Synthesis of Pyrrolidinols by Radical Additions to Carbonyls Groups †
Abstract
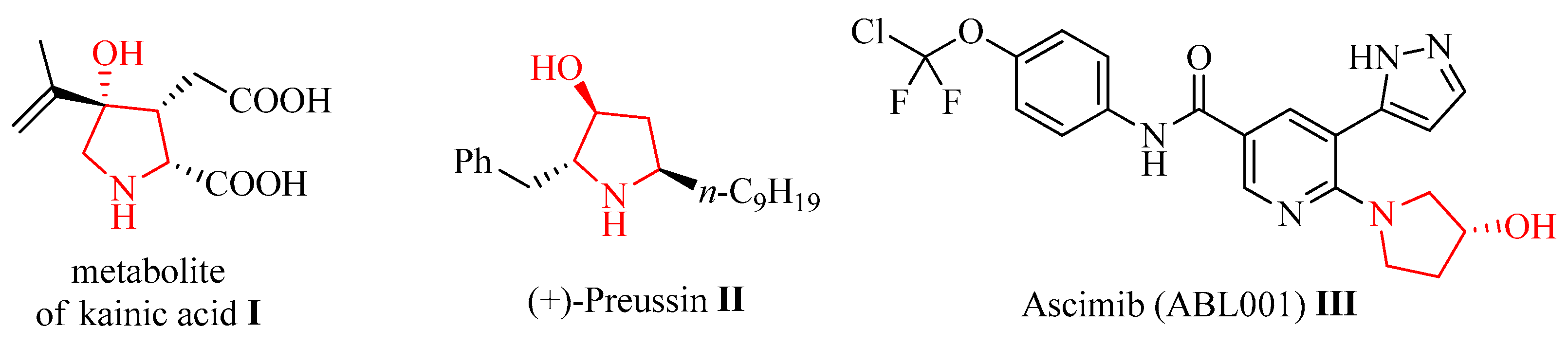
:1. Introduction
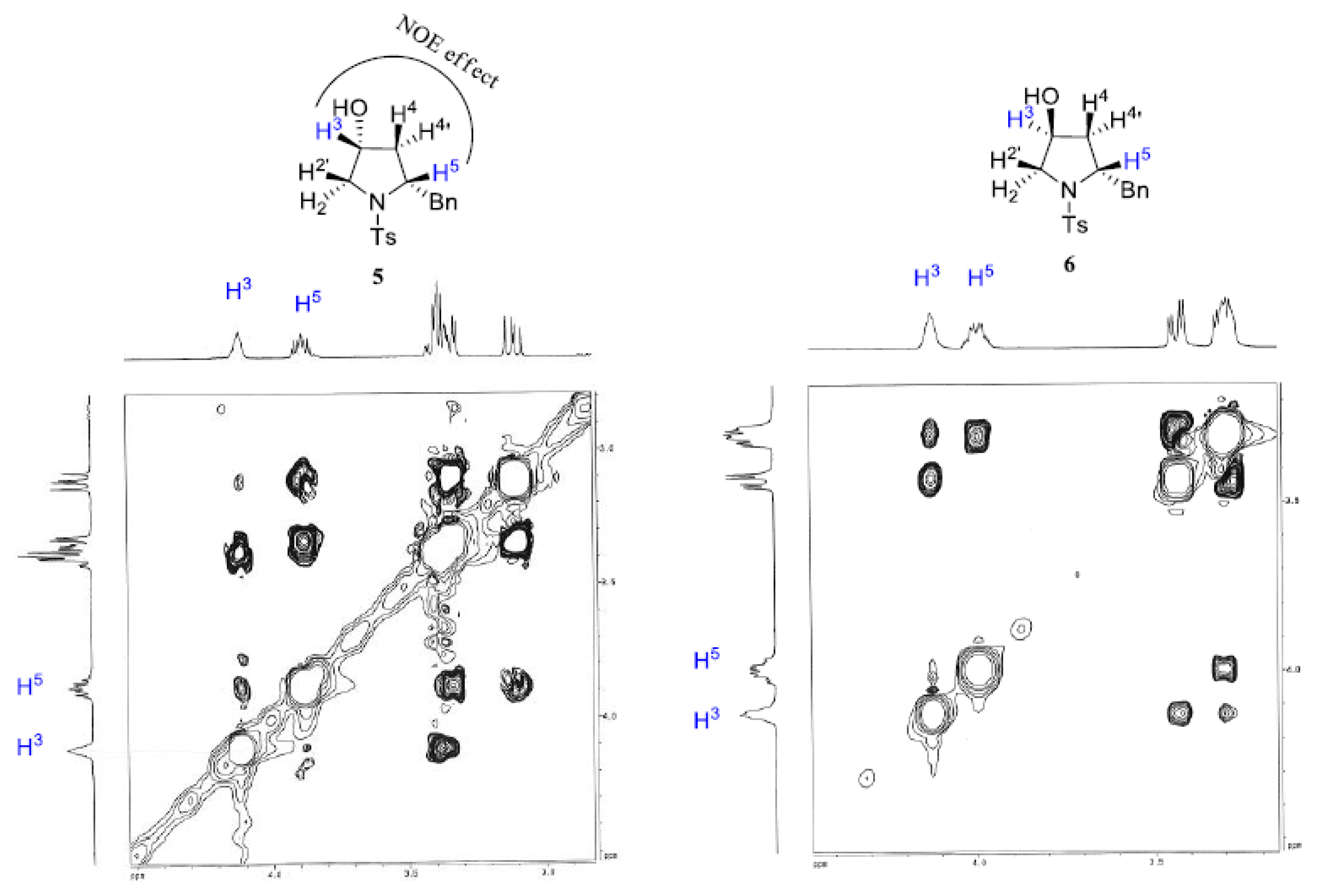
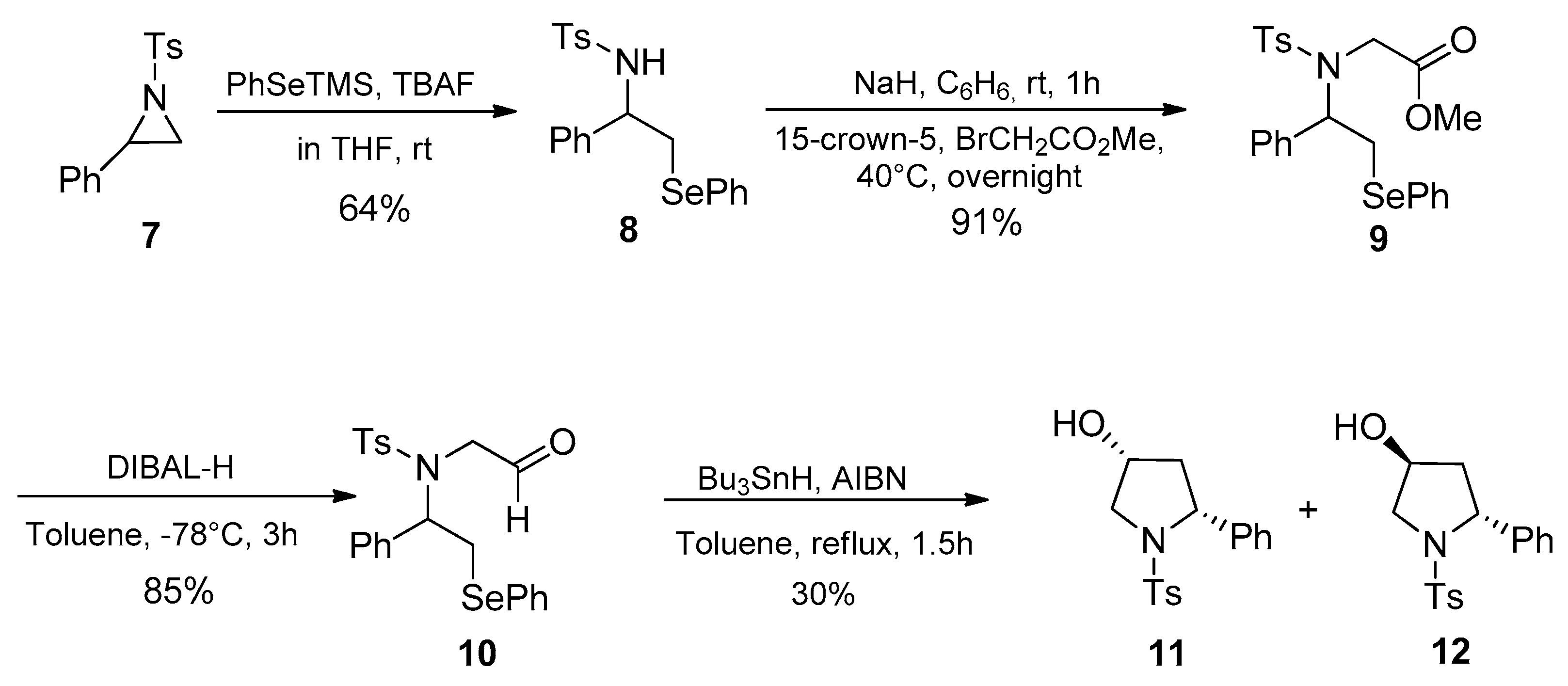
2. Results and Discussion
3. Conclusions
Funding
Acknowledgments
Conflicts of Interest
References
- Bowman, W.R.; Cloonan, M.O.; Krintel, S.L. Synthesis of Heterocycles by radical cyclization. J. Chem. Soc. Perkin Trans. 2001, 1, 2885–2902. [Google Scholar] [CrossRef]
- Bowman, W.R.; Fletcher, A.J.; Potts, G.B.S. Synthesis of heterocycles by radical cyclisation. J. Chem. Soc. Perkin Trans. 2002, 1, 2747–2762. [Google Scholar] [CrossRef]
- Renaud, P. Radical reactions using selenium precursors. In Organoselenium Chemistry: Modern Developments in Organic Synthesis; Wirth, T., Ed.; Springer-Verlag: Berlin/Heidelberg, Germany, 2000; pp. 81–112. [Google Scholar]
- Bowman, W.R. Selenum compounds in radical reactions. In Organoselenium Chemistry: Synthesis and Reactions; Wirth, T., Ed.; Wiley-VCH: Weinheim, Germany, 2012; pp. 111–146. [Google Scholar]
- Beckwith, A.L.J.; Page, D.M. Formation of Some Oxygen-Containing Heterocycles by Radical Cyclization: The Stereochemical Influence of Anomeric Effects. J. Org. Chem. 1998, 63, 5144–5153. [Google Scholar] [CrossRef]
- Engman, L.; Gupta, V. Tetrahydrofuran Derivatives from Epoxides via Group Transfer Cyclization or Reductive Radical Cyclization of Organotellurium and Organoselenium Intermediates. J. Org. Chem. 1997, 62, 157–173. [Google Scholar] [CrossRef] [PubMed]
- Engman, L.; Ericsson, C. Diastereocontrol by Trialkylaluminums in the Synthesis of Tetrahydrofurans via Radical Cyclization. Org. Lett. 2001, 3, 3459–3462. [Google Scholar] [CrossRef]
- Engman, L.; Gupta, V.; Besev, M. Pyrrolidines from olefins via radical cyclization. Tetrahedron Lett. 1998, 39, 2429–2432. [Google Scholar] [CrossRef]
- Engman, L.; Besev, M. Pyrrolidines from β-Aminoselenides via Radical Cyclization. Diastereoselectivity Control by the N-Substituent. Org. Lett. 2000, 2, 1589–1592. [Google Scholar] [CrossRef]
- Engman, L.; Bresev, M. Diastereocontrol by a Hydroxyl Auxiliary in the Synthesis of Pyrrolidines via Radical Cyclization. Org. Lett. 2002, 4, 3023–3025. [Google Scholar] [CrossRef]
- Kumamoto, H.; Ogamino, J.; Tanak, H.; Suzuki, H.; Haraguchi, K.; Miyasaka, T.; Yokomatsu, T.; Shibuya, S. Radical-mediated furanose ring reconstruction from 2′,3′-seco-uridine. Tetrahedron 2001, 57, 3331–3341. [Google Scholar] [CrossRef]
- Wirth, T.; Kulicke, K.J.; Fragale, G. Chiral Diselenides in the Total Synthesis of (+)-Samin. J. Org. Chem. 1996, 61, 2686–2689. [Google Scholar] [CrossRef] [PubMed]
- Uchiyama, M.; Hirai, M.; Nagata, M.; Katoh, R.; Ogawa, R.; Ohta, A. Stereoselective synthesis of optically active perhydrofuro[2,3-b]furan derivatives. Tetrahedron Lett. 2001, 42, 4653–4656. [Google Scholar] [CrossRef]
- Tiecco, M.; Testaferri, L.; Marini, F.; Sternativo, S.; Santi, C.; Bagnoli, L.; Temperini, A. Intramolecular addition of carbon radicals to aldehydes: Synthesis of enantiopure tetrahydrofuran-3-ols. Tetrahedron 2007, 63, 5482–5489. [Google Scholar] [CrossRef]
- Palomba, M.; Scarcella, E.; Sancineto, L.; Bagnoli, L.; Santi, C.; Marini, F. Synthesis of SpirooxindoleOxetanes Through a Domino Reaction of 3-Hydroxyoxindoles and Phenyl Vinyl Selenone. Eur. J. Org. Chem. 2019, 2019, 5396–5401. [Google Scholar] [CrossRef]
- Palomba, M.; Sancineto, L.; Marini, F.; Santi, C.; Bagnoli, L. A domino approach to pyrazino- indoles and pyrroles using vinyl selenones. Tetrahedron 2018, 74, 7156–7163. [Google Scholar] [CrossRef]
- Sternativo, S.; Marini, F.; Del Verme, F.; Calandriello, A.; Testaferri, L.; Tiecco, M. One-pot synthesis of aziridines from vinyl selenones and variously functionalized primary amines. Tetrahedron 2010, 66, 6851–6857. [Google Scholar] [CrossRef]
- Tiecco, M.; Testaferri, L.; Bagnoli, L.; Scarponi, C.; Temperini, A.; Marini, F.; Santi, C. Selenium promoted synthesis of enantiopure pyrrolidines starting from chiral aminoalcohols. Tetrahedron: Asymm. 2007, 18, 2758–2767. [Google Scholar] [CrossRef]
- Maeno, Y.; Terada, R.; Kotaki, Y.; Cho, Y.; Konoki, K.; Yotsu-Yamashita, M. Possible Biosynthetic Products and Metabolites of Kainic Acid from the Red Alga Digenea simplex and Their Biological Activity. J. Nat. Prod. 2019, 82, 1627–1633. [Google Scholar] [CrossRef] [PubMed]
- Hausherr, A.; Siemeisterb, G.; Reissig, H.-U. Alkoxyallene-based syntheses of preussin and its analogs and their cytotoxicity. Org. Biomol. Chem. 2019, 17, 122–134. [Google Scholar] [CrossRef] [PubMed]
- Schoepfer, J.; Jahnke, W.; Berellini, G.; Buonamici, S.; Cotesta, S.; Cowan-Jacob, S.W.; Dodd, S.; Drueckes, P.; Fabbro, D.; Gabriel, T.; et al. Discovery of Asciminib (ABL001), an Allosteric Inhibitor of the Tyrosine Kinase Activity of BCR-ABL1. J. Med. Chem. 2018, 61, 8120–8135. [Google Scholar] [CrossRef] [PubMed]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Marini, F.; Palomba, M.; Bagnoli, L.; Santi, C. Synthesis of Pyrrolidinols by Radical Additions to Carbonyls Groups. Proceedings 2019, 41, 20. https://doi.org/10.3390/ecsoc-23-06606
Marini F, Palomba M, Bagnoli L, Santi C. Synthesis of Pyrrolidinols by Radical Additions to Carbonyls Groups. Proceedings. 2019; 41(1):20. https://doi.org/10.3390/ecsoc-23-06606
Chicago/Turabian StyleMarini, Francesca, Martina Palomba, Luana Bagnoli, and Claudio Santi. 2019. "Synthesis of Pyrrolidinols by Radical Additions to Carbonyls Groups" Proceedings 41, no. 1: 20. https://doi.org/10.3390/ecsoc-23-06606
APA StyleMarini, F., Palomba, M., Bagnoli, L., & Santi, C. (2019). Synthesis of Pyrrolidinols by Radical Additions to Carbonyls Groups. Proceedings, 41(1), 20. https://doi.org/10.3390/ecsoc-23-06606







