Abstract
This study investigated the effect of different monomer compositions of acrylonitrile (AN) and methyl methacrylate (MMA) on the synthesis and expansion performance of thermally expandable microspheres (TEMs). TEMs with different monomer ratios, specifically AN to MMA ratios of 100:0, 90:10, 80:20, and 70:30, were synthesized via free radical suspension polymerization. The inner morphology, crystallinity, blowing agent encapsulation efficiency, and expansion ratio of the microspheres were analyzed using scanning electron microscopy (SEM), Fourier-transform infrared spectroscopy (FTIR), thermogravimetric analysis (TGA), and other characterization techniques. The results showed that as the MMA content and reaction time increased, the internal structure of the microsphere shell became more uniform, and its thickness increased. Notably, the P(AN:MMA)(90:10) microspheres exhibited the lowest expansion temperature and the highest expansion ratio. This study provides a theoretical basis for the further optimization of TEM synthesis processes.
1. Introduction
Thermally expandable microspheres (TEMs) consist of a thermoplastic polymer shell encapsulating a core of low-boiling-point alkane (blowing agent), with particle sizes typically ranging from 5 to 100 μm. During heating, two pivotal transitions occur: the thermoplastic shell undergoes softening, while the encapsulated core material vaporizes. The internal pressure generated by the gasified blowing agent drives the outward expansion of the softened shell layer, ultimately inducing microsphere inflation, as illustrated in Figure 1. Upon cooling, the expanded structure remains stabilized, with its density dramatically decreasing from 1100 kg/m3 to approximately 20–30 kg/m3 [1]. TEMs have demonstrated exceptional utility across multiple applications, including weight reduction [2,3,4], detachable adhesives [5], coatings [6], thermal insulation [7,8,9], and textiles [10].
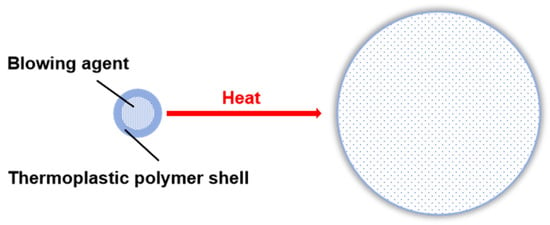
Figure 1.
Expansion of unexpanded microspheres under the application of heat.
TEMs are synthesized via suspension polymerization [11,12,13]. Since their initial disclosure by the Dow Chemical Company [14], extensive investigations have focused on optimizing synthesis parameters such as monomer combinations, blowing agent selection, crosslinker ratios, and stirring rates. Changing the monomer combination of the polymer shell during synthesis is a critical method to obtain TEMs with different properties. Yasuhiro Kawaguchi et al. [15] investigated the optimal polymer shell monomer composition of acrylonitrile/methacrylonitrile/vinyl acetate (AN/MAN/VAc) and the effects of crosslinker on expansion performance. In another study [16], Yasuhiro Kawaguchi et al. investigated the effect of chemical structure on the heat resistance of microspheres. Seven derivatives of methacrylic acid (MAA), along with MAA itself and acrylic acid (AA), which are nitrile-free monomers, were individually synthesized into TEMs with AN and MAN. The results showed that the addition of polymer monomers with relatively strong intermolecular forces and high Tg values, such as MAA, was suitable for the formation of heat-resistant microspheres. Kim et al. [11] investigated the AN/methyl methacrylate (MMA)/MAA system, which is a group of monomer combinations with a better expansion performance, after using a variety of monomer combinations. They started with the AN/methyl acrylate (MA) system to study the performance of microspheres at different ratios and confirmed that the mass ratio of AN:MA = 1:1 had the optimal yield and performance. This mass ratio was used to study TEMs synthesis with AN with ethyl acrylate (EA), butyl acrylate (BA), MMA, ethyl methacrylate (EMA), butyl methacrylate (BMA), acryl amide (AM), ethyl vinyl ether (EVE), butyl vinyl ether (BVE), AA, and MAA, respectively. However, only a few monomer combinations could form TEMs. Jasmine C. Gomez et al. [17] reported the incorporation of 2-hydroxyethyl methacrylate (HEMA) into TEM formulations. The synthesized AN/MMA/HEMA-based TEMs had a starting expansion temperature around 90 °C through changing the blowing agent. In a recent study, Maryam Mousa et al. [18] investigated α-methylene γ-valerolactone (MeMBL), a biobased monomer with a similar structure to MMA, to synthesize TEMs using either AN or MMA monomers. The synthesis of TEMs using MeMBL alone resulted in the poor formation of core–shell structures, but in conjunction with either AN or MMA, the resulting TEMs demonstrated some expansion properties.
The TEM synthesis process involves the dispersion of oil-phase droplets containing blowing agent, monomers, crosslinkers, and initiators within an aqueous suspension medium stabilized by suspending agents. Each droplet acts as an isolated micro-reactor where polymerization proceeds. As polymerization advances, polymeric material precipitates and accumulates at the water/oil interface, forming a triphasic water/oil/polymer system. The successful encapsulation of the blowing agent critically depends on the formation of a continuous polymeric shell at this interface. Notably, not all monomer combinations could form a uniform core–shell structure.
The formation of a core–shell structure is the most crucial step in the synthesis of thermally expandable microspheres. On the one hand, the formation of the core–shell structure is a necessary condition for the successful encapsulation of the blowing agent; on the other hand, the uniformity of the core–shell structure is essential for efficient foaming. Although numerous studies on core–shell structure formation provide evidence that the morphology of the shell layer is diverse, no specific analysis or discussion has been conducted on the factors influencing the internal morphology of AN-MMA-based TEMs. As mentioned earlier, TEM shells commonly employ monomers including AN, MAN, vinylidene chloride (VAC), and MMA, and MAA as an auxiliary component; systematic research on the widely used AN/MMA monomer system remains limited, despite existing reports on the core–shell formation mechanisms of MMA/styrene (MMA/St) [19], VDC/AN/MMA [20], and AN/MAN systems [1].
While numerous studies have explored TEM synthesis through the incorporation of third or fourth monomers into AN/MMA-based systems, fundamental investigations specifically focused on the core–shell formation mechanisms in binary AN/MMA systems remain scarce. In this study, we investigated the effects of monomer composition and reaction time on the internal morphology of TEMs and the encapsulation content of the blowing agent in the AN/MMA system. The aim was to obtain microspheres with a well-defined core–shell structure. Additionally, we evaluated the expansion ratio of the synthesized TEMs under different monomer compositions.
2. Experimental
2.1. Materials
Acrylonitrile (AN), 99%, was purchased from Energy Chemical (Shanghai, China). Isooctane (IO), 98%, polyvinylpyrrolidone K30 (PVP K30), 99.8%, and sodium chloride, ≥99.5%, were purchased from Sinopharm Chemical Reagent Co., Ltd. (Shanghai, China). Methyl methacrylate (MMA), >99.5%, stabilized with 30 ppm hydroquinone monomethyl ether; ethylene glycol dimethacrylate (EGDMA), 98%, stabilized with 90–110 ppm hydroquinone monomethyl ether; sodium nitrite (NaNO2), 99% metals basis; and 2,2′-azobis(2-methylpropionitrile), 98%, were purchased from Shanghai Macklin Biochemical Technology Co., Ltd. (Shanghai, China). Colloidal silica was purchased from Guangzhou Jinsheng Chemical Industries Co., Ltd. (Guangzhou, China) All chemicals were used as received.
2.2. Suspension Polymerization
Preparation of the oil phase: In a beaker, 13.6 g of total monomers (AN and MMA), 0.0544 g of EGDMA, 0.136 g of AIBN, and 3.4 g of IO was mixed to prepare the oil phase. The mass ratios of AN to MMA used in the experiment were 100:0, 90:10, 85:15, 80:20, and 70:30.
Preparation of the water phase: In another beaker, 0.0708 g of PVP K30 (36 wt% aqueous solution), 50 g of deionized water, 18 g of NaCl, 0.0136 g of NaNO2, and 5.66 g of colloidal silica (30 wt%) was mixed to prepare the water phase.
Polymerization: The oil phase was then poured into the water phase. The mixture was emulsified using a high-speed homogenizer (FJ200-SH, Shanghai Specimen Model Factory) at 9000 rpm for 3 min. Polymerization was carried out in a 150 mL flask at 63 °C, with mechanical stirring at 200–400 rpm for 15 h.
After the reaction, the mixture was cooled to room temperature and filtered. After drying in an oven at 50 °C for 24 h, the TEMs were separated by a 150-mesh sieve. Figure 2 is a schematic diagram of the preparation of the TEMs.
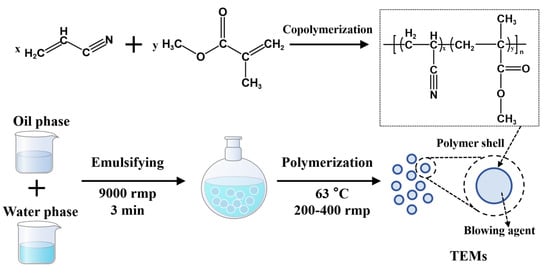
Figure 2.
Schematic diagram of preparation of TEMs.
2.3. Characterization
SEM (FE-SEM, Gemini 500, ZEISS, Oberkochen, Germany) was used to characterize the microscopic morphology of the TEMs. Gold spray was required to increase the conductivity of the sample before the test. Particles were molded into an epoxy matrix at room temperature for 24 h to enable studies of particle cross-sections. The cross-section was obtained by liquid nitrogen embrittlement. The elemental composition of the material surface was characterized by an energy-dispersive spectrometer (EDS, Ultim Max 170, Oxford Instruments, Oxford, UK).
The crystallinity of the material was obtained by X-ray diffraction (XRD, Rigaku Smartlab9KW, Rigaku Corporation, Tokyo, Japan). The TEMs were placed on a glass plate, and a Cu Kα radiation source was employed for the testing at a voltage of 40 kV and a current of 150 mA, with a scanning range of 2θ between 5–70°. The crystallinity Xc of the polymer is usually calculated by the following formula:
where Ac represents the area of the crystallization region, and Aa represents the area of the amorphous region.
A Fourier-transform infrared spectrometer (FTIR, Bruker TensorII, Bruker Corporation, Karlsruhe, Germany) revealed the molecular structure and chemical composition. At room temperature, the sample was processed by the potassium bromide pellet method, and the test range was 4000–400 cm−1.
The thermal gravimetric analysis (TGA) was conducted on a PerkinElmer TGA8000 (PerkinElmer, Waltham, MA, USA) to determine blowing agent content. Under a N2 atmosphere, the sample was heated from 50 to 350 °C at 10 °C/min. To eliminate the influence of residual moisture, the content of the blowing agent was defined as the mass loss between 110 °C and the subsequent plateau region in the thermogravimetric curve. The encapsulation ratio of the blowing agent was defined as the ratio between the weight of the blowing agent encapsulated in the microspheres and the weight of the microspheres. The encapsulation efficiency was defined as the percentage of the amount of blowing agent encapsulated in the microspheres to the amount of blowing agent actually added.
The maximum volume expansion ratio of microspheres was measured with a graduated cylinder. The unexpanded sample was added to the cylinder, and the volume of the sample was recorded as V1 (mL). The measuring cylinder containing the sample was heated to 200 °C in an oil bath and kept there for 1 min to ensure that the microspheres fully expanded. The volume of the expanded sample in the cylinder was recorded as V2 (mL). Obviously, the value of V2/V1 was the maximum volume expansion ratio (Vv)
A polarizing optical microscope (POM, Axio Scope A1 pol, ZEISS, Oberkochen, Germany) was employed to obtain the starting expansion temperature (Tstart). Tstart is the temperature at which the volume of the microspheres begins to change.
A laser particle size analyzer (LPSA, Bettersize2600E, Bettersize Instruments, Dandong, China) was used to measure the particle size and particle size distribution of the TEMs using the wet method. Particle size distribution was represented by Span, calculated as Span = (D90 − D10)/D50, where D90, D50, and D10 represented the particle size of the sample when the percentage of cumulative particle size distribution reached 90%, 50%, and 10%, respectively. D50, the median particle size, was used to represent the sample’s particle size.
Differential scanning calorimetry (DSC) measurements were performed using a PerkinElmer DSC8000 (PerkinElmer, Waltham, MA, USA). TEMs required the removal of the blowing agent for the DSC analysis. The DSC results were derived from TEM samples compressed under a hydraulic press at 20 MPa for 1 min and subsequently dried in an oven at 60 °C for 24 h. About 2–4 mg of the sample was placed into a DSC pan and sealed with an aluminum lid, then compacted under pressure. Under a nitrogen flow, the sample was maintained at 30 °C for 5 min and then heated to 130 °C at 10 °C/min.
3. Results and Discussion
3.1. Chemical Structure of TEMs
An FTIR is conducted to analyze the chemical structures of the TEMs synthesized with different monomer compositions, as shown in Figure 3. The absorption peak at 2244 cm−1 is attributed to the C≡N bond in the monomer AN. The absorption peak at 1730 cm−1, observed only in the spectra of curves (b), (c), and (d) of Figure 3, corresponds to the C=O bond in MMA [21,22]. This indicates that in the P(AN:MMA)(90:10), (80:20), and (70:30) samples, AN and MMA undergo copolymerization, and the polymer shell consists of the copolymer of AN and MMA. In contrast, the polymer shell of P(AN:MMA)(100:0) is composed solely of PAN.
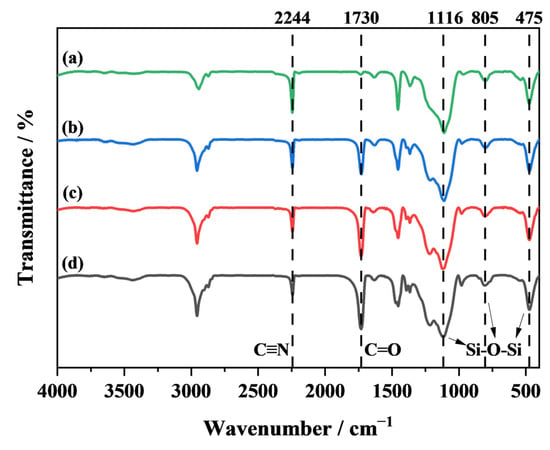
Figure 3.
FTIR spectra of (a) P(AN:MMA)(100:0), (b) P(AN:MMA)(90:10), (c) P(AN:MMA)(80:20), and (d) P(AN:MMA)(70:30).
Furthermore, in the FTIR, the intensity of the peaks is related to the relative content of the corresponding functional groups [23]. In curves (b), (c), and (d) of Figure 3, the characteristic peak for the C=O bond gradually becomes more intense relative to the C≡N bond. The ratios of transmittance of the characteristic peaks C=O/C≡N are shown in Table 1. This suggests that as the MMA monomer content increases, the relative content of MMA in the polymer shell also increases.

Table 1.
The particle size and distribution, transmittance ratio, and crystallinity of the thermally expandable microspheres.
It is worth noting that due to the use of colloidal silica as the suspending agent during polymerization, silica particles tend to adhere to the surface of the polymer shell after the reaction, making it difficult to completely remove them during the post-treatment process. As shown in the EDS of P(AN:MMA)(100:0) in Figure 4, a significant amount of the Si element is present on the polymer surface. Therefore, in Figure 3, curves (a), (b), (c), and (d), absorption peaks at 475 cm−1, 805 cm−1, and 1116 cm−1 are observed, which correspond to the stretching vibrations of the Si-O bond in the Si-O-Si fragments [24].
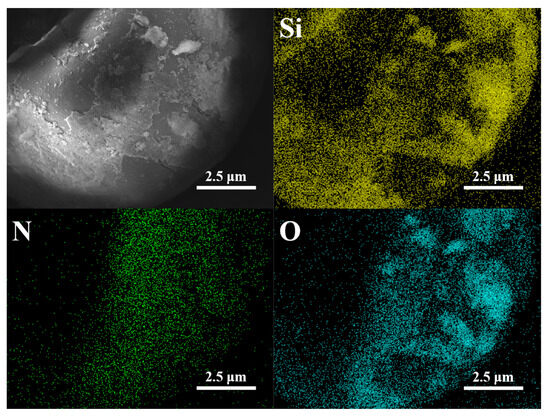
Figure 4.
SEM and EDS analysis of P(AN:MMA)(100:0).
3.2. Effect of the Feeding Monomer Composition on the Inner Surface Morphology of TEMs
In this section, we synthesize AN/MMA-based thermally expandable microspheres via free radical suspension polymerization using AN and MMA. The microspheres are encapsulated in epoxy resin, and TEM cross-sectional SEM images are obtained through liquid nitrogen embrittlement. The internal morphology of the core–shell structure is then analyzed.
At 63 °C, with a reaction time of 15 h, TEMs with a core–shell structure are successfully synthesized for all compositions: P(AN:MMA)(100:0), (90:10), (80:20), and (70:30). However, variations in monomer composition lead to changes in the physical properties of the polymer shell, resulting in differences in internal morphology. The characteristic internal morphologies of the microspheres under different monomer ratios are shown in Figure 5.
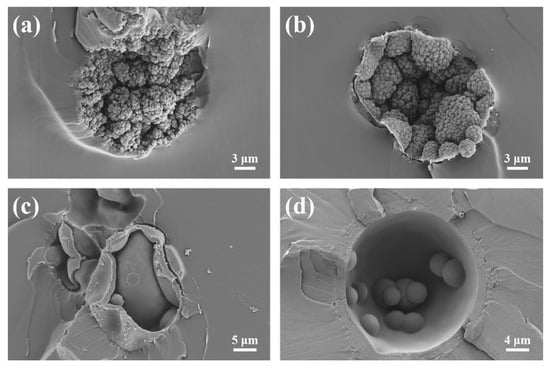
Figure 5.
SEM images of particle cross-sections of TEMs: (a) P(AN:MMA)(100:0), (b) P(AN:MMA)(90:10), (c) P(AN:MMA)(80:20), and (d) P(AN:MMA)(70:30).
When AN is used as the sole monomer, the core–shell structure is less distinct. The shell consists of tiny primary particles (<0.5 μm), consistent with observations by Jonsson et al. [1]. These primary particles coalesce, forming a cauliflower-like structure that extends toward the microsphere interior (Figure 5a). For P(AN:MMA)(90:10), a more defined core–shell structure is observed (Figure 5b), where the polymer shell is composed of numerous particles. The primary particles inside the microspheres grow in size and fuse into larger secondary particles (~2 μm).
With an increasing MMA content, P(AN:MMA)(80:20) and P(AN:MMA)(70:30) exhibit a thicker and more uniform polymer shell with a smoother inner surface. Simultaneously, the distinction between primary and secondary particles becomes less apparent, and the particle size increases (>2 μm) (Figure 5c,d).
Figure 6 illustrates the effect of different monomer feeds on the crystallinity of TEMs. The crystallinity (Xc) of TEMs under different monomer ratios is calculated using Equation (1), as shown in Table 1. During polymerization, the formed polymer precipitates out due to its low solubility in the reaction medium, a process known as precipitation polymerization [25,26]. Polyacrylonitrile (PAN) is insoluble in both AN monomer and water, indicating that PAN polymerization follows a precipitation mechanism when using water as the polymerization medium [27].
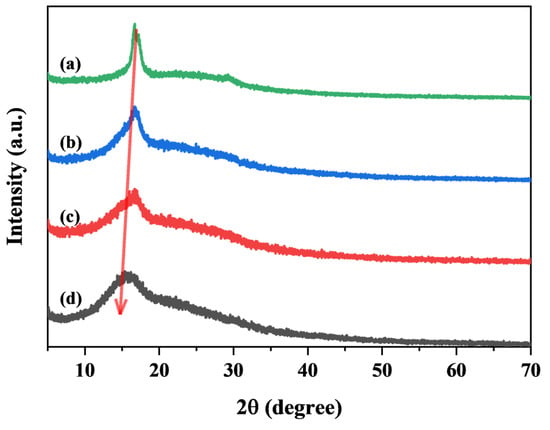
Figure 6.
XRD patterns of (a) P(AN:MMA)(100:0), (b) P(AN:MMA)(90:10), (c) P(AN:MMA)(80:20), and (d) P(AN:MMA)(70:30).
Notably, due to the strong polarity of the C≡N groups in PAN, PAN molecular chains tend to precipitate from the monomer in the form of polymer crystallites [28]. As shown in Figure 6, P(AN:MMA)(100:0) exhibits a sharp polymer crystallization peak at 2θ = 16.9°, corresponding to the (100) crystal plane, while a less pronounced peak at 2θ = 29.3° corresponds to the (110) crystal plane [29,30]. Since PAN is a semicrystalline polymer and does not swell during polymerization, the mobility of polymer chains is restricted. This explains why the shell of P(AN:MMA)(100:0) microcapsules appears rough, composed of uniform but non-smooth primary particles [31]. The limited chain mobility makes shell homogenization difficult, leading to the continuous stacking of semicrystalline primary particles upon contact, ultimately forming the cauliflower-like internal structure observed in the microspheres.
Polymethyl methacrylate (PMMA), on the other hand, is an amorphous polymer. As the MMA feed ratio increases from 0 wt% to 30 wt%, the crystallinity of P(AN:MMA) decreases significantly, dropping from 46.4% to 10.8%, as shown in Figure 6 and Table 1. The sharp crystallization peak at 16.9° gradually transitions into a broad amorphous peak. The bulky side groups of MMA introduce steric hindrance, reducing the regularity of PAN polymer chains and the content of polar functional groups, thereby lowering the crystallinity of the copolymer [32]. Additionally, the incorporation of MMA increases the lattice spacing of crystalline PAN, causing the peak at 2θ = 16.9° to shift toward smaller angles [33].
Unlike PAN, PMMA undergoes a different polymerization process. During suspension polymerization, the formed PMMA polymer can swell in the monomer [1]. With an increasing MMA content, the crystallinity of the P(AN:MMA) copolymer decreases, making the polymer more susceptible to swelling, which enhances the mobility of polymer chains. The combined effects of these factors lead to the formation of larger primary particles in P(AN:MMA)(90:10), (80:20), and (70:30) compared to P(AN:MMA)(100:0) and a smoother polymer inner wall.
Furthermore, increased polymer chain mobility facilitates particle fusion into the polymer shell, resulting in a progressively thicker microsphere shell. In P(AN:MMA)(70:30), the incorporation of particles into the shell is clearly visible (Figure 5d), which is a phenomenon consistent with that observed by Safajou-Jahankhanemlou et al. [34] in PMMA-based microspheres.
As shown in Figure 7, the incorporation of MMA not only reduces the crystallinity of the TEMs but also decreases the glass transition temperature (Tg) of the polymer shell. PAN typically exhibits two Tgs [35,36], whereas in P(AN:MMA)(100:0) TEMs, only a single Tg at 81.3 °C is observed, with no second Tg detected within the test temperature range (30–130 °C). This discrepancy may be attributed to the second Tg of P(AN:MMA)(100:0) exceeding the maximum DSC testing temperature (130 °C). Notably, as reported by Mousa et al. [18], the second Tg of PAN occurs at 150 °C. Upon the introduction of MMA, the structural regularity of the polymer chains is disrupted, and interchain interactions are weakened, collectively lowering the Tg to fall within the DSC testing temperature range. The Tg of P(AN:MMA)(90:10) is 103 °C, whereas the Tg of P(AN:MMA)(70:30) decreases to 91.3 °C. The reduced Tg enhances the segmental mobility of the polymer chains at the given polymerization temperature, thereby promoting the homogenization of the polymeric shell.
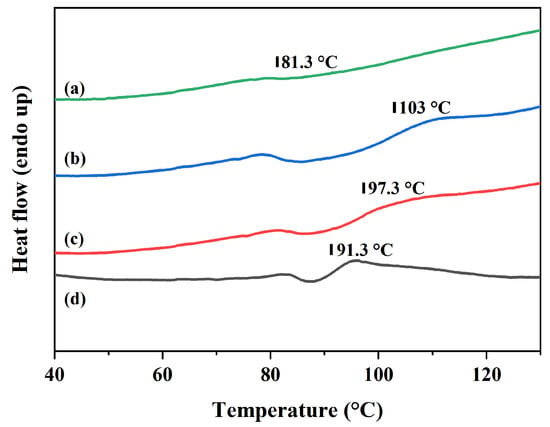
Figure 7.
DSC curves of (a) P(AN:MMA)(100:0), (b) P(AN:MMA)(90:10), (c) P(AN:MMA)(80:20), and (d) P(AN:MMA)(70:30).
It is noteworthy that the transition in the internal morphology of the TEMs occurs quite rapidly. To investigate this further, we conducted EDS point scanning on both the inner and outer surfaces of the shell (with the outer surface selected from a clean region), as shown in Figure 8 and Table 2.
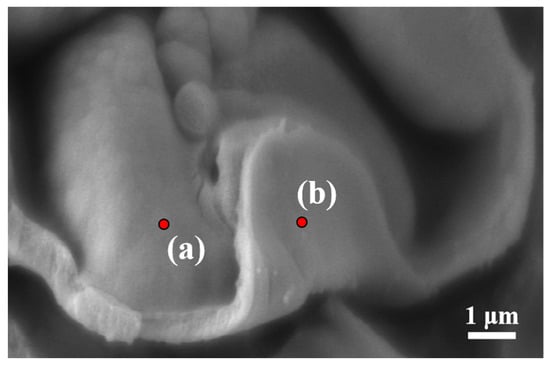
Figure 8.
SEM and EDS analysis of P(AN:MMA)(85:15) microspheres in different regions prepared at 63 °C, reaction time for 13 h: (a) inner surface, (b) outer surface.

Table 2.
Element composition of P(AN:MMA)(85:15) microspheres in different regions prepared at 63 °C, reaction time for 13 h.
The elemental composition of the TEMs differs between the surface and the interior. The oxygen content on the surface of the polymer shell is higher than that on the inner surface, while the nitrogen content on the outer surface is noticeably lower than that on the inner surface. This is due to the higher polymerization activity of MMA compared to AN. According to reports on the free radical copolymerization of AN and MMA [37,38,39,40], the reactivity ratios are reported as rAN = 0.16 and rMMA = 1.33 [41]. The AN radicals tend to copolymerize with MMA, while the MMA radicals prefer to homopolymerize with MMA [41,42]. As a result, a significant amount of MMA is synthesized on the outer surface of the polymer shell during the early stages of polymerization. The high PMMA content in the shell enhances the swelling of the shell by the monomer in the oil phase. This explains why the internal morphology of the shell changes so rapidly from P(AN:MMA)(100:0) to P(AN:MMA)(70:30). It also accounts for the increasing thickness of the microsphere shell with a higher MMA content.
3.3. Effect of the Reaction Time on the Inner Surface Morphology of TEMs
To investigate the effect of reaction time on the internal morphology of TEMs, the polymerization process of P(AN:MMA)(85:15) TEMs is studied. The optical microscope and SEM images are shown in Figure 9.
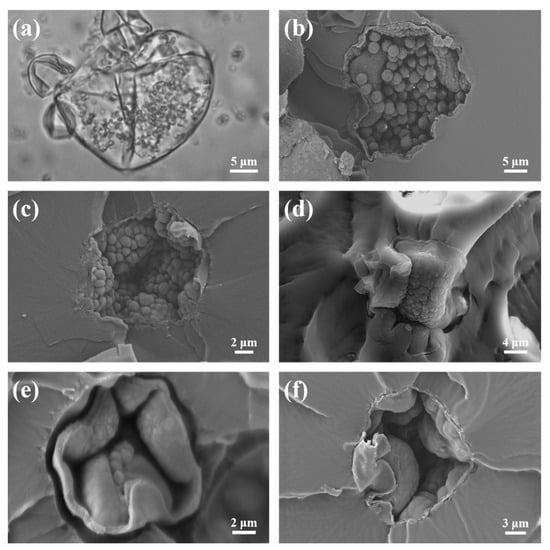
Figure 9.
POM and SEM images of the TEMs during the polymerization of P(AN:MMA)(85:15): (a) 3 h, (b) 7 h, (c) 9 h, (d) 11 h, (e) 13 h, and (f) 15 h.
After 3 h of reaction, as shown in Figure 9a, the microspheres display a clear core–shell structure. Under the optical microscope, it is evident that the polymer shell contains particles that precipitate from the oil phase. However, due to the relatively short reaction time, the viscosity of the polymer shell is high, and after drying, it is almost impossible to obtain microspheres that are independently distributed in powder form.
After 7 h of reaction, the viscosity of the polymer shell decreases, and through post-processing, independently distributed microspheres can be obtained. As shown in Figure 9b, unfused particles within the microsphere shell can be observed, which is consistent with the findings in Figure 9a. The inner surface of the shell exhibits small and uniform protrusions. These are the primary particles that precipitate from the oil phase and accumulate at the water/oil interface, forming the microsphere’s surface. As the reaction progresses, the particles inside the microspheres are swollen and continuously fuse into the shell, causing the diameter of the protrusions on the inner surface of the microsphere to increase and the inner surface to become rougher, as shown in Figure 9c.
The polymer shell, swollen by the monomer, gradually consumes the monomer, while the particles merge with each other. The surface of the polymer shell transitions from an uneven, rough state to a smooth and uniform one. During this process, the shell of the microspheres continues to thicken, becoming more uniform and smoother, as shown in Figure 9d–f. Ultimately, the microspheres form a closed structure with a smooth and uniform inner surface morphology.
3.4. Blowing Agent Encapsulation Content and Efficiency of TEMs
Through thermogravimetric analysis, we examine the encapsulation content of the blowing agent in microspheres with different monomer compositions, as shown in Figure 10. The encapsulation content and encapsulation efficiency of the blowing agent are listed in Table 3. P(AN:MMA)(90:10), (80:20), and (70:30) TEMs form clear shell structures, thus achieving a good blowing agent encapsulation, with encapsulation efficiencies all above 80%. Considering that the boiling point of IO is 99 °C, the temperature increase during the reaction causes some liquid IO to evaporate into the gas phase. Therefore, an encapsulation efficiency of no less than 80% is acceptable. However, the brittleness of pure PAN leads to the formation of many cracks on the P(AN:MMA)(100:0) microsphere surface, resulting in the poor encapsulation ability of the blowing agent, as shown in Figure 4.
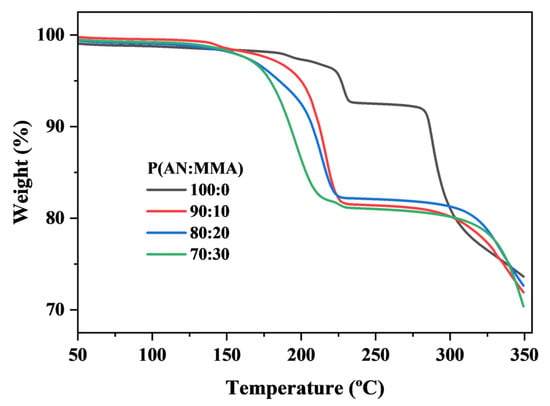
Figure 10.
TGA curve for P(AN:MMA) with different monomer compositions.

Table 3.
Encapsulation ratio and efficiency of isooctane in P(AN:MMA) microspheres synthesized with different monomer compositions.
3.5. Expansion Capacity of the TEMs.
Figure 11 shows digital photos of unexpanded and expanded thermally expandable microspheres under different monomer combinations. Table 4 lists the Tstart and expansion ratios of P(AN:MMA) microspheres under different monomer feed ratios. P(AN:MMA)(100:0) microspheres exhibit no expansion. Surprisingly, P(AN:MMA)(90:10) has the lowest expansion temperature and the highest expansion ratio (Vv = 4.4). In contrast, the expansion performance of P(AN:MMA)(80:20) and (70:30) gradually decreases. This is due to the increasing thickness of the shell, which restricts the expansion of the microspheres.

Figure 11.
Photos of different P(AN:MMA) microsphere samples before and after expansion. The top image shows the unexpanded microspheres; the bottom image shows the expanded microspheres.

Table 4.
The Tstart and expansion ratio of P(AN:MMA) microspheres under different monomer feed ratios.
3.6. The Internal Morphology Model of TEMs
Based on the above research results, the internal microstructure of TEMs is analyzed, and the process of internal morphology changes in microspheres with different monomer ratios is summarized. A model for the internal morphology of AN-MMA-based microspheres is established, as shown in Figure 12.
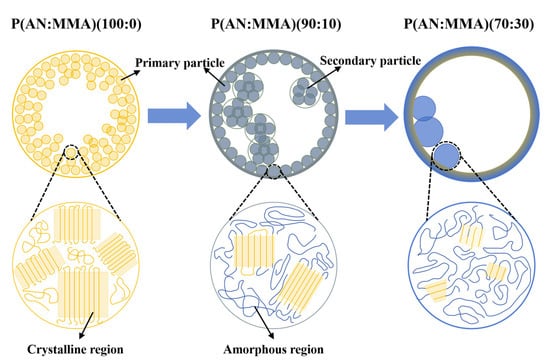
Figure 12.
The microstructure model of TEMs.
P(AN:MMA)(100:0) microspheres exhibit high crystallinity (Xc = 46.4%) and a high Tg (>130 °C). Additionally, PAN cannot be swollen by the monomers in the oil phase. These factors result in poor polymer chain mobility. A large number of primary particles precipitate and accumulate from the oil phase, forming a cauliflower-like structure, but the core–shell structure is unclear.
For P(AN:MMA)(90:10), the addition of MMA significantly reduces the copolymer crystallinity to 25.9% and lowers the Tg to 103 °C, leading to an increase in polymer chain mobility. The copolymer can be partially swollen by the monomer. The swollen primary particles merge to form a clear core–shell structure, but the inner wall still retains a particulate characteristic. The size of the primary particles increases, and they fuse with each other to form secondary particles.
Since the reactivity of MMA is higher than that of AN (r_AN = 0.16 and r_MMA = 1.33), for P(AN:MMA)(70:30), increasing the amount of MMA results in a thicker shell. Meanwhile, the copolymer crystallinity decreases to 10.8%, and the Tg drops to 91.3 °C. At the same time, the copolymer is more easily swollen by the monomer, and the distinction between primary and secondary particles disappears, resulting in a more uniform and smooth inner wall for the microsphere.
4. Conclusions
Suspension polymerizations of AN and MMA were used to prepare TEMs. We investigated the effect of different monomer compositions on the internal morphology and expansion performance of TEMs. The research found that monomer composition and reaction time significantly influenced the internal morphology of the microsphere shell. Additionally, monomer composition had a notable impact on the encapsulation efficiency of the blowing agent and the expansion performance. As the MMA content in the feed increased from 0% to 30%, the crystallinity of the TEMs decreased from 46.4% to 10.8%. Meanwhile, the glass transition temperature (Tg) of the polymer shell dropped from above 130 °C to 91.1 °C. The shell structure became more uniform and thicker, leading to an initial increase in expansion performance, followed by a subsequent decline. The internal morphology of the P(AN:MMA) (85:15) microspheres changed gradually from a cauliflower-like structure to a uniform and flat structure with the extension of the reaction time. P(AN:MMA) (90:10) microspheres exhibited the best expansion performance, with a Tstart of 137.4 °C and an expansion ratio of 4.4, which was closely related to their thinner shell structure. This study provides important experimental data and theoretical support for controlling the performance of thermally expandable microspheres, especially in the synthesis of core–shell structures.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/jcs9040163/s1, Figure S1: Particle size distribution curve of P(AN:MMA) TEMs.
Author Contributions
Conceptualization, D.Y. and Y.W. (Yanxiang Wang); methodology, D.Y. and Y.F.; software, B.D.; validation, D.Y.; formal analysis, J.G., H.J. and Y.W. (Yongbo Wang); investigation, D.Y., S.D., Y.S. and Y.W. (Yanxiang Wang); resources, S.D.; data curation, D.Y.; writing—original draft preparation, D.Y. and Y.F.; writing—review and editing, D.Y. and Y.W. (Yanxiang Wang); visualization, D.Y.; supervision, Y.W. (Yanxiang Wang) and H.J.; project administration, Y.W. (Yanxiang Wang). All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the Natural Science Foundation of Shandong Province (2024TSGC0550, 2023TSGC0545, 2023TATSGC025).
Data Availability Statement
The data that support the findings of this study are available on request from the corresponding author, Yanxiang Wang, upon reasonable request.
Acknowledgments
The authors thank the editor and the anonymous reviewers for their valuable comments on this manuscript. The authors also acknowledge the support of technical staff for assisting in preparing the samples and analyzing them.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Jonsson, M.; Nordin, O.; Malmström, E.; Hammer, C. Suspension polymerization of thermally expandable core/shell particles. Polymer 2006, 47, 3315–3324. [Google Scholar] [CrossRef]
- Kalia, K.; Francoeur, B.; Amirkhizi, A.; Ameli, A. In Situ Foam 3D Printing of Microcellular Structures Using Material Extrusion Additive Manufacturing. ACS Appl. Mater. Interfaces 2022, 14, 22454–22465. [Google Scholar] [CrossRef] [PubMed]
- Kalia, K.; Ameli, A. Understanding the process-microstructure-property relationships in material extrusion additive manufacturing of polylactic acid microcellular foams. Addit. Manuf. 2023, 72, 103636. [Google Scholar] [CrossRef]
- Kang, X.; Li, X.; Li, Y.; Duan, Y. Strengthening and toughening 3D printing of photocured resins by thermal expansion microspheres. J. Appl. Polym. Sci. 2022, 140, e53516. [Google Scholar] [CrossRef]
- Wanghofer, F.; Wolfberger, A.; Oreski, G.; Neumaier, L.; Schlögl, S. Investigation of expandable fillers for reversible adhesive bonding in photovoltaic modules. Int. J. Adhes. Adhes. 2023, 126, 103454. [Google Scholar] [CrossRef]
- Zhao, T.; Zhang, X.; Chang, G.; Li, R. Study of ZnO-coated Modified Hollow Microsphere Insulation Coated Fabrics. ChemistrySelect 2023, 8, e202300670. [Google Scholar] [CrossRef]
- Lamm, M.E.; Li, K.; Atchley, J.; Shrestha, S.S.; Mahurin, S.M.; Hun, D.; Aytug, T. Tailorable thermoplastic insulation foam composites enabled by porous-shell hollow glass spheres and expandable thermoplastic microspheres. Polymer 2023, 267, 125652. [Google Scholar] [CrossRef]
- Zhou, S.; Xu, Y.; Tang, J.; Qian, K.; Zhao, J.; Wang, J.; Gao, H.; Li, Z. Expansion force induced in situ formation of a 3D boron nitride network for light-weight, low-k, low-loss, and thermally conductive composites. J. Mater. Chem. A 2022, 10, 14336–14344. [Google Scholar] [CrossRef]
- Xu, Y.; Li, W.; Zhu, M.; Yue, X.; Wang, M. Novel porous fiber-based composites with excellent sound-absorbing and flame-retardant properties. J. Wood Chem. Technol. 2020, 40, 285–293. [Google Scholar] [CrossRef]
- Jiao, S.-Z.; Sun, Z.-C.; Li, F.-R.; Yan, M.-J.; Cao, M.-J.; Li, D.-S.; Liu, Y.; Li, L.-H. Preparation and Application of Conductive Polyaniline-Coated Thermally Expandable Microspheres. Polymers 2018, 11, 22. [Google Scholar] [CrossRef]
- Kim, H.T.; Jaladi, A.K.; Lee, Y.J.; An, D.K. Thermal Expansion Behavior of Thermally Expandable Microspheres Prepared by Suspension Polymerization Using P(AN-MMA-MAA) Core/Shell. Bull. Korean Chem. Soc. 2020, 41, 190–195. [Google Scholar] [CrossRef]
- Kim, H.T.; Jaladi, A.K.; Kim, J.H.; Gundeti, S.; An, D.K. Suspension Polymerization of Thermally Expandable Microspheres Using Cinnamonitrile and Diethyl Fumarate as Crosslinking Agents. Bull. Korean Chem. Soc. 2018, 40, 45–50. [Google Scholar] [CrossRef]
- Zhou, S.; Zhou, Z.; Xu, W.; Ma, H.; Ren, F.; Shen, H. Water as Blowing Agent: Preparation of Environmental Thermally Expandable Microspheres via Inverse Suspension Polymerization. Polym.-Plast. Technol. Eng. 2017, 57, 1026–1034. [Google Scholar] [CrossRef]
- Morehouse, D.S., Jr.; Tetreault, R.J. Expansible Thermoplastic Polymer Particles Containing Volatile Fluid Foaming Agent and Method of Making the Same. U.S. Patent 3,615,972, 26 October 1971. [Google Scholar]
- Kawaguchi, Y.; Oishi, T. Synthesis and properties of thermoplastic expandable microspheres: The relation between crosslinking density and expandable property. J. Appl. Polym. Sci. 2004, 93, 505–512. [Google Scholar] [CrossRef]
- Kawaguchi, Y.; Itamura, Y.; Onimura, K.; Oishi, T. Effects of the chemical structure on the heat resistance of thermoplastic expandable microspheres. J. Appl. Polym. Sci. 2005, 96, 1306–1312. [Google Scholar] [CrossRef]
- Gomez, J.C.; Vishnosky, N.S.; Grafstein, J.T.; Kim, S.T.; Steinhardt, R.C. Thermally expandable microspheres with high expansion ratios: Design of core and shell for largest size change. J. Appl. Polym. Sci. 2022, 139, e52517. [Google Scholar] [CrossRef]
- Mousa, M.; Jonsson, M.; Granbom, L.; Larsson Kron, A.; Malmström, E. Thermally expandable microspheres based on fully or partially bio-based polymers. J. Appl. Polym. Sci. 2024, 141, e55368. [Google Scholar] [CrossRef]
- Tian, S.-c.; Cao, J.-x.; Xie, G.m.; Wang, M.-w.; Shi, Y.-y.; Yi, Y.; Yang, C.-l.; Xiao, Y.-h.; Wei, X.-l.; Tian, B.-m.; et al. Study on preparation and process of poly(MMA-St) thermally expandablecore-shellmicrospheres. J. Appl. Polym. Sci. 2020, 138, 49927. [Google Scholar] [CrossRef]
- Xie, G.; Pan, P.; Bao, Y. Morphology and blowing agent encapsulation efficiency of vinylidene chloride copolymer microspheres synthesized by suspension polymerization in the presence of a blowing agent. J. Appl. Polym. Sci. 2016, 134. [Google Scholar] [CrossRef]
- Zhao, T.; Yang, H.; Xu, X.; Chang, G.; Li, R. Modification of Hollow Expanded Microspheres with Superior Thermal Insulation Properties and Their Applications. Chem.-Asian J. 2023, 18, e202201233. [Google Scholar] [CrossRef]
- Zhang, J.; Zhou, Y.; Huang, B.; Lv, S.; Ma, X.; Tang, J. Synthesis and characterization of high temperature resistant thermal expansion microsopheres with P(acrylonitrile/methacrylic acid/N,N-dimethylacrylamide/n-butylacrylate) shell. SN Appl. Sci. 2019, 1, 923. [Google Scholar] [CrossRef]
- Yi, Q.; Li, J.; Zhang, R.; Ma, E.; Liu, R. Preparation of small particle diameter thermally expandable microspheres under atmospheric pressure for potential utilization in wood. J. Appl. Polym. Sci. 2020, 138, e49734. [Google Scholar] [CrossRef]
- Toledo-Manuel, I.; Pérez-Alvarez, M.; Cadenas-Pliego, G.; Cabello-Alvarado, C.J.; Tellez-Barrios, G.; Ávila-Orta, C.A.; Ledezma-Pérez, A.S.; Andrade-Guel, M.; Bartolo-Pérez, P. Sonochemical Functionalization of SiO2 Nanoparticles with Citric Acid and Monoethanolamine and Its Remarkable Effect on Antibacterial Activity. Materials 2025, 18, 439. [Google Scholar] [CrossRef] [PubMed]
- Arshady, R. Suspension, emulsion, and dispersion polymerization: A methodological survey. Colloid Polym. Sci. 1992, 270, 717–732. [Google Scholar] [CrossRef]
- Zhang, R.; Gao, R.; Gou, Q.; Lai, J.; Li, X. Precipitation Polymerization: A Powerful Tool for Preparation of Uniform Polymer Particles. Polymers 2022, 14, 1851. [Google Scholar] [CrossRef]
- Yan, H.; Jiao, Y.; Jin, B.; Zhang, H.; Fu, Z.; Jia, S.; Deng, Y. Insights of mechanism and kinetics of acrylonitrile aqueous precipitation polymerization. J. Macromol. Sci. Part A 2024, 61, 805–821. [Google Scholar] [CrossRef]
- Landfester, K.; Antonietti, M. The polymerization of acrylonitrile in miniemulsions: “Crumpled latex particles” or polymer nanocrystals. Macromol. Rapid Commun. 2000, 21, 820–824. [Google Scholar] [CrossRef]
- Wang, C.; Xin, Z. Effects of itaconic acid and ammonium itaconic acid on cyclization and thermal stabilization of narrow polydispersity polyacrylonitrile terpolymers synthesized in continuous flow. J. Polym. Res. 2025, 32, 42. [Google Scholar] [CrossRef]
- Suo, R.; Xie, L.; Liao, J.; Chen, J.; Lu, C.-Z. Enhanced Charge Separation in a PAN/ZnO Nanocomposite for Promoted Photocatalytic Hydrogen Evolution. ACS Appl. Energy Mater. 2024, 7, 5668–5678. [Google Scholar] [CrossRef]
- Shi, S.; Kuroda, S.; Hosoi, K.; Kubota, H. Poly(methyl methacrylate)/polyacrylonitrile composite latex particles with a novel surface morphology. Polymer 2005, 46, 3567–3570. [Google Scholar] [CrossRef]
- Hu, W.-B. Polymer Features in Crystallization. Chin. J. Polym. Sci. 2022, 40, 545–555. [Google Scholar] [CrossRef]
- Ali, A.; Chiang, Y.W.; Santos, R.M. X-ray Diffraction Techniques for Mineral Characterization: A Review for Engineers of the Fundamentals, Applications, and Research Directions. Minerals 2022, 12, 205. [Google Scholar] [CrossRef]
- Safajou-Jahankhanemlou, M.; Abbasi, F.; Salami-Kalajahi, M. Synthesis and characterization of thermally expandable PMMA-based microcapsules with different cross-linking density. Colloid Polym. Sci. 2016, 294, 1055–1064. [Google Scholar] [CrossRef]
- Bashir, Z. Polyacrylonitrile, an unusual linear homopolymer with two glass transitions. Indian J. Fibre Text. Res. 1999, 24, 1–9. [Google Scholar]
- Bashir, Z. The Hexagonal Mesophase in Atactic Polyacrylonitrile: A New Interpretation of the Phase Transitions in the Polymer. J. Macromol. Sci. Part B 2007, 40, 41–67. [Google Scholar] [CrossRef]
- Simionescu, C.; Asandei, N.; Liga, A. Research in the field of terpolymers. I. Copolymerization of acrylonitrile/methaerylic acid/methyl methacrylate. Die Makromol. Chem. 2003, 110, 278–290. [Google Scholar] [CrossRef]
- Naguib, H.F.; Mokhtar, S.M.; Khalil, N.Z.; Elsabee, M.Z. Polymerization kinetics of indene, methyl methacrylate and acrylonitrile and characterization of their terpolymer. J. Polym. Res. 2009, 16, 693–702. [Google Scholar] [CrossRef]
- Fan, L.; Zhao, D.; Bian, C.; Wang, Y.; Liu, G. Glass transition temperatures of copolymers from methyl methacrylate, styrene, and acrylonitrile: Binary copolymers. Polym. Bull. 2011, 67, 1311–1323. [Google Scholar] [CrossRef]
- Reddy, G.V.R.; Magesh, C.; Sriram, R. Microemulsion co-polymerization of methyl methacrylate with acrylonitrile. Des. Monomers Polym. 2012, 8, 75–89. [Google Scholar] [CrossRef][Green Version]
- Jonsson, M.; Nordin, O.; Kron, A.L.; Malmström, E. Influence of crosslinking on the characteristics of thermally expandable microspheres expanding at high temperature. J. Appl. Polym. Sci. 2010, 118, 1219–1229. [Google Scholar] [CrossRef]
- Mohy Eldin, M.S.; Elaassar, M.R.; Elzatahry, A.A.; Al-Sabah, M.M.B. Poly (acrylonitrile-co-methyl methacrylate) nanoparticles: I. Preparation and characterization. Arab. J. Chem. 2017, 10, 1153–1166. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).