Abstract
Voltammetric sensors chemically modified with combinations of two electrocatalytic materials: tetraoctylammonium bromide capped gold nanoparticles (AuNPNBr) and a sulphur containing zinc phthalocyanine derivative (ZnPcRS) are reported. The electrocatalytic effects in the detection of catechol have been analyzed in sensors obtained by direct mixing (AuNPNBr/ZnPcRS) and in sensors modified with an adduct where both components are linked covalently (AuNPNBr-S-ZnPcR). Results demonstrate that the nature of the interaction between both components modifies the electrocatalytic properties. The AuNPNBr/ZnPcRS mixture improves the electron transfer rate of the catechol reduction, with limits of detection of 10−6 M. The covalent adduct AuNPNBr-S-ZnPcR enhances the response rate of the oxidation of the catechol with limits of detection of 10−7 M.
1. Introduction
Phenols, one of the most important classes of antioxidants present in foods, have been successfully assessed using electrodes chemically modified with a variety of materials [1].
Phthalocyanines have attracted considerable attention as chemical modifiers in electrochemical sensors dedicated to the detection of phenols. This is due to their well-established electrocatalytic activity and their versatility [2,3]. Similarly, the electrocatalytic properties of AuNPs have been well established. Their catalytic properties combined with their biocompatibility make them ideal electron mediators in biosensors. Some examples of capped AuNPs have been successfully used for the detection of phenols [4]. Improvement in performance of electrochemical sensors can be achieved by using combinations of electrocatalytic materials due to synergistic effects [5]. Such synergistic effects can be obtained by bringing together components just by mixing, by means of co-electrodeposition, by self assembling or by other methods such as in Langmuir-Blodgett films. An aspect that remains unexplored is the effect of covalent link in the electrocatalytic activity and in the synergistic properties.
The objective of this work is to obtain improved voltammetric sensors towards catechol by means of synergistic effects between phthalocyanines and nanoparticles. For this purpose, a complex formed by tetraoctylammonium bromide-gold nanoparticles (AuNPNBr) linked covalently to 2-{2′-[(5″-Acetylthiopentyloxo)amino]ethoxy}-9(10),16(17),23(24)-tri-tert-butylphthalocyaninate Zn(II) (ZnPcRS) through thiol bonds was obtained (AuNPNBr-S-ZnPcR). The sensing properties of spin-coated films towards catechol were analyzed. Similarly, the sensing properties of substrates modified with a mixture of both compounds (not covalently linked) (AuNPNBr/ZnPcSR) were also tested. Limits of detection and have been evaluated. Studies at increasing scan rates, have been carried out to evaluate the improvement of the charge transfer rates.
2. Materials and Methods
All chemicals were of reagent grade and used as supplied (Aldrich Chemical Ltd., St. Louis, MO, USA). Solutions were obtained by dilution in deionized water (resistivity 18.2 mΩ·cm−1).
The complex formed by tetraoctylammonium bromide-gold nanoparticles (AuNPNBr) was synthesized using an adaptation of the method described by Brust et al. [6]. 2-{2′-[(5″-Acetylthiopentyloxo)amino]ethoxy}-9(10),16(17),23(24)-tri-tert-butylphalocyaninate Zn(II) (ZnPcSR) was synthetized according to [7] and solved in toluene. The mixture AuNPNBr/ZnPcSR was prepared by mixing the AuNPNBr toluene colloid (Abs 398 nm = 3.5 ua) with ZnPcSR (6.5 × 10−5 mol∙L−1, in toluene) in proportion 2:1. The mixture was kept in dark during 1 h before use. The hybrid AuNPNBr-S-ZnPcR was obtained by a chemical reaction between AuNPNBr and ZnPcSR to obtain a complex covalently linked [6]. For this purpose 4 mL of a toluene solution of the phthalocyanine (1.3 × 10−3 mol∙L−1) were mixed with 4 mL of the toluene colloid of the nanoparticles (Abs 398 nm = 3.5 ua) and stirred for 24 h under inert atmosphere, in darkness and room temperature. Then the product was added drop by drop to pentane. The precipitate was solved in methane and kept at −20 °C overnight. After centrifugation, the new precipitate of AuNPNBr-S-ZnPcR was resuspended in toluene (Figure 1).
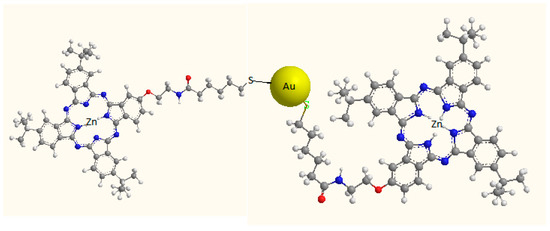
Figure 1.
Representation of the AuNPNBr-S-ZnPcR adduct.
Sensors were prepared by spin coating (spin coater model 1H-D7, Micasa Co., Tokyo, Japan). For this purpose, 50 µL of the corresponding material were deposited onto an ITO glass substrate (1 cm2 surface) using 120 s slope and 120 s at 1000 rpm. Sensing materials and films were characterized by UV-Vis spectroscopy (UV-2600, Shimadzu, Kyoto, Japan) and TEM microscopy (JEOL-FS2200 HRP. 200 kV emission). The sensing behavior of the chemically modified films was characterized by cyclic voltammetry. Electrochemical measurements were carried out in a PGSTAT 128N potentiostat (Autolab Metrohm, Utrecht, The Netherlands) using a three-electrode cell. The reference electrode was an Ag|AgCl/KCl sat. and the counter electrode was a platinum sheet. Modified ITO films were used as working electrodes. The electrochemical response was tested towards catechol 10−3 mol∙L−1 in phosphate buffer.
3. Results
The UV-Vis spectra of the AuNPNBr colloid showed an intense peak corresponding to the plasmon resonance at 398 nm with a small shoulder at 485 nm. Absorption spectra of the ZnPcR toluene solutions presented the characteristic Q band at 689 nm, accompanied by an intense shoulder at 675 typical of the existence of dimers in the solution (H-aggregates). The UV-vis spectrum of the mixture AuNPNBr/ZnPcSR showed features corresponding to both components. Finally, absorption UV spectra of the covalently linked composite AuNPBe-S-ZnPcR was similar to the spectrum registered in the mixture, but a drastic increase in the intensity of the band at 393 nm (corresponding to the overlapping of the B band and the plasmon band) was observed, confirming the existence of a covalent interaction that modifies the π–π transition. The core diameter of the AuNBrNP estimated from TEM images was 2–3 nm. TEM images of the AuNPNBr-S-ZnPcR adduct showed an average of 4 nm. Images also showed the existence of a lighter halo surrounding the nanoparticles which was produced by the presence of phthalocyanine molecules around the nanoparticle.
The sensing properties towards catechol of ITO glass modified with the AuNPNBr/ZnPcSR mixture and the AuNPNBr-S-ZnPcR adduct were analyzed using cyclic voltammetry. Voltammograms were registered from −0.5 to 1.2 V at a scan rate of 0.1 V∙s−1. (Figure 2). Voltammograms showed an anodic peak at positive potentials, which corresponds to the oxidation of catechol to 1,2 benzoquinone. In the negative region the cathodic peak produced by the reversal reduction of benzoquinone to catechol was observed. These peaks are quite weak when a bare ITO glass was used as electrode. The intensity was slightly increased when the ITO glass was covered with AuNPNBr. However, when ITO glass was covered with phthalocyanine, ZnPcSR the peaks increased clearly in intensity: the anodic wave increased from 3 μA observed in ITO to 30 μA in films covered with ZnPcSR. Similarly, the cathodic wave increased from −7 μA to −45 μA, confirming the strong electrocatalytic effect shown by the phthalocyanine compound. The electrocatalytic effect observed in sensors chemically modified with the AuNPNBr/ZnPcSR mixture, was similar to the observed in the ZnPcSR modified sensors. The effect of the AuNPNBr in the mixture was almost negligible. Using the covalent adduct AuNPNBr-S-ZnPcR, results were clearly different from those obtained with the mixture and the anodic wave increased even further its intensity while an important shift to lower potentials was observed. In contrast, electrocatalytic effect was not observed in the cathodic wave.
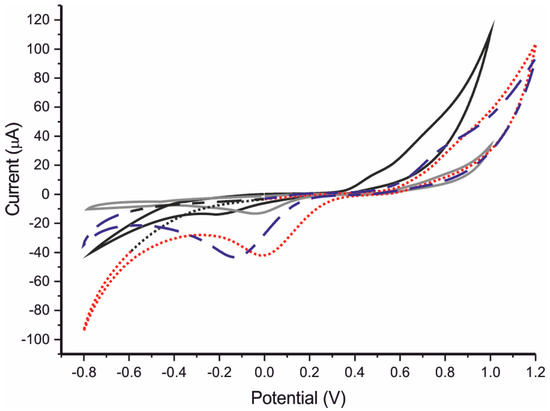
Figure 2.
Cyclic voltammograms registered in in catechol 10−3 mol∙L−1 (0.01 M phosphate buffer as electrolyte): AuNPNBr (light grey), ZnPcSR (dashed blue), AuNPNBr/ZnPcSR (dashed red) and AuNPNBr-S-ZnPcR (black). Scan rate 100 mV∙s−1.
The limits of detection (LD) were calculated from the responses of the sensors immersed in catechol solutions with concentrations ranging from 4 × 10−6 to 1.40 × 10−4 mol∙L−1 (Table 1 For the regression plot of Ia (or Ic) versus catechol concentration, sensitivity and detection limits were calculated. The LDs were dependent on the type of composite. When calculations were carried out using the Ic taken form the cathodic wave, the sensor modified with the AuNPNBr/ZnPcSR mixture provided the lowest LD. The detection limit (3σ) was 1.51 × 10−6 mol∙L−1, which is lower than the LD of 8.35 × 10−6 mol∙L−1 obtained using the adduct AuNPNBr-S-ZnPcR. On the contrary, when calculations were carried out using the anodic peak, the sensor modified with the adduct provided the lowest LD. The detection limit (3σ) was 1.46 × 10−7 mol∙L−1, which is lower than the LD of 5.45 × 10−6 mol∙L−1 obtained using the the mixture.

Table 1.
Sensitivity, LDs and regression coefficients calculated form the calibration curves.
4. Conclusions
This study reported the ability to modify the electrocatalytic activity of gold nanoparticles and phthalocyanines composites obtained by simple mixing AuNPNBr/ZnPcSR or by means of the establishment of a covalent bond AuNPNBr-S-ZnPcR. Studies at increasing scan rates, confirmed the improvement of the charge transfer rates caused by the composite.
The sensor obtained by simple mixture improves the electron transfer rate of the reduction of catechol at the electrode surface allowing limits of detection of 10−6 mol∙L−1 to be attained. The oxidation of catechol at the surface of a AuNPNBr-S-ZnPcR modified ITO glass, shows enhanced charge transfer rate, high sensitivity with limits of detection of 10−7 mol∙L−1.
Acknowledgments
Financial support by MINECO-FEDER (AGL2015-67482-R) and the Junta de Castilla y Leon FEDER (VA-032U13) is gratefully acknowledged. Celia Garcia-Hernandez would also like to thank Junta de Castilla y León for a grant (BOCYL-D-4112015-9).
Conflicts of Interest
The authors declare no conflict of interest. The founding sponsors had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, and in the decision to publish the results.
References
- Blasco, A.J.; Crevillén, A.G.; González, M.C.; Escarpa, A. Direct electrochemical Sensing and Detection of Natural Antioxidants and Antioxidant Capacity in Vitro Systems. Electroanalysis 2007, 19, 2275–2286. [Google Scholar] [CrossRef]
- Rodriguez-Mendez, M.L.; de Saja, J.A. Nanostructured thin films based on phthalocyanines: Electrochromic displays and sensors J. Porphyr. Phthaloc. 2009, 13, 606–615. [Google Scholar] [CrossRef]
- Zagal, J.H.; Griveau, S.; Silva, J.F.; Nyokong, T.; Bedioui, F. Metallophthalocyanine-based molecular materials as catalysts for electrochemical reactions Coord. Chem. Rev. 2010, 254, 2755–2794. [Google Scholar] [CrossRef]
- Saha, K.; Agasti, S.S.; Kim, C.; Li, X.; Rotello, V.M. Gold Nanoparticles in Chemical and Biological Sensing Chem. Rev. 2012, 112, 2739–2779. [Google Scholar] [CrossRef]
- Medina-Plaza, C.; Furini, L.N.; Constantino, J.C.L.; de Saja, J.A.; Rodríguez-Mendez, M.L. Synergistic electrocatalytic effect of nanostructured mixed films formed by functionalised gold nanoparticles and bisphthalocyanines. Anal. Chim. Acta 2014, 851, 95–102. [Google Scholar] [CrossRef] [PubMed]
- Brust, M.; Walker, M.; Bethell, D.; Schiffrin, D.J.; Whyman, R.J. Synthesis of thiol-derivatised gold nanoparticles in a two-phase liquid–liquid system Chem. Soc. Chem. Commun. 1994, 7, 801–802. [Google Scholar] [CrossRef]
- Blas-Ferrando, V.M.; Ortiz, J.; Fernández-Lázaro, F.; Sastre-Santos, A. Synthesis and characterization of a sulfur-containing phthalocyanine-gold nanoparticle hybrid J. Porphyr. Phthaloc. 2015, 19, 1–9. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).