How Clinical Research Can Contribute to Strengthening Health Systems in Low Resource Countries
Abstract
:1. Introduction
- the quality of the information produced;
- its relevance to significant health problems;
- its contribution to the creation or evaluation of interventions, policies, or practices that promote individual or public health.
2. Materials and Methods
3. Results
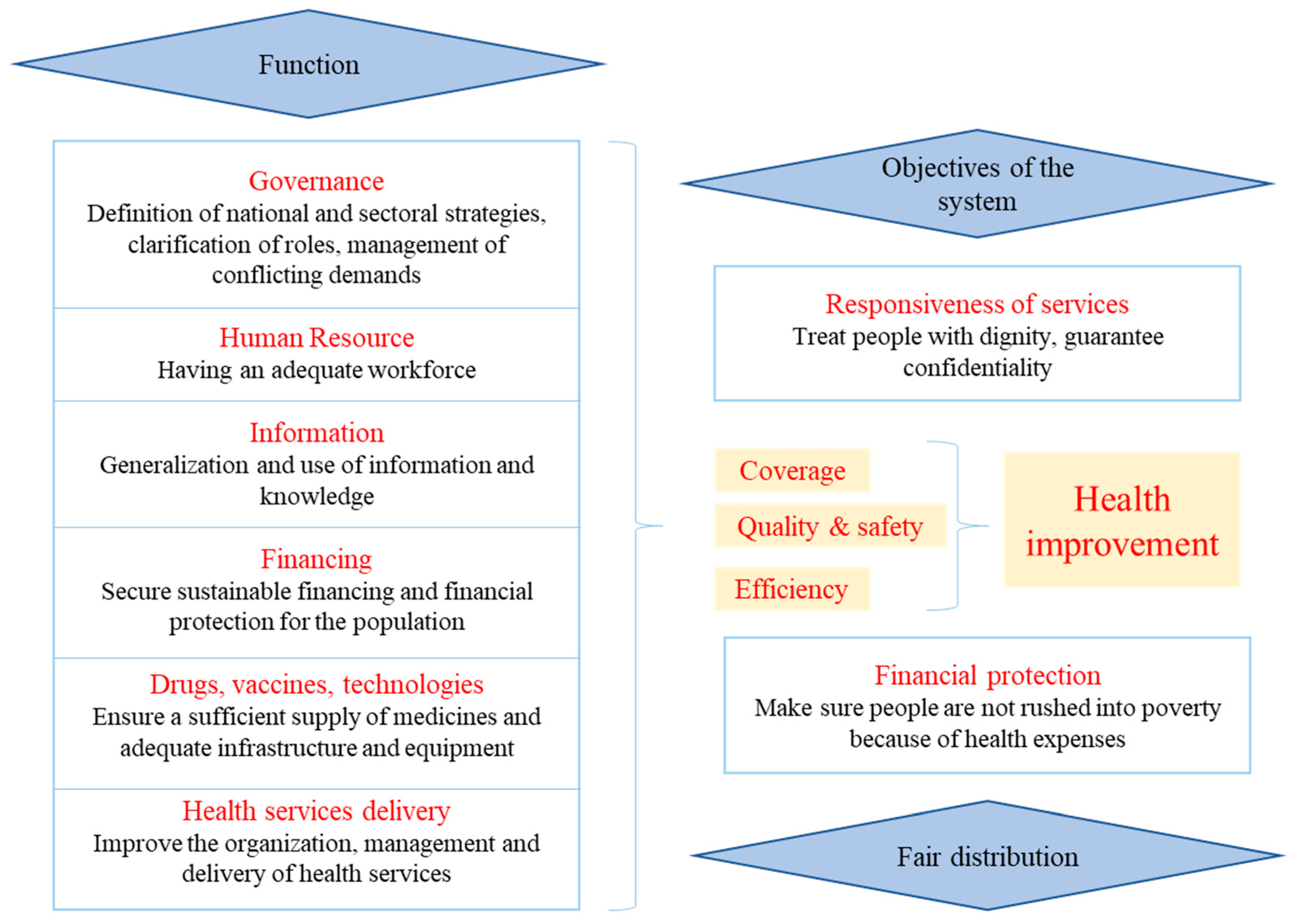
3.1. Leadership and Governance
3.2. Human Resources
3.3. Organization of Services
3.4. Organization of Health Information
3.5. Material Resources
3.6. Funding
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- CIOMS/WHO. International Ethical Guidelines for Health-Related Research Involving Humans; World Health Organization: Geneva, Switzerland, 2016. [Google Scholar]
- Hotez, P.J.; Pecoul, B. “Manifesto” for Advancing the Control and Elimination of Neglected Tropical Diseases. PLoS Negl. Trop. Dis. 2010, 4, e718. [Google Scholar] [CrossRef]
- Amidou, B.; Saidou Pathé, B.; Kandjoura, D. Renforcement des systèmes de santé dans les pays de la région africaine de l’OMS: Répondre au défi. Afr. Health Monit. 2012, 14, 4–13. Available online: http://afrolib.afro.who.int/index.php?lvl=notice_display&id=29235 (accessed on 4 March 2020).
- WHO. Renforcer la Gestion Dans Les Pays à Faible Revenu, Document de Travail No. 1; WHO/EIP: Geneva, Switzerland, 2005. [Google Scholar]
- Mbo, F.; Valverde, O. The human African trypanosomiasis (HAT) platform. In Trop Med International Health, Proceedings of the 10th ECTMIH, Antwerp, Belgium, 11–19 October 2017; John Wiley & Sons Ltd.: Hoboken, NJ, USA, 2017; Volume 22, (Suppl. S1), pp. 115–345. [Google Scholar]
- WHO. Rapport Mondial sur la Santé Dans le Monde 2000: Pour un Système de Santé Performant; World Health Organization: Geneva, Switzerland, 2000. [Google Scholar]
- Valverde, O. Impact des études cliniques visant à développer des médicaments trypanocides sur les efforts d’élimination de la maladie. In Proceedings of the 4th Joint HAT Platform-EANETT Scientific Meeting, Conakry, Guinea, 16 September 2016; Available online: https://tinyurl.com/syc5637 (accessed on 17 March 2020).
- WHO. La Santé Des Populations—Rapport Sur la Santé Dans la Région Africaine; OMS, Bureau régional de l’Afrique: Brazzaville, Congo, 2006. [Google Scholar]
- Mesu, V.K.B.K.; Kalonji, W.M.; Bardonneau, C.; Mordt, O.V.; Blesson, S.; Sc, F.S.; Delhomme, S.; Bernhard, S.; Kuziena, W.; Med, J.-P.F.L.M.; et al. Oral fexinidazole for late-stage African Trypanosoma brucei gambiense trypanosomiasis: A pivotal multicentre, randomised, non-inferiority trial. Lancet 2018, 391, 144–154. [Google Scholar] [CrossRef]
- Drugs for Neglected Diseases Initiative. Available online: https://www.dndi.org/strengthening-capacity/hat-platform/ (accessed on 16 January 2020).
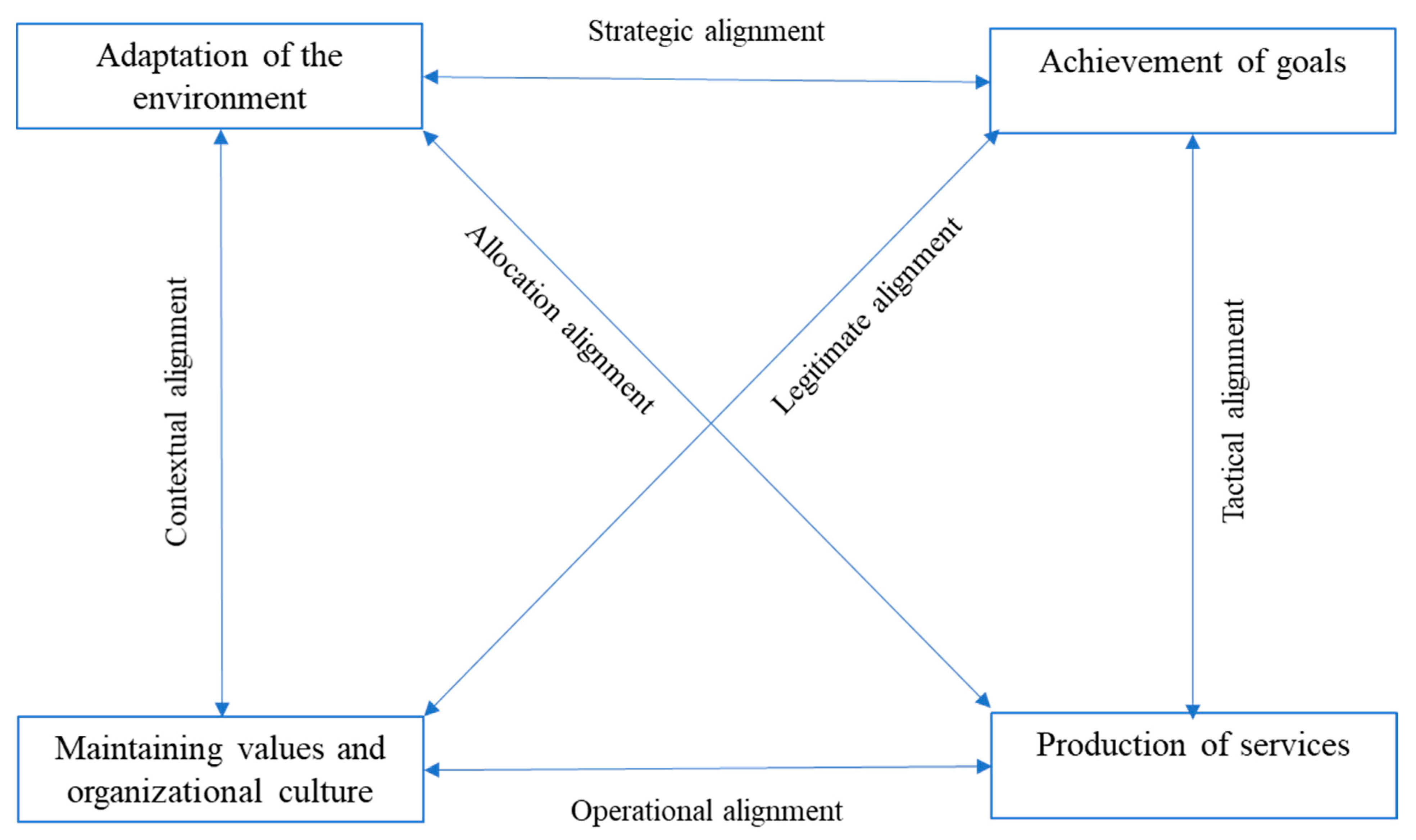
- Sicotte, C.; Champagne, F.; Contandriopoulos, A.P.; Barnsley, J.; Béland, F.; Leggat, S.G.; Denis, J.L.; Bilodeau, H.; Langley, A.; Brémond, M.; et al. A conceptual framework for the analysis of health care organizations’ performance. Health Serv. Manag. Res. 1998, 11, 24–41. [Google Scholar] [CrossRef] [PubMed]
| Trainings Conducted (Number of People Trained) | Venue and Year |
|---|---|
| Training in ethical review of research (142) | Kinshasa 2007 |
| Khartoum 2007 | |
| Kampala 2007 | |
| Luanda 2008 | |
| Juba 2009 | |
| Bangui 2010 | |
| Training of physicians in good clinical practice (GCP) (96) | Nairobi 2006, Kinshasa 2011 and 2012, Juba 2012 |
| Training of physicians on clinical examination of the patient (25) | Kinshasa 2007 |
| Training of clinical monitors (13) | Kampala 2008 |
| Participation at ICAT6 and -7 (26) | Kinshasa 2014, Kampala 2017 |
| HAT training in Dinamadji health district (30) | Dinamadji 2015 |
| HAT clinical training in South Sudan (41) | Juba 2015 |
| Training of Guinean physician in DRC (1) | Kinshasa 2014 |
| Training of laboratory technicians from South Sudan in DRC (3) | Kinshasa 2016 |
| Training of mobile team technicians on HAT diagnosis in DRC (36) | Kinshasa 2016 |
| Waste management training at clinical trial sites in DRC (182) | Mushie, Vanga, Bagata, Masi 2016 |
| Material Need. | Provision |
|---|---|
| Food | Provided for all HAT patients, irrespective of trial participation |
| Accommodation | Beds repaired or purchased; new mattresses and mosquito nets; dedicated wards repainted; floors, windows repaired |
| Personal hygiene | Latrines and shower blocks built; water supply arranged, including rainwater reservoirs, for the benefit of staff and patients |
| Office space | A nursing room, investigator’s office, lockable cupboards, and furniture were provided for the research team and trial documentation |
| Waste management | A closed waste disposal area with three separated pits was provided or improved; incinerators were built or rehabilitated |
| Laboratory space | Refurbishment through rebuilding of interiors, including working surfaces and necessary equipment |
| Energy | Generators and solar systems for lightning, electric equipment, and a cold chain were provided. |
| Communication equipment | Computers, printers, internet access, and telephone cards were provided |
| Items | Unit Cost in USD (Euro Converted at USD 1.1) |
|---|---|
| Rehabilitation Works | |
| Preparation and construction of waste areas | USD 10,150 |
| Latrine and shower construction | USD 8730 |
| Rainwater collection system | USD 5000 |
| Preparation of investigators’ offices | USD 750 |
| Lab preparation for routine exams | USD 17,500 |
| 5kva solar panels | USD 21,000 |
| Medical Equipment | |
| Pavilion equipment with 12 beds | USD 1800 |
| Foldable examination table | USD 164 |
| Mechanical weight and height scale | USD 227 |
| Life support equipment | USD 2443 |
| Emergency bag kit | USD 811 |
| Laboratory Equipment | |
| Microscopes with Camera | USD 3470 |
| 8-tube electric centrifuges | USD 1122 |
| Electric Hematocrit Centrifuges | USD 1467 |
| HemoCue Hb 301 | USD 548 |
| Eppendorf" automatic pipette | USD 242 |
| Cold Chain | |
| Vestfrost refrigerator | USD 895 |
| Cold chain (Freezer + specific solar panels + batteries) | USD 24,074 |
| Transport and Office Equipment | |
| Motorbike Yamaha AG100 | USD 4600 |
| Laptop | USD 1000 |
| Internet connection kit | USD 2760 |
| Printer, scanner, photocopier | USD 300 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mbo, F.; Mutombo, W.; Ngolo, D.; Kabangu, P.; Valverde Mordt, O.; Wourgaft, N.S.; Mwamba, E. How Clinical Research Can Contribute to Strengthening Health Systems in Low Resource Countries. Trop. Med. Infect. Dis. 2020, 5, 48. https://doi.org/10.3390/tropicalmed5020048
Mbo F, Mutombo W, Ngolo D, Kabangu P, Valverde Mordt O, Wourgaft NS, Mwamba E. How Clinical Research Can Contribute to Strengthening Health Systems in Low Resource Countries. Tropical Medicine and Infectious Disease. 2020; 5(2):48. https://doi.org/10.3390/tropicalmed5020048
Chicago/Turabian StyleMbo, Florent, Wilfried Mutombo, Digas Ngolo, Patrice Kabangu, Olaf Valverde Mordt, Nathalie Strub Wourgaft, and Erick Mwamba. 2020. "How Clinical Research Can Contribute to Strengthening Health Systems in Low Resource Countries" Tropical Medicine and Infectious Disease 5, no. 2: 48. https://doi.org/10.3390/tropicalmed5020048
APA StyleMbo, F., Mutombo, W., Ngolo, D., Kabangu, P., Valverde Mordt, O., Wourgaft, N. S., & Mwamba, E. (2020). How Clinical Research Can Contribute to Strengthening Health Systems in Low Resource Countries. Tropical Medicine and Infectious Disease, 5(2), 48. https://doi.org/10.3390/tropicalmed5020048







