Abstract
Synthesis of hardly available 2-alkylsemicarbazides and their hydrochlorides from semicarbazide hydrochloride has been developed. This general and efficient protocol is based on preparation of acetone semicarbazone, its N2-alkylation in the presence of sodium hydride, and hydrolysis under mild conditions.
1. Introduction
Acyclic semicarbazides are versatile reagents commonly utilized for preparation of various acyclic and heterocyclic nitrogen-containing compounds, e.g., semicarbazones, azapeptides, hydantoines, pyrazoles, 1,2,4-triazoles, 1,2,4-triazines, 1,2,4-triazepines, pyrimidines, 1,3,4-oxadiazoles, azamacrocycles [1], etc. Semicarbazides are also used for synthesis of various semicarbazide-containing substances with remarkable biological properties, particularly, analogs of antimicrobial nitrofurazone and nitrofurantoin [2], selective peroxisome proliferator-activated receptor hPPARα agonist [3], inhibitors of MALT1 protease [4], azapeptide activators of apoptosis mediated by caspase-9 in cancer cells [5], etc.
Semicarbazide hydrochloride is commercially available and, as a rule, many other acyclic semicarbazides can be readily prepared. However, no general and convenient approaches to 2-alkylsemicarbazides have been developed. Scheme 1 shows two commonly used methods of 2-alkylsemicarbazide synthesis. The first is based on nitrosonation of N-alkylureas with nitrous acid or its anhydride, followed by reduction of the nitroso group in the obtained N-Alkyl-N-nitrosoureas with Zn in aqueous AcOH [6,7,8], H2 over Pd/C [9], or using the electrochemical method [10]. The second approach involves carbamoylation of the corresponding monosubstituted hydrazines with trimethylsilyl isocyanate [4,11,12,13], urea [14], or cyanic acid generated by reaction of Brønsted acid and metal cyanate [15,16,17,18].
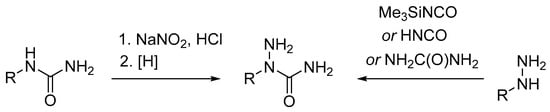
Scheme 1.
Commonly used approaches to 2-alkylsemicarbazides.
In addition, there are some particular syntheses of 2-alkylsemicarbazides. For example, 2-methylsemicarbazide was prepared by the reaction of benzaldehyde methylhydrazone with phosgene and NH3, followed by acid-catalyzed hydrolytic cleavage of benzylidene group [19]. A derivative of 2-benzylsemicarbazide was synthesized by N-alkylation of tert-butoxycarbonyl hydrazine, followed by successive reactions with triphosgene, NH3, HCl in MeOH, and aqueous NaHCO3 [20]. Synthesis of the hydromethanesulfonate salt of 2-(4-methylbenzyl) semicarbazide involved reaction of tert-butoxycarbonyl hydrazine with 4-methylbenzaldehyde to give the corresponding Boc-protected hydrazine, which was reduced with H2/Pd into 1,2-disubstituted hydrazine, followed by treatment with trimethylsilyl isocyanate, and then with MeSO2OH [21].
All of the above approaches to 2-alkylsemicarbazides are based on construction of semicarbazide fragment by N–N or С–N bond formation and have such drawbacks as multistep procedures, laborious isolation of products, low yields, use of highly toxic reagents, poor scalability, etc.
We hypothesized that general and preparative synthesis of 2-alkylsemicarbazides could be developed starting from commercially available unsubstituted semicarbazide. However, higher basicity and nucleophilicity of the nitrogen N1 in semicarbazide compared with the amide nitrogens N2 and N4 (e.g., pKa = 3.86 [22] and pKa = 0.053 [23] for protonated semicarbazide and urea, respectively, in water at 25 °C) inhibits direct alkylation at the nitrogen N2. Therefore, the N(1)H2 group should be protected with an electron-withdrawing alkylidene or arylidene group, which results in a decrease in nucleophilicity of the nitrogen N1 and a significant increase in acidity of the N(2)H group. Deprotonation of the N(2)H group with appropriate base, followed by alkylation and deprotection, would provide the target products. To our knowledge, there is only one report describing the application of this approach [24]. Namely, 2-{3-[4-(3-chlorophenyl)piperazin-1-yl]prop-1-yl}semicarbazide was prepared by deprotonation of benzaldehyde semicarbazone with NaNH2 (1,4-dioxane, reflux, 1 h), followed by alkylation with 3-[4-(3-chlorophenyl)piperazin-1-yl]propyl chloride (reflux, 18 h) and hydrolysis (water, H2C2O4, reflux) with removal of benzaldehyde formed by steam distillation. However, reaction time of the last step and isolation and purification of the target semicarbazide, as well as yields in the alkylation and hydrolysis steps, were not described. Since the acid-catalyzed hydrolysis of semicarbazones of aromatic aldehydes is known to proceed under drastic conditions, along with the formation of side products, e.g., hydrazines from initially formed semicarbazides [18], we supposed that hydrolytically labile semicarbazones of aliphatic ketones would be the best starting materials.
Herein, we report a reliable method for selective N2-alkylation of semicarbazones to give 2-alkylsemicarbazones. A general three-step synthesis of 2-alkylsemicarbazides from semicarbazide hydrochloride involving preparation of acetone semicarbazone, followed by its alkylation and mild hydrolysis, is also described.
2. Results and Discussion
The first step of our approach to 2-alkylsemicarbazides was the development of selective alkylation of semicarbazones at the N2-nitrogen. Clearly, this alkylation can proceed only via formation of a conjugate base of the starting material. Various base/solvent combinations for deprotonation of semicarbazones (e.g., MeONa/DMF [25,26], Et4NOH/THF [27,28,29], NaOH/EtOH-H2O [30], t-BuOK/THF [5,31,32,33], NaH/DMF [34], K2CO3/DMF [35,36,37], Cs2CO3/MeCN [38], tert-butylimino-tri(pyrrolidino)phosphorane/THF [39]), followed by treatment with alkylating reagents, were reported. We tested some base/solvent combinations for the alkylation of semicarbazones of aromatic aldehydes (E)-1a–c as model compounds (Scheme 2).
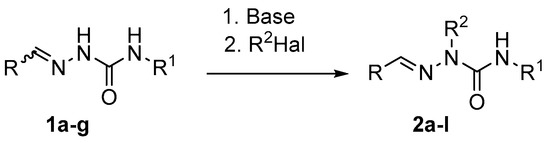
Scheme 2.
Synthesis of 2-alkylsemicarbazones by alkylation of 2-unsubstituted semicarbazones.
Treatment of (E)-1a with BuBr in the presence of K2CO3 under the described conditions [37] (DMF, rt, 12 h) failed to give N-butyl derivative 2a (NMR data) (Table 1, entry 1), while the yield of 2a was reported to be 60%. Prolongation of the reaction time (15 h and 23 h) also did not result in product formation.

Table 1.
Synthesis of 2-alkylsemicarbazones 2b–l by alkylation of semicarbazones 1b–g.a
Next, we reacted (E)-1b with significantly more active alkylating reagent MeI under the above conditions (K2CO3/DMF, rt, 17 h), and again, no expected N-methylated product 2b was formed (NMR data) (Table 1, entry 2). Thus, the conditions described in ref. 37 are inapplicable for N2-alkylation of semicarbazones.
We suppose that basicity of K2CO3 in DMF is not sufficient to generate essential concentrations of semicarbazone conjugated bases. It is noteworthy that the nature of semicarbazones, in particular their solubility in reaction media, may also play a role in the alkylation. Indeed, 4-substituted semicarbazone (E)-1c [40], which is more soluble in organic solvents than (E)-1a,b, was found to react with MeI in the presence of K2CO3 in DMF at room temperature to give the expected N-methylated product 2c, although the rate of this reaction is very low. According to NMR spectroscopic data, conversion of (E)-1c into 2c was 39% after 22 h, and 50% after 5 days (entry 4). The rate of methylation of (E)-1c with MeI in the presence of DBU (DMF, rt, 24 h) was also low, and only 14% conversion of the starting material into 2c was observed (NMR data) (entry 5). Use of MeONa in MeOH failed to give compound 2c.
We found that NaH in MeCN is the best choice for complete and selective N2-deprotonation of various semicarbazones. Treatment of (E)-1c with NaH (1.1 equiv.) in MeCN at room temperature smoothly gave the corresponding conjugated base which was reacted with excess of MeI (MeCN, rt, 2 h) to provide semicarbazone 2c in 97% yield (entry 6). Analogously, after deprotonation with NaH in MeCN, semicarbazone (E)-1b was alkylated with MeI (rt, 5.3 h) to afford compound 2b in 95% yield (entry 3), and semicarbazone (E)-1d was alkylated with MeI, EtI, BuI, or PhCH2Br to give the corresponding compounds 2d–g in 72–96% yields (entries 7–10). According to the 1H NMR spectroscopic data, semicarbazones 2b–g were obtained as a single stereoisomer with (E)-configuration, the same as in the starting materials 1b–d.
Similarly, 2-benzyl- 2h,i,k and 2-(4-methoxybenzyl)-substituted semicarbazones of aliphatic aldehydes 2j,l were prepared in 66–78% yields by alkylation of semicarbazones of propanal, butanal, or 2-methylpropanal 1e,f, (E)-1g after their deprotonation with NaH in MeCN (entries 11–15). Only a single stereoisomer of 2h–l, presumably with (E)-configuration, was obtained in each case. Interestingly, while semicarbazones of propanal (1e) and butanal (1f) used for the alkylation were mixtures of (E)- and (Z)-isomers in a ratio of 86:14 and 74:26, respectively (NMR data), the corresponding alkylated products 2h–g were isolated as (E)-isomers. It could be explained either by Z/E-isomerization in the course of the alkylation or by the fact that the minor (Z)-isomers were not alkylated and were lost during work up of reaction mixtures.
The most plausible Z/E-isomerization pathway in semicarbazones involves inversion at the N1 nitrogen atom [41]. We estimated energy barrier for the inversion in the conjugated base of ethanal semicarbazone using the DFT B3LYP/6-311++G (d, p) calculations. The IRC (Intrinsic Reaction Coordinate) analysis demonstrated that the found transition state connect the desired minima. The data obtained show that energy barrier for the conversion of (Z)-isomer into (E)-isomer (Scheme 3) is relatively high (39.35 kcal/mol).
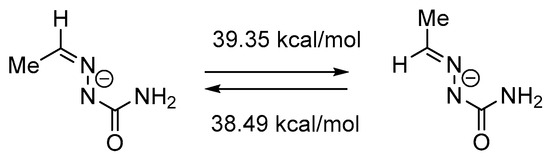
Scheme 3.
Transformation of the (Z)-isomer of the conjugated base of ethanal semicarbazone into the (E)-isomer via inversion pathway.
Since the alkylation of the conjugated base of 1e with PhCH2Br proceeds at room temperature (Table 1, entry 11), Z/E-isomerization can be excluded. Thus, we suppose that the isolation of only (E)-isomers of 2h–j is due to the fact that (Z)-isomers of the starting materials 1e,f were not alkylated, presumably due to steric hindrance.
Next we applied the above conditions to the N2-alkylation of hydrolytically labile acetone semicarbazone (3). Starting, compound 3 was readily prepared from semicarbazide hydrochloride and acetone in the presence of sodium acetate (H2O, rt) according to routine procedure in excellent yield (Scheme 4).
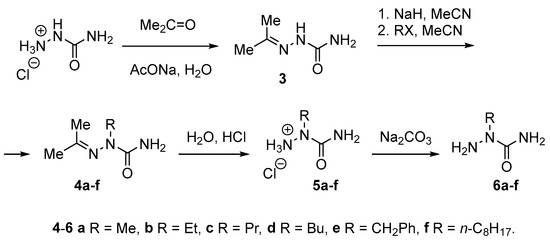
Scheme 4.
Synthesis of 2-alkylsemicarbazides 6a–f and their hydrochlorides 5a–f from semicarbazide hydrochloride.
Compounds 4a–f were synthesized by the treatment of 3 with NaH (1.05–1.07 equiv.) in MeCN at room tempetature for 40–60 min, followed by the reaction of the generated conjugated base with excess of appropriate alkylating reagent. The degree of conversion of 3 into 4a–f was determined by 1H NMR spectroscopic data for crude products isolated after removal of all volatiles under reduced pressure.
Reaction of the conjugated base of 3 with methyl iodide (10 equiv.) completed in MeCN at room temperature for 4 h. The resulting solution was evaporated to dryness under vacuum, the residue was dissolved in H2O, the solution was heated at 60 °С for 10–15 min, and the solvent was removed under vacuum. The obtained oily residue was triturated with Et2O/EtOH mixture (1:1) to give a solid product. 1H NMR spectroscopic data showed that the isolated product was 2-methylsemicarbazide (6a) resulted from hydrolysis of 4a upon water treatment. According to the data of elemental analysis, the crystallized from EtOH or MeCN 6a contained 33 mol% of NaI. Therefore, we supposed that compound 6a formed a stable complex with NaI. Since 2-methylsemicarbazide is highly soluble in water, aqueous work up of crude product to remove NaI became inacceptable in contrast to 2b–d. Treatment of water solution of crude 6a with lead nitrate for the same purpose was inefficient.
Next we used dimethyl sulfate as methylating reagent instead of MeI. The reaction of the conjugated base of 3 with dimethyl sulfate (1.06 equiv.) smoothly proceeded at room temperature for 17 h to give semicarbazone 4a. After removal of the solvent under reduced pressure, compound 4a was readily hydrolyzed with excess of hydrochloric acid followed by evaporation of the solution formed under vacuum. Treatment of the obtained residue with cold i-PrOH afforded easy to handle crystalline 2-methylsemicarbazide hydrochloride (5a) in 72% yield (based on 3) (Table 2, entry 1).

Table 2.
Synthesis of 2-alkylsemicarbazides hydrochlorides 5a–f by alkylation of the conjugated base of acetone semicarbazone (3) in MeCN followed by treatment with excess of hydrochloric acid (60 °С).
Analogously, hydrochlorides of 2-ethyl- (5b), 2-propyl- (5c), and 2-butylsemicarbazides (5d) were prepared in 59–71% yields by the treatment of conjugated base of 3 with excess (5–10 equiv.) of the corresponding alkyl bromides (MeCN, reflux, 9 h), followed by the acidic workup (Table 2, entries 2–4).
Alkylation with benzyl bromide (1.06 equiv.) with the subsequent acidic treatment gave hydrochloride of 2-benzylsemicarbazide (5e) in 60% yield (entry 5).
It should be noted that the 2-alkylated semicarbazides can be also isolated as free bases 6a–e by treatment of reaction mixtures after their evaporation with aqueous Na2CO3, followed by extraction with EtOAc. However, it was more difficult to handle and purify free bases 6a–e compared with hydrochlorides 5a–e. In contrast, our attempts to obtain the analytically pure sample of hydrochloride 2-octylsemicarbazide (5f) prepared by the alkylation of 3 with octyl bromide (5.0 equiv.) (MeCN, reflux, 6.5 h) failed, while free base 6f was isolated in 58% yield (based on 3) (Table, entry 6) and readily purified.
3. Conclusions
An effective method for selective N2-alkylation of semicarbazones to give 2-alkylsemicarbazones has been developed. It involves deprotonation of semicarbazones with sodium hydride in MeCN, followed by treatment with alkylating reagents. This method was applied to general and convenient synthesis of 2-alkylsemicarbazides and their hydrochlorides, starting from commercially available semicarbazide hydrochloride. It is based on selective N2-alkylation of acetone semicarbazone under the action of sodium hydride and dimethyl sulfate or alkyl bromides. The resulting acetone 2-alkylsemicarbazones were hydrolyzed by fast heating (60 °С) with 17–36% hydrochloric acid to give the target products in 58–72% yields. Alkylation and hydrolytic steps were conveniently performed in one reaction flask, making the described approach very simple and preparative.
Funding
This work was supported by the Russian Foundation for Basic Research (grant No. 19-316-70006).
References
- Belletire, J.L.; Rauh, R.J.; Huérou, Y.L. Semicarbazide. In The Electronic Encyclopedia of Reagents for Organic Synthesis (e-EROS); John Wiley & Sons: New York, NY, USA, 2006. [Google Scholar] [CrossRef]
- Vass, M.; Hruska, K.; Franek, M. Nitrofuran antibiotics: a review on the application, prohibition and residual analysis. Vet. Med. 2008, 53, 469–500. [Google Scholar] [CrossRef]
- Xu, Y.; Mayhugh, D.; Saeed, A.; Wang, X.; Thompson, R.C.; Dominianni, S.J.; Kauffman, R.F.; Singh, J.; Bean, J.S.; Bensch, W.R.; et al. Design and synthesis of a potent and selective triazolone-based peroxisome proliferator-activated receptor α agonist. J. Med. Chem. 2003, 46, 5121–5124. [Google Scholar] [CrossRef] [PubMed]
- Krappmann, D.; Nagel, D.; Schlauderer, F.; Lammens, K.; Hopfner, K.-P.; Chrusciel, R.A.; Kling, D.L.; Bedore, M.W. Inhibitors of MALT1 propease. Patent WO 2014086478; Chem. Abstr. 2014, 161, 86223. [Google Scholar]
- Bourguet, C.B.; Boulay, P.-L.; Claing, A.; Lubell, W.D. Design and synthesis of novel azapeptide activators of apoptosis mediated by caspase-9 in cancer cells. Bioorg. Med. Chem. Lett. 2014, 24, 3361–3365. [Google Scholar] [CrossRef]
- Smith, P.A.S.; Clegg, J.M.; Lakritz, J. Preparation of alkyl azides from hydrazine derivatives. J. Org. Chem. 1958, 23, 1595–1599. [Google Scholar] [CrossRef]
- Vogelesang, C. Methylated semicarbazides. Rec. Trav. Chim. 1943, 62, 5–11. [Google Scholar] [CrossRef]
- Gabriel, S. Über einige Hydrazin-Derivate. Chem. Ber. 1914, 47, 3028–3033. [Google Scholar] [CrossRef]
- Lum, D.W.; Mador, I.L. Preparation of substituted semicarbazides. Patent US 2959615; Chem. Abstr. 1961, 55, 22440. [Google Scholar]
- Backer, H.J. Réductions électrochimiques, Troisième Mémoire. Réduction des nitrosamines. Rec. Trav. Chim. 1913, 32, 39–47. [Google Scholar] [CrossRef]
- Szimhardt, N.; Stierstorfer, J. Methylsemicarbazide as a ligand in late 3d transition metal complexes. Chem. Eur. J. 2018, 24, 2687–2698. [Google Scholar] [CrossRef]
- Viner, R.C.; Rzepa, P.R.; Mitchell, G. Pyridazinone derivatives as herbicides. Patent WO 2014154828; Chem. Abstr. 2014, 161, 551169. [Google Scholar]
- Shibayama, A.; Kajiki, R.; Kobayashi, M.; Mitsunari, T.; Nagamatsu, A. 6-Acyl-1,2,4-triazine-3,5-dione derivative and herbicides. Patent WO 2012002096; Chem. Abstr. 2012, 156, 122559. [Google Scholar]
- Maliga, P.; Allison, L.A.; Hajdukiewicz, P.T. Nuclear-encoded transcription system in plastids of higher plants. Patent WO 9706250; Chem. Abstr. 1997, 126, 234441. [Google Scholar]
- Argentine, M.D.; Braden, T.M.; Czarnik, J.; Conder, E.W.; Dunlap, S.E.; Fennell, J.W.; LaPack, M.A.; Rothhaar, R.R.; Scherer, R.B.; Schmid, C.R.; et al. The role of new technologies in defining a manufacturing process for PPARα agonist LY518674. Org. Process Res. Dev. 2009, 13, 131–143. [Google Scholar] [CrossRef]
- Bonnard, H.; Lecomte, L.; Senet, J.-P. Verfahren zur Synthese von 1-Alkyl-3-hydroxy-5-halogen-1,2,4-triazolen sowie neue Hydrazinderivate. Patent DE 4416868; Chem. Abstr. 1995, 122, 133194. [Google Scholar]
- Gever, G.; O’Keefe, C.; Drake, G.; Ebetino, F.; Michels, J.; Hayes, K. Chemotherapeutic nitrofurans. I. Some derivatives of 3-amino-2-oxazolidone. J. Am. Chem. Soc. 1955, 77, 2277–2281. [Google Scholar] [CrossRef]
- Hale, W.J.; Lange, N.A. Four-membered cyclic ureas. III. The condensation of isocyanic acid with alkyl Schiff bases and related compounds. J. Am. Chem. Soc. 1920, 42, 107–116. [Google Scholar] [CrossRef]
- Ciba Limited. New hydrazine carboxylic acid halides and process for preparing same. Patent GB 898419; Chem. Abstr. 1963, 58, 52963. [Google Scholar]
- Fauber, B.; Laddywahetty, T.; Rene, O. Heteroarylalkylene aryl sultam derivatives as RORc modulators. Patent WO 2016096936; Chem. Abstr. 2016, 165, 127244. [Google Scholar]
- Braden, T.M.; Coffey, D.S.; Doecke, C.W.; LeTourneau, M.E.; Martinelli, M.J.; Meyer, C.L.; Miller, R.D.; Pawlak, J.M.; Pedersen, S.W.; Schmid, C.R.; et al. A convergent kilogram-scale synthesis of the PPARα agonist LY518674: Discovery of a novel acid-mediated triazolone synthesis. Org. Process Res. Dev. 2007, 11, 431–440. [Google Scholar] [CrossRef]
- Jencks, W.P.; Gilchrist, M. Nonlinear structure-reactivity correlations. The reactivity of nucleophilic reagents toward esters. J. Am. Chem. Soc. 1968, 90, 2622–2637. [Google Scholar] [CrossRef]
- Grant, H.M.; McTigue, P.; Ward, D.G. The basicities of aliphatic amides. Aust. J. Chem. 1983, 36, 2211–2218. [Google Scholar] [CrossRef]
- Sigma-Tau Industrie Farmaceutiche Riunite, S.p.A. Dérivés de triazolinone et procédé de préparation. Patent BE 821084; Chem. Abstr. 1975, 83, 179071. [Google Scholar]
- Hrebabecky, H.; Beranek, J. Isomerisation, alkylation, and cyclisation of glyoxylic acid semicarbazone derivatives. Coll. Czech. Chem. Commun. 1975, 40, 2364–2377. [Google Scholar] [CrossRef]
- Garcia Mellado, O.; Cortes Cortes, E. Derivatives of 5-R1-2[(N-R2)-furfuryliden; thiophenyliden] semicarbazones and thiosemicarbazones, method for obtaining and using the same for the preparation of a drug for the Chagas disease. Patent MX 2007013128; Chem. Abstr. 2009, 155, 11750. [Google Scholar]
- Douchez, A.; Lubell, W.D. Chemoselective alkylation for diversity-oriented synthesis of 1,3,4-benzotriazepin-2-ones and pyrrolo[1,2][1,3,4]benzotriazepin-6-ones, potential turn surrogates. Org. Lett. 2015, 17, 6046–6049. [Google Scholar] [CrossRef]
- Doan, N.-D.; Zhang, J.; Traoré, M.; Kamdem, W.; Lubell, W.D. Solid-phase synthesis of C-terminal azapeptides. J. Pept. Sci. 2015, 21, 387–391. [Google Scholar] [CrossRef]
- Garcia-Ramos, Y.; Lubell, W.D. Synthesis and alkylation of aza-glycinyl dipeptide building blocks. J. Pept. Sci. 2013, 19, 725–729. [Google Scholar] [CrossRef]
- Noyáček, A.; Sedláčková, V.; Vondráček, B.; Šeyčík, B.; Bedrník, P.; Gut, J. Synthesis of 1-benzyl-6-azauracil derivatives, chlorinated in the nucleus. Coll. Czech. Chem. Commun. 1981, 46, 2203–2206. [Google Scholar] [CrossRef]
- Bourguet, C.B.; Proulx, C.; Klocek, S.; Sabatino, D.; Lubell, W.D. Solution-phase submonomer diversification of aza-dipeptide building blocks and their application in aza-peptide and aza-DKP synthesis. J. Pept. Sci. 2010, 16, 284–296. [Google Scholar] [CrossRef]
- Proulx, C.; Lubell, W.D. Aza-1,2,3-triazole-3-alanine synthesis via copper-catalyzed 1,3-dipolar cycloaddition on aza-progargylglycine. J. Org. Chem. 2010, 75, 5385–5387. [Google Scholar] [CrossRef] [PubMed]
- Sabatino, D.; Proulx, C.; Klocek, S.; Bourguet, C.B.; Boeglin, D.; Ong, H.; Lubell, W.D. Exploring side-chain diversity by submonomer solid-phase aza-peptide synthesis. Org. Lett. 2009, 11, 3650–3653. [Google Scholar] [CrossRef] [PubMed]
- Qi, B.; Tao, H.; Wu, D.; Bai, J.; Shi, Y.; Gong, P. Synthesis and biological evaluation of 4-phenoxy-6,7-disubstituted quinolines possessing semicarbazone scaffolds as selective c-Met inhibitors. Arch. Pharm. 2013, 346, 596–609. [Google Scholar] [CrossRef] [PubMed]
- Gillespie, E.; Damoiseaux, R.; Ho, C.-L. C.; Chamberlain, B.T.; Jung, M.E.; Bradley, K.A. Protective molecules against anthrax toxin. Patent WO 2014113607; Chem. Abstr. 2014, 161, 275703. [Google Scholar]
- Jung, M.E.; Chamberlain, B.T.; Ho, C.-L. C.; Gillespie, E.J.; Bradley, K.A. Structure–activity relationship of semicarbazone EGA furnishes photoaffinity inhibitors of anthrax toxin cellular entry. ACS Med. Chem. Lett. 2014, 5, 363–367. [Google Scholar] [CrossRef][Green Version]
- Brondani, D.J.; Moreira, D.R.M.; de Farias, M.P.A.; Souza, F.R.S.; Barbosa, F.F.; Leite, A.C.L. A new and efficient N-alkylation procedure for semicarbazides/semicarbazones derivatives. Tetrahedron Lett. 2007, 48, 3919–3923. [Google Scholar] [CrossRef]
- Mederski, W.W.K.R.; Germann, M. A general synthesis of 1-aryl carbamoyl-2-alkyl-4-aryl substituted semicarbazides as nonbasic factor Xa inhibitors. Bioorg. Med. Chem. Lett. 2003, 13, 3715–3718. [Google Scholar] [CrossRef]
- Kurian, L.A.; Silva, T.A.; Sabatino, D. Submonomer synthesis of azapeptide ligands of the insulin receptor tyrosine kinase domain. Bioorg. Med. Chem. Lett. 2014, 24, 4176–4180. [Google Scholar] [CrossRef]
- Fesenko, A.A.; Yankov, A.N.; Shutalev, A.D. An efficient and stereoselective approach to 14-membered hexaaza macrocycles using novel semicarbazone-based amidoalkylation reagents. Tetrahedron Lett. 2016, 57, 5784–5787. [Google Scholar] [CrossRef]
- Kessler, H. Detection of hindered rotation and inversion by NMR spectroscopy. Angew. Chem. Internat. Edn. 1970, 9, 219–235. [Google Scholar] [CrossRef]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).