applications on the surface properties and staining susceptibility of dentalcomposites. J. Wuhan. Univ. Technol. Mater. Sci. Ed. 2016, 31, 677–683. [Google Scholar] [CrossRef]
- Borges, A.B.; Marsilio, A.L.; Pagani, C.; Rodrigues, J.R. Surface roughness of packable composite resins polished with various systems. J. Esthet. Restor. Dent. 2004, 16, 42–47. [Google Scholar] [CrossRef]
- Guler, A.U.; Yilmaz, F.; Kulunk, T.; Guler, E.; Kurt, S. Effects of different drinks on stainability of composite resin provisional restorative materials. J. Prosthet. Dent. 2005, 94, 118–124. [Google Scholar] [CrossRef] [PubMed]
- Bagheri, R.; Burrow, M.F.; Tyas, M. Influence of food simulating solutions and surface finish on susceptibility to staining of aesthetic restorative materials. J. Dent. 2005, 33, 389–398. [Google Scholar] [CrossRef]
- Vidal, M.L.; Pecho, O.E.; Collares, K.; Brandeburski, S.; Bona, A.D. Color Change of Resin-based Composites After In Vitro Bleaching Protocols: A Systematic Review and Meta-analysis. Oper. Dent. 2022, 47, 149–162. [Google Scholar] [CrossRef]
- Barczyk, I.; Masłyk, D.; Walczuk, N.; Kijak, K.; Skomro, P.; Gronwald, H.; Pawlak, M.; Rusińska, A.; Sadowska, N.; Gronwald, B.; et al. Potential Clinical Applications of Ozone Therapy in Dental Specialties—A Literature Review, Supported by Own Observations. Int. J. Environ. Res. Public Health 2023, 20, 2048. [Google Scholar] [CrossRef] [PubMed]
- Moureu, S.; Violleau, F.; Ali Haimoud-Lekhal, D.; Calmon, A. Ozonation of sunflower oils: Impact of experimental conditions on the composition and the antibacterial activity of ozonized oils. Chem. Phys. Lipids 2015, 186, 79–85. [Google Scholar] [CrossRef] [PubMed]
- Scribante, A.; Gallo, S.; Pascadopoli, M.; Soleo, R.; Di Fonso, F.; Politi, L.; Venugopal, A.; Marya, A.; Butera, A. Management of Periodontal Disease with Adjunctive Therapy with Ozone and Photobiomodulation (PBM): A Randomized Clinical Trial. Photonics 2022, 9, 138. [Google Scholar] [CrossRef]
- Sen, S.; Sen, S. Ozone therapy a new vista in dentistry: Integrated review. Med. Gas Res. 2020, 10, 189–192. [Google Scholar] [CrossRef]
- Alsakr, A.; Gufran, K.; Alqahtani, A.S.; Alasqah, M.; Alnufaiy, B.; Alzahrani, H.G.; Alahmari, A.A.; Alhumaidani, F.K.; Alhumaidani, R.K.; Althobiti, M.J. Ozone Therapy as an Adjuvant in the Treatment of Periodontitis. J. Clin. Med. 2023, 12, 7078. [Google Scholar] [CrossRef]
- Scribante, A.; Gallo, S.; Pascadopoli, M.; Frani, M.; Butera, A. Ozonized gels vs chlorhexidine in non-surgical periodontal treatment: A randomized clinical trial. Oral Dis. 2024, 30, 3993–4000. [Google Scholar] [CrossRef]
- Choudhary, A.; Rajasekar, A. Efficacy of Ozonated Olive Oil Gel in the Management of Peri-Implant Mucositis. J. Long. Term. Eff. Med. Implants. 2024, 34, 69–73. [Google Scholar] [CrossRef] [PubMed]
- Santos, G.M.; Pacheco, R.L.; Bussadori, S.K.; Santos, E.M.; Riera, R.; de Oliveira Cruz Latorraca, C.; Mota, P.; Benavent Caldas Bellotto, E.F.; Martimbianco, A.L.C. Effectiveness and Safety of Ozone Therapy in Dental Caries Treatment: Systematic Review and Meta-analysis. J. Evid. Based Dent. Pract. 2020, 20, 101472. [Google Scholar] [CrossRef]
- Makeeva, M.K.; Daurova, F.Y.; Byakova, S.F.; Turkina, A.Y. Treatment of an Endo-Perio Lesion with Ozone Gas in a Patient with Aggressive Periodontitis: A Clinical Case Report and Literature Review. Clin. Cosmet. Investig. Dent. 2020, 12, 447–464. [Google Scholar] [CrossRef] [PubMed]
- Suh, Y.; Shrey, P.; Re, K.; Gandhi, J.; Joshi, G. Clinical utility of ozone therapy in dental and oral medicine. Med. Gas. Res. 2019, 9, 163–167. [Google Scholar]
- Berezow, A.B.; Darveau, R.P. Microbial shift and periodontitis. Periodontology 2000 2011, 55, 36–47. [Google Scholar] [CrossRef]
- El Meligy, O.A.; Elemam, N.M.; Talaat, I.M. Ozone Therapy in Medicine and Dentistry: A Review of the Literature. Dent. J. 2023, 11, 187. [Google Scholar] [CrossRef]
- Abd Elhamid, M.; Mosallam, R. Effect of bleaching versus repolishing on colour and surface topography of stained resin composite. Aust. Dent. J. 2010, 55, 390–398. [Google Scholar] [CrossRef] [PubMed]
- Gallo, S.; Colombo, M.; Poggio, C.; Scribante, A.; Saracino, M.; Beltrami, R. Bleaching Effect of Ozonized Substances on Resin Composite: A New Potentiality for Ozone Therapy in Dentistry. Appl. Sci. 2023, 13, 2149. [Google Scholar] [CrossRef]
- Agnihotry, A.; Gill, K.S.; Singhal, D.; Fedorowicz, Z.; Dash, S.; Pedrazzi, V. A comparison of the bleaching effectiveness of chlorine dioxide and hydrogen peroxide on dental composite. Braz. Dent. J. 2014, 25, 524–527. [Google Scholar] [CrossRef]
- Rode, K.M.; Kawano, Y.; Turbino, M.L. Evaluation of curing light distance on resin composite microhardness and polymerization. Oper. Dent. 2007, 32, 571–578. [Google Scholar] [CrossRef]
- Zorzin, J.; Maier, E.; Harre, S.; Fey, T.; Belli, R.; Lohbauer, U.; Petschelt, A.; Taschner, M. Bulk-fill resin composites: Polymerization properties and extended light curing. Dent. Mater. 2015, 31, 293–301. [Google Scholar] [CrossRef] [PubMed]
- Poggio, C.; Lombardini, M.; Gaviati, S.; Chiesa, M. Evaluation of Vickers hardness and depth of cure of six composite resins photo-activated with different polymerization modes. J. Conserv. Dent. 2012, 15, 237. [Google Scholar] [CrossRef] [PubMed]
- Alrahlah, A.; Silikas, N.; Watts, D.C. Post-cure depth of cure of bulk fill dental resin composites. Dent. Mater. 2014, 30, 149–154. [Google Scholar] [CrossRef] [PubMed]
- Luo, M.R.; Cui, G.; Rigg, B. The development of the CIE 2000 colour-difference formula: CIEDE2000. Color. Res. Appl. 2001, 26, 340–350. [Google Scholar] [CrossRef]
- Randi, C.J.; Heiderich, C.M.C.; Serrano, R.V.; Morimoto, S.; de Moraes, L.O.C.; Campos, L.; Palma, L.F. Use of Ozone Therapy in Implant Dentistry: A Systematic Review. Oral. Maxillofac. Surg. 2024, 28, 39–49. [Google Scholar] [CrossRef]
- Kumar, A.; Srivastava, A.; Sah, N.; Sah, S.; Nair, V.I.N.; Das, A.; Singh, A.K. Evidence-Based Effectiveness of Ozone Therapy in the Treatment for Oral Lichen Planus—A Systematic Review. Natl. J. Maxillofac. Surg. 2024, 15, 18–22. [Google Scholar] [CrossRef]
- Liu, J.; Huang, Y.; Huang, J.; Yang, W.; Tao, R. Effects of Ozone Therapy as an Adjuvant in the Treatment of Periodontitis: A Systematic Review and Meta-Analysis. BMC Oral Health 2025, 25, 335. [Google Scholar] [CrossRef]
- Colombo, M.; Gallo, S.; Garofoli, A.; Poggio, C.; Arciola, C.R.; Scribante, A. Ozone Gel in Chronic Periodontal Disease: A Randomized Clinical Trial on the Anti-Inflammatory Effects of Ozone Application. Biology 2021, 10, 625. [Google Scholar] [CrossRef]
- Johnston, W.M. Color Measurement in Dentistry. J. Dent. 2009, 37 (Suppl. 1), e2–e6. [Google Scholar] [CrossRef]
- Talic, N.F.; Almudhi, A.A. The effect of dietary pigmentation on the esthetic appearance of clear orthodontic elastomeric modules. J. Orthod. Sci. 2016, 5, 70–73. [Google Scholar]
- Ludovichetti, F.S.; Zerman, N.; Stellini, E.; Zambon, G.; Mazzoleni, S.; Zuccon, A. Dental Bleaching: Patient Perception and Satisfaction. Minerva Dent. Oral Sci. 2024, 73, 217–223. [Google Scholar] [CrossRef] [PubMed]
- Demirkol, D.; Aksu, S.; Çalışkan, S.; Tüloğlu, N. Evaluation of pediatric dentists’ knowledge and approaches to tooth discoloration. J. Clin. Pediatr. Dent. 2024, 48, 129–135. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez-Chavez, J.A.; Soto-Barreras, U.; Perez-Aguirre, B.; Nevarez-Rascon, M.; Villegas-Mercado, C.E.; Dominguez-Perez, R.A. Reliability of Dental Shade Selection Methods: Agreement among Spectrophotometer, Intraoral Scanner, and Cross-Polarization Photography. J. Esthet. Restor. Dent. 2025, in press. [CrossRef] [PubMed]
- Joiner, A. Tooth color: Review of the literature. J. Dent. 2004, 32, 3–12. [Google Scholar] [CrossRef]
- O’Brien, W.J. Dental Materials and Their Selection, 3rd ed.; Quint Pub Inc.: Chicago, IL, USA, 2002; p. 28. [Google Scholar]
- International Commission on Illumination. Colorimetry: Official Recommendations of the Interntional Commission on Illumination, 2nd ed.; Bureau Central de la CIE: Vienna, Austria, 1986. [Google Scholar]
- Pereira-Lores, P.; Gancedo-Gancedo, T.; Martín-Biedma, B.; Varela-Aneiros, I.; Dablanca-Blanco, A.B.; Villasenín-Sánchez, C.; Martín-González, J.; Alonso de la Peña, V.; Castelo-Baz, P. Is At-Home Bleaching More Effective on the Upper Arch than the Lower Arch? A Prospective Cohort Study. J. Dent. 2025, 157, 105729. [Google Scholar] [CrossRef]
- Peker, O.; Bolgul, B. Evaluation of surface roughness and color changes of restorative materials used with different polishing procedures in pediatric dentistry. J. Clin. Pediatr. Dent. 2023, 47, 72–79. [Google Scholar] [CrossRef] [PubMed]
- Hashemikamangar, S.S.; Farahani, S.; Khoshgoo, S.; Doroudgar, P. Comparative Efficacy of Four Stain Removal Methods for Bleach-Shade Composite Resins after Immersion in Staining Solutions: An In Vitro Study. Int. J. Dent. 2023, 2023, 8909288. [Google Scholar] [CrossRef]
- Dietrich, L.; de Assis Costa, M.D.M.; Blumenberg, C.; Nascimento, G.G.; Paranhos, L.R.; da Silva, G.R. A meta-analysis of ozone effect on tooth bleaching. Sci. Rep. 2021, 11, 13177. [Google Scholar] [CrossRef]
- Fidan, M.; Çankaya, N. Effect of food-simulating liquids and polishing times on the color stability of microhybrid and nanohybrid resin composites. Discov. Nano. 2025, 20, 43. [Google Scholar] [CrossRef]
- Zupo, R.; Castellana, F.; De Nucci, S.; Dibello, V.; Lozupone, M.; Giannelli, G.; De Pergola, G.; Panza, F.; Sardone, R.; Boeing, H. Beverages Consumption and Oral Health in the Aging Population: A Systematic Review. Front. Nutr. 2021, 8, 762383. [Google Scholar] [CrossRef]
- Alkahtani, R.; Stone, S.; German, M.; Waterhouse, P. A Review on Dental Whitening. J. Dent. 2020, 100, 103423. [Google Scholar] [CrossRef]
- Alqahtani, M.Q. Tooth-Bleaching Procedures and Their Controversial Effects: A Literature Review. Saudi Dent. J. 2014, 26, 33–46. [Google Scholar] [CrossRef] [PubMed]
- Carvalho, R.R.; Carlos, N.R.; Campos, F.U.; Turssi, C.P.; Vieira Júnior, W.F.; Amaral, F.L.D.; Basting, R.T. Ozone Gas Therapy for Tooth Bleaching Preserves Enamel Microhardness, Roughness, and Surface Micromorphology. Acta Odontol. Latinoam. 2023, 36, 15–23. [Google Scholar] [CrossRef] [PubMed]
- Bin Hassan, S.A. Tooth Sensitivity Following Hydrogen Peroxide Bleaching with and without Ozone: A Randomized Controlled Trial: Tooth Sensitivity Following H2O2 versus H2O2/Ozone Bleaching. Pain. Res. Manag. 2024, 2024, 2695533. [Google Scholar] [CrossRef] [PubMed]
- Erdem, R.Z.; Çellik, Ö. Investigation of the Bleaching Efficiencies of Different Office-Type Bleaching Techniques and the Changes Caused on the Enamel Surface. Lasers Med. Sci. 2023, 38, 211. [Google Scholar] [CrossRef]
- Akl, M.A.; Sim, C.P.C.; Nunn, M.E.; Zeng, L.L.; Hamza, T.A.; Wee, A.G. Validation of Two Clinical Color Measuring Instruments for Use in Dental Research. J. Dent. 2022, 125, 104223. [Google Scholar] [CrossRef]
- Santos, M.; Leandro, F.; Barroso, H.; Delgado, A.H.S.; Proença, L.; Polido, M.; Vasconcelos e Cruz, J. Antibacterial Effect of Ozone on Cariogenic Bacteria and Its Potential Prejudicial Effect on Dentin Bond Strength—An In Vitro Study. Pharmaceutics 2024, 16, 614. [Google Scholar] [CrossRef]
- Pérez Mdel, M.; Ghinea, R.; Rivas, M.J.; Yebra, A.; Ionescu, A.M.; Paravina, R.D.; Herrera, L.J. Development of a customized whiteness index for dentistry based on CIELAB color space. Dent. Mater. 2016, 32, 461–467. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).