Abstract
Morphological and structural properties of layered Au, Pt-YSZ mixed-potential gas sensing electrodes (APE) prepared under different temperature treatments were studied by environmental scanning electron microscope (ESEM) and glow-discharge optical emission spectroscopy (GD-OES), and correlated with open-circuit potential (OCP) and cyclic voltammetry (CV) measurements under gas exposures at elevated temperatures. The OCP response of the APE sintered at 1050 °C is clearly higher than that of the APE sintered at 850 °C, and can be well correlated with a smaller oxygen reduction reaction (ORR) observed in the related CV diagrams. Moreover, a transition from the charge transfer reaction kinetics to the diffusional transport controlled mixed-potential formation was found between 550 °C and 650 °C.
Keywords:
solid electrolyte gas sensor; mixed potential; Au, Pt-YSZ electrode; OCP; CV; ESEM; GD-OES 1. Introduction
Mixed potential (MP) type gas sensors based on YSZ solid electrolyte, have been extensively studied over the last three decades due to their promising features related to analysis of combustible gas components in high-temperature applications [1]. In the recent development of such type of sensors, the combination of Au/AuPt-alloy and oxides was found to be a promising MP-electrode material. With this kind of material combination, satisfactory sensing characteristics were achieved using different kinds of electrode design, including uniformly mixing Au/AuPt-alloy with oxides or applying a Au mesh onto a Pt-YSZ electrode [2]. However up to now, there is only few information available about the electrochemical characteristics of these kinds of electrodes.
Recently, we reported that a thick film Pt-YSZ electrode modified by a thin-film Au layer deposited on top surprisingly showed MP characteristic as well, and the electrochemical behaviors of such kind of electrode are strongly influenced by the thickness of the Au layer [3]. To attain deeper knowledge about the role of Au addition on the electrode processes contributing to the mixed potential formation, combined structural and electrochemical investigations were carried out. Especially, the influence of the sintering temperature on the Au distribution in the MP-electrode was studied by ESEM, XRD and GD-OES material analytical methods, and related to the corresponding OCP sensing response and CV characteristic.
2. Materials and Methods
2.1. Sensor Element
The sensor element (CarboSen) studied in this work is commercially available from Lamtec GmbH, Walldorf, Germany. As schematically shown in Figure 1a, the CarboSen sensor element is comprised of two identical layered Au, Pt-YSZ electrodes on top of a porous YSZ layer, a buried Pt reference electrode (Pt-RE) located between an alumina substrate and the YSZ-layer and a Pt heater structured on the reverse side of the substrate. The APEs were prepared layer by layer (Figure 1b) in two steps: (i) the thick-film Pt-YSZ parts were first screen printed and sintered; (ii) Afterwards the thin gold films were deposited on top by PVD-technique and again sintered. According to different sintering processes for the as-prepared APEs, the sensors are classified into two types (type I: sintered at 850 °C for 10 min; type II: sintered at 1050 °C for 4 h). More technical details of the sensor element are described elsewhere [3].
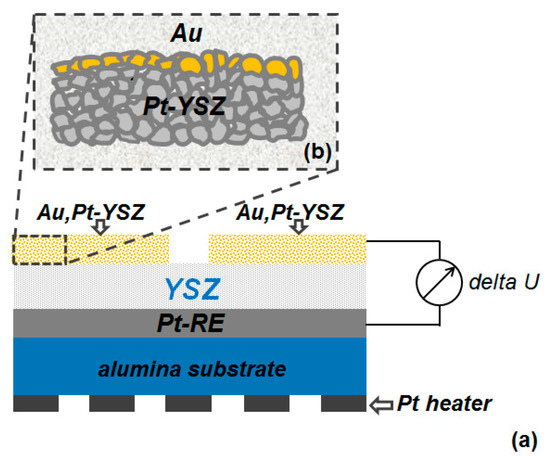
Figure 1.
(a) Schematic drawing of the CarboSen sensor element (cross-section view); (b) the layer structure of the as-prepared Au, Pt-YSZ mixed-potential sensing electrode before sintering.
2.2. Electrode Material Characterization
The surface morphological structures of both types of the APEs were studied with an ESEM type XL 30 FEG, Philips Inc. equipped with an EDX system. In addition, for further studies of the crystal structure on the surface of the APEs, and the Au distribution from the top surface into the bulk of the APEs, two Au, Pt-YSZ model samples with enlarged area were self-prepared in analogy to the type I- and the type II-electrode of the commercial sensors, respectively. The XRD study was carried out with a MiniFlex X-ray diffractometer (Rigaku Inc., Tokyo, Japan), and the Au distribution analysis was done by GD-OES using a GD Profiler 2TM (HORIBA, Kyoto, Japan).
2.3. OCP and CV Measurements
OCP and the CV measurements were carried out using an automated gas mixing system (described elsewhere [3]) outfitted with an electrochemical working station (MultiEmStat, PalmSens, Houten, The Netherlands). The sensing behavior of both types of sensors was studied by measuring the potential difference (∆U) between the APE and the Pt-RE (Figure 1) under exposure to various concentrations of CO balanced with synthetic air at 550 °C, 600 °C and 650 °C separately, while the CV measurements were only conducted in synthetic air at 600 °C.
3. Results and Discussion
Figure 2 shows the ESEM images of the APEs of both types of sensors after different sintering processes (Section 2.1). A porous electrode covered with an inhomogeneous bright Au layer (confirmed by EDX analysis) was found for the type I sensor. However, such a layer seems to be more uniformly distributed at the type II APE. The XRD study showed that only at the type I APE (sintered at 850 °C) Au formed a separate crystalline phase on the surface (not shown here). These differences in the electrode surface morphology and crystallization might indicate that the Au was probably more intensively alloyed with Pt and better equilibrated with the inner parts of the electrode by diffusion during the sintering process (1050 °C) of type II APE.
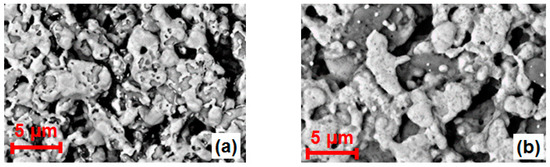
Figure 2.
(a) ESEM image (top view) of the type I APE; (b) ESEM image (top view) of the type II APE.
In addition, the GD-OES analysis experiments confirm that the Au distribution from the top surface into the bulk of the Au, Pt-YSZ electrodes is strongly dependent on the sintering conditions (Figure 3). A more even Au distribution was found for the type II-APE compared to that of the type I-APE. These clear differences in the morphological, crystal and structural properties of the APEs are assumed to be related to different catalytic activities over the electrode layer, and probably to their electrochemical behavior as well.
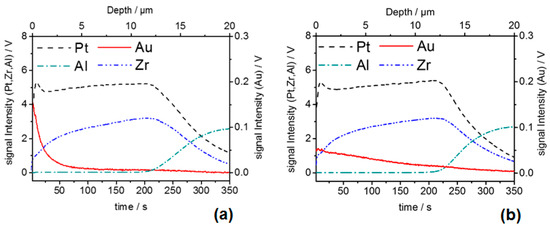
Figure 3.
(a) GD-OES analysis of elemental depth profile of the self-prepared model sample sintered at 850 °C for 10 min; (b) elemental depth profile of the model sample sintered at 1050 °C for 4 h.
The response behaviors of the sensors at 600 °C are shown in Figure 4a. Both types of sensors showed stable and reversible negative OCP response to stepwise variation of CO concentration in the upward and downward change. The response of the type II sensor, however, was found to be clearly higher than that of the type I sensor. Correspondingly, the current in the CV measurement is much smaller for the type II sensor compared to that for the type I sensor (Figure 4b). This indicates that the catalytic activity of the type II APE for oxygen reaction is significantly lower compared to that of the type I APE, which is related to the more uniform Au-distribution over the inner parts of the type II APE. This clear correlation between the catalytic activity of the APE and the OCP response is in good agreement with the sensitivity model for the APE proposed in [3].
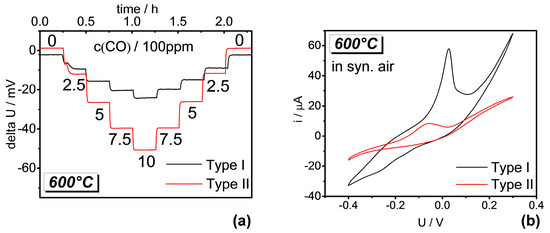
Figure 4.
(a) Responses of both types of sensors under exposure to CO at different concentrations (conc.) balanced with synthetic air; (b) related cyclic voltammograms in synthetic air at 600 °C.
The study of the dependence of sensitivity behavior on temperature disclosed an interesting sensing characteristic of the type I sensor (Figure 5). As expected, at the higher temperature the response to CO is significantly reduced. However, a logarithmic dependence of the delta U on CO concentration was found only at 550 °C (Figure 5a), while a linear dependence was observed at 650 °C (Figure 5b). The change from the logarithmic to the linear dependence of delta U indicates a transition from the charge transfer reaction kinetics to the diffusional transport controlled mixed-potential formation between 550 °C and 650 °C as described in [4].
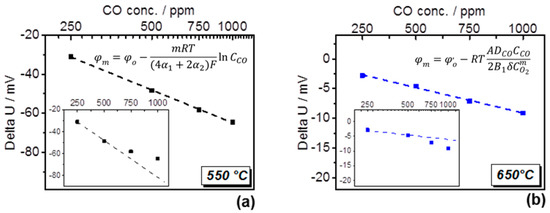
Figure 5.
Type I sensor: (a) dependence of delta U on CO conc. at 550 °C on logarithmic and linear (inset) scale, and its mathematical expression; (b) dependence of delta U on CO conc. at 650 °C on linear and logarithmic (inset) scale, and its mathematical expression.
4. Conclusions
The layered Au, Pt-YSZ electrodes under different temperature treatments were studied by different kinds of material analytical tools and electrochemical methods. The morphological and structural properties of the electrodes and their electrochemical behaviors are highly influenced by the electrode sintering temperature. The electrode sintered at 1050 °C provided a more uniform Au distribution not only on the surface but also in the inner part of the electrode, and showed a better response to CO than that sintered at 850 °C. The better sensing characteristics of the electrode sintered at 1050 °C are probably attributed to its reduced catalytic activity for ORR. In addition, for the electrode sintered at 850 °C, a transition from the charge transfer reaction kinetics to the diffusional transport controlled mixed-potential formation was found between 550 °C and 650 °C.
Author Contributions
Those authors addressed in the affiliations contributed to this publication.
Acknowledgments
The authors wish to acknowledge the support with sensor elements for investigation and technical information by Marko Völkel and Frank Hammer (Lamtec GmbH, Walldorf, Germany).
Conflicts of Interest
The authors declare no conflict of interest.
References
- Riegel, J.; Neumann, H.; Wiedenmann, H.M. Exhaust gas sensors for automotive emission control. Solid State Ion. 2002, 152–153, 783–800. [Google Scholar] [CrossRef]
- Miura, N.; Sato, T.; Anggraini, S.A.; Ikeda, H.; Zhuiykov, S. A review of mixed-potential type zirconia-based gas sensors. Ionics 2014, 20, 901–925. [Google Scholar] [CrossRef]
- Zhang, X.; Kohler, H.; Schwotzer, M.; Wu, Y.H.; Guth, U. Mixed-potential gas sensor with layered Au, Pt-YSZ electrode: Investigating the sensing mechanism with steady state and dynamic electrochemical methods. Sens. Actuators B Chem. 2017, 252, 554–560. [Google Scholar] [CrossRef]
- Garzon, F.H.; Mukundan, R.; Brosha, E.L. Solid-state mixed potential gas sensors: Theory, experiments and challenges. Solid State Ion. 2000, 136–137, 633–638. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).